General Categorization
This is an optional component of TBS 2001.8 It was designed to assist clinicians and/or their staff to triage cervical smear reports adequately and includes:
- No intraepithelial lesion/malignancy: encompasses the previous TBS 1991 categories
of “within normal limits” and “benign
cellular change/organisms” and “reactive cellular change.” These
cytologic changes can be described under this category
as “nonneoplastic findings.”
- Epithelial cell abnormalities: further specified as squamous, glandular
or extrauterine/other.
- Other: this was added as a category for cases in which there are no cytologic
abnormalities per se in the cells, however the finding may indicate
some risk of a preneoplastic or neoplastic condition (e.g., endometrial
cells in a woman >40 years of age).
These three general categories are mutually exclusive; thus, if several findings are present, the general categorization should be based on the most clinically significant result.8
Interpretation/Result
The unanimous opinion at the 2001 Bethesda workshop was that cervical cytology is primarily a screening test, which in certain instances may serve as a medical consultation, by providing an interpretation/result that contributes to a diagnosis.8 Because an individual patient’s final diagnosis and subsequent management, should integrate the clinical and laboratory results, in TBS 2001, the term “diagnosis” has been replaced by “interpretation” or “result.”8
Nonneoplastic Changes
Reporting of certain organisms (Trichomonas, fungi (yeast), shift in flora, Actinomyces, Herpes) is done by most laboratories; however reporting of other non-neoplastic findings is variable, and optional under TBS 2001 guidelines.8
ORGANISMS.
The vaginal flora is composed of multiple microorganisms. The most common are Lactobacillus vaginalis, Streptococcus viridans, and Staphylococcus epidermidis. They thrive in an environment in which balance is established by hormones as well as physical influences, such as intrauterine devices. Occasionally, intrauterine devices influence the balance of the vaginal flora, causing symptoms and infection with actinomyces.23
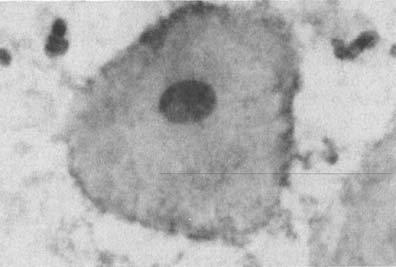
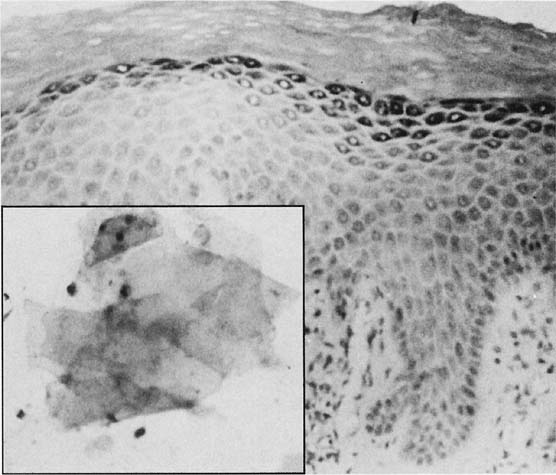
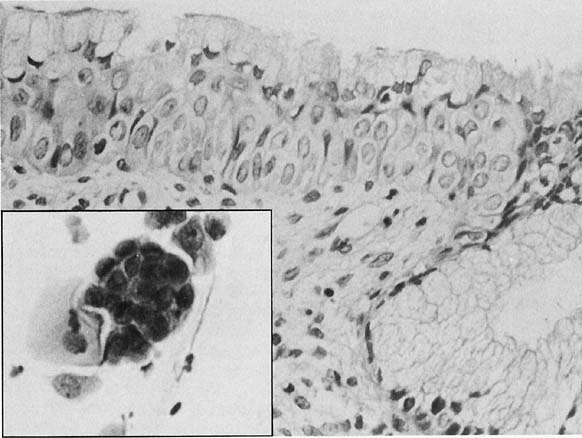
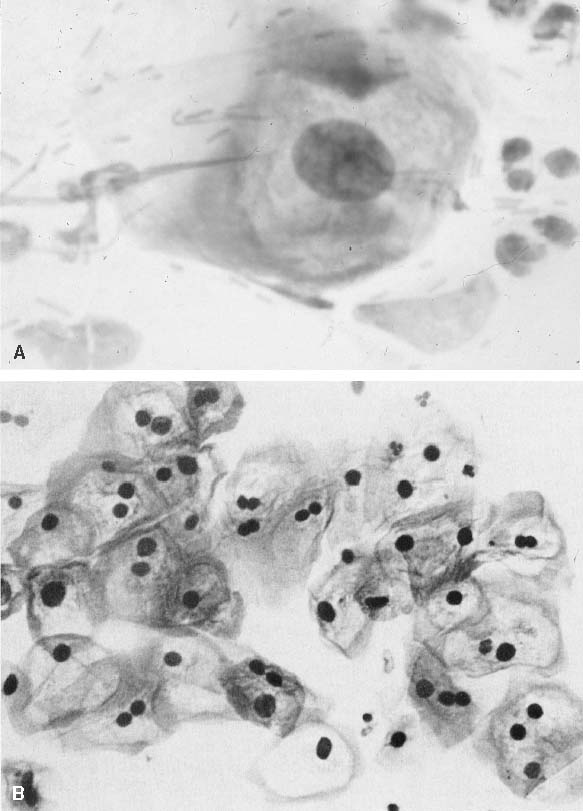
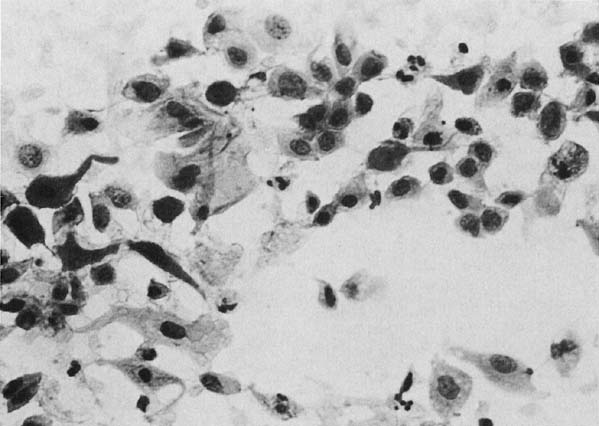
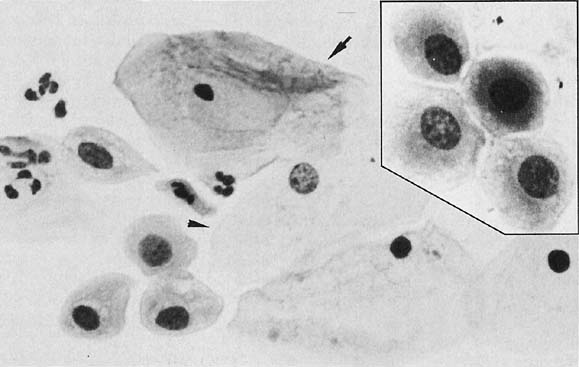
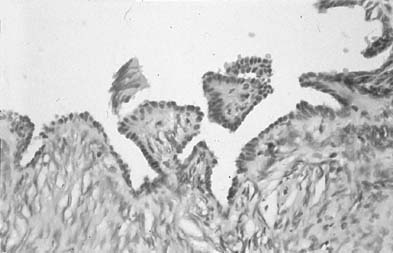
Changes caused by inflammation include metaplasia, surface reactions, such as hyperkeratosis and parakeratosis, and reparative processes.23 Frequently, the nuclei of the epithelial cells show enlargement, but the chromatin is fine, and cytoplasmic changes are variable (Fig. 1). In other instances, degenerative changes occur.23 The background may show numerous polymorphonuclear leukocytes and debris, a large number of bacterial forms, and histiocytes. When this exudate obscures the surface of the epithelial cells, it is advisable to treat the infection and repeat the smears because dysplastic or malignant cells may be hiding beneath the white blood cells, bacteria, and debris. Nonspecific vaginitis is commonly seen in patients harboring flora such as mixed bacteria or coccobacilli. Occasionally, cells show inflammatory changes in a background of rod bacteria that appear to be Döderlein’s bacilli. These bacteria belong to a heterogeneous group of organisms, and they morphologically resemble other microorganisms that may cause inflammatory changes in the epithelial cells. The presence of some polymorphonuclear leucocytes by itself is not an indication of inflammation, reactive changes in the epithelial cells are necessary.
|
Bacteria.
Gardnerella vaginalis, mobilincus, and other anaerobic bacteria (bacterial vaginosis) may be found in asymptomatic women (40% to 50% of patients), and other women may develop leukorrhea and an inflamed vaginal mucosa (see Fig. 1).23 Smears show cells covered by bacteria, so called clue cells, against a background of feathery cocobacillary forms (see Fig. 1).
Actinomyces species occur frequently in association with intrauterine device usage. Less frequently, the use of a pessary, tampons, or any type of foreign body left in place for long periods in the cervicovaginal area may elicit infection with this bacteria. Pelvic infection may occur in some patients. In Papanicolaou-stained smears, filamentous bacteria are seen, usually in clumps as well as single forms.
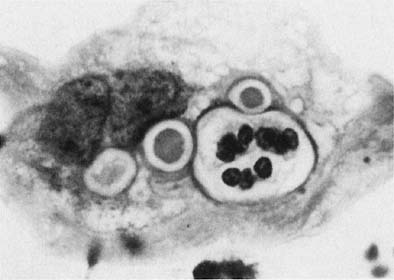
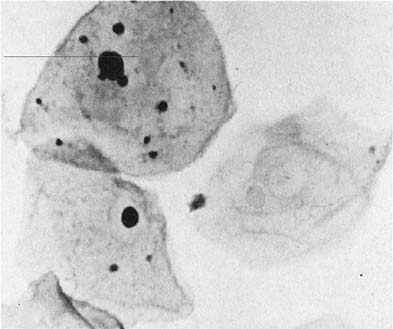
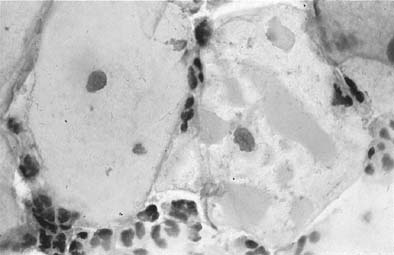
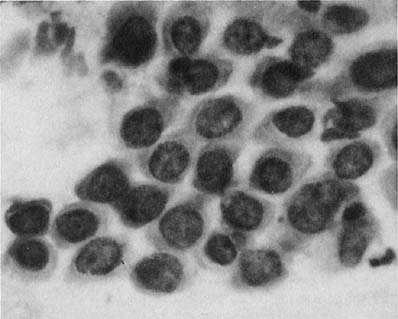
Chlamydia trachomatis infection is seen in cervicovaginal smears as cytoplasmic bacterial inclusions (Fig. 2). The false-positive rate is high for morphologic diagnosis, and mucus secretion in immature metaplastic cells, phagocytosed debris, and degenerative changes may be the source of erroneous diagnosis of C. trachomatis infection. Because the distinction between these entities is impossible on a morphologic basis alone, more specific microbiologic assays (polymerase chain reaction [PCR], enzyme immunoassay [EIA]) are now used for identification of chlamydia. The FDA recently approved testing for chlamydia out of the liquid-based collection devices.
|
Fungi (yeast).
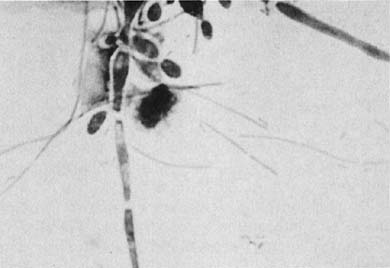
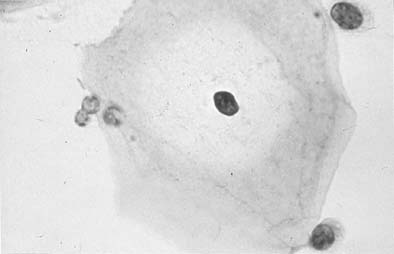
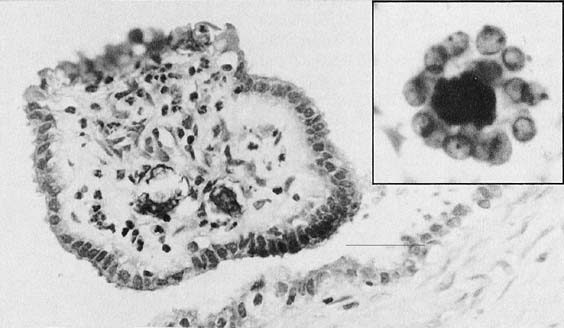
Candida albicans is the most common fungus found in the cervicovaginal smear (Fig. 3). Yeast forms as well as pseudohyphae are commonly encountered. Diabetes, pregnancy, birth control pills, and antibiotic use may change the vaginal flora, and predispose patients to infection by this fungal agent. Fewer than 10% of mycotic vaginal infections are caused by another Candida species, Torulopsis glabrata, which is morphologically similar to the yeast form of C. albicans.
|
Flagellates.
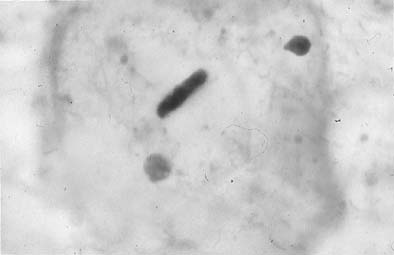
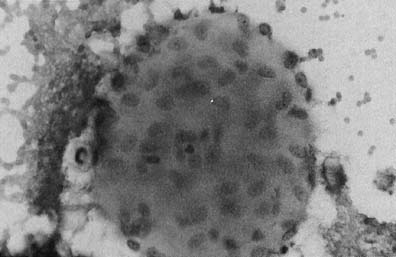
Trichomonas vaginalis infection may cause changes that range from minimal to severe. Fifty percent of infected women are asymptomatic.23 The classic signs of inflammation are present in the epithelial cells and also in the background of the smears that contain variable amounts of debris and WBCs. Small perinuclear halos may be seen in the squamous cells, but this finding is not pathognomonic of this infectious process. The organisms are seen as pear-shaped structures with a punctate, almond-shaped nucleus. Flagella rarely are seen in smears prepared with the Papanicolaou technique. Without the presence of nuclei, a diagnosis of T. vaginalis cannot be made because fragments of cytoplasm of the intermediate squamous cells may resemble these flagellates. Leptothrix (Leptotrichia buccalis) are filamentous bacteria that frequently are associated with T. vaginalis (70% to 80% of cases) (Fig. 3). Certain forms of Döderlein’s bacilli are morphologically identical to Leptotrichia, and only by culture can they be identified adequately.
Viruses.
The most common viral infections encountered in cervicovaginal smears are caused by Herpes genitalis and papillomaviruses. Herpes genitalis infection occurs primarily by sexual transmission. Clinically symptomatic eruptions may occur. Usually, they are related to stress, the menstrual period, or unrelated diseases. Cytologically, the epithelial cells show multinucleation, the chromatin assumes a ground-glass appearance, and intranuclear inclusions may appear in some cells.
Over the years, there have been several reports in the literature suggesting that microorganisms that induce changes in the cervical epithelium, may be a potentiating cause for the development of cervical neoplasia. Trichomonas vaginalis,24 Chlamydia trachomatis,25 and Herpes genitalis26,27 cause inflammatory changes that not infrequently associated with SIL and may produce fertile ground for the development of preneoplastic and neoplastic squamous lesions. Immunosuppression may be a setting for viral infection, such as HPV and herpes simplex, and also for the development of neoplasia.28 However, it was not until the 1970s that the tools of modern molecular biology were applied to the molecular characterization of the papillomavirus family that HPV,29 with more than 100 molecular types, emerged as the major player in the etiology of cervical neoplasia.
BENIGN/REACTIVE CHANGES.
Inflammation and Regenerative Changes.
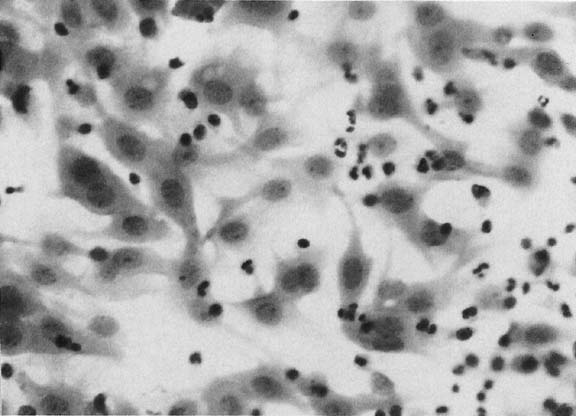
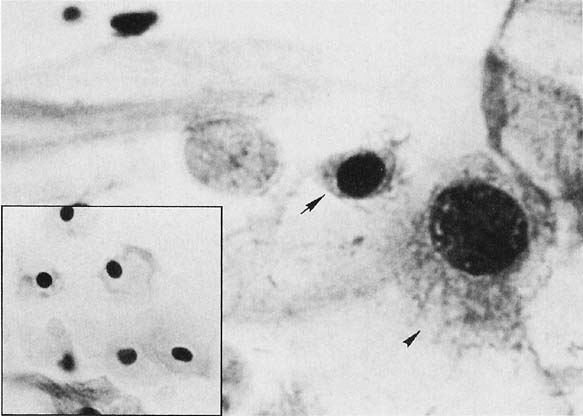
A common finding in cervicovaginal smears is reparative change as a result of a variety of processes30,31 that may involve the glandular, metaplastic, and squamous epithelium. These changes are seen after damage to the mucosa and after radiation, chemotherapy, infections, or any condition that alters the tissue integrity. Inflammation of the squamous epithelium can be seen in cells of metaplastic or mature squamous origin. When a reparative process is present, monolayers of cells are seen with enlarged nuclei and nucleoli, variable chromatin content, and delicate cytoplasm with ill-defined borders. Isolated atypical cells of a reparative nature are rare. The cytologic features include enlargement of the nuclei without relative increase in chromatin content. The cytoplasm may acquire increased thickness, with a variable staining quality (Fig. 4). Inflammatory changes in epithelial cells are differentiated from dysplasias and carcinomas by the relative low content of chromatin, symmetrical nuclear outline, and low nuclear-cytoplasmic ratio. The cytoplasm may be thickened and the nuclei and nucleoli enlarged; however, the chromatin is powdery and distributed regularly (Fig. 4). When inflammatory/reparative changes cause epithelial atypia, in either squamous or endocervical cells, the changes maybe classified as atypical squamous cells (ASC) or atypical glandular cells (ACG). Follow-up is needed in these cases to make sure the atypia has regressed.
|
Differences exist between these proliferating cells and invasive squamous carcinoma, such as the absence of single atypical cells, macronucleoli, a low nuclear-cytoplasmic ratio, and the absence of tumor diathesis (Fig. 4), and are important for the differentiation of repair from nonkeratinized squamous carcinoma and, rarely, from sarcomas.30,31
Therapy Changes.
Several modalities of therapy affect malignant and benign cells, the latter at the vicinity or at a distance from the malignant neoplasm. The types of therapy include chemotherapy, immunotherapy, radiation therapy, and mechanical therapy.32 Therapy of any modality may cause changes in benign cells that can resemble malignant changes.
By examining cervicovaginal smears, short- and long-term radiation changes can be observed. In benign cells, short-term changes usually are found within 6 months of irradiation. These changes consist of nuclear enlargement, with condensation of chromatin, abnormal mitosis, and multinucleation. At the cytoplasmic level, there are varying degrees of vacuolization. Cellular enlargement occurs, and a low nuclear-cytoplasmic ratio is maintained. This feature helps the cytopathologist to recognize the affected cells as benign. Short-term changes can also be encountered in malignant cells shortly after treatment. However, with effective treatment, they regress approximately 1 month after the completion of therapy because of their exquisite sensitivity to radiation.33 Malignant cells that show radiation changes 6 to 8 weeks after treatment indicate persistence of the neoplasm. After this time, the presence of malignant cells without radiation changes indicates recurrence.
The features seen with long-term radiation changes are reflected in cytologic smears by aberrant cells from the basal layers, cellular enlargement, and atrophy. These changes can persist for several years, and vary from patient to patient. Nuclear, nucleolar, and cytoplasmic alterations reflect the destruction of chromatin,34 increased ribonucleic acid synthesis followed by decreased DNA synthesis,35 and abnormal levels of various cytoplasmic elements, respectively. The end result of these changes is seen at the ultrastructural level as destructive changes of the nuclear and cytoplasmic organelles.34 Under light microscopy, cytoplasmic vacuolization starts at the parabasal layers, and engulfment of polymorphonuclear leukocytes within some of the vacuoles are seen. Some of the cells acquire an abnormal staining quality referred to as amphophilia. The most important diagnostic characteristic change is significant enlargement of the epithelial cells, with otherwise normal features. Giant cell histiocytes, and reparative changes,36 are usually encountered. The effectiveness of radiation can be monitored by the assessment of several cellular features in cervico- vaginal smears.33,34,37,38 The cytopathologist should observe the postradiation smear for increased cellular maturation, which is a warning sign for the development of de novo intraepithelial neoplasia. Equally important is the detection of tumor diathesis, which may indicate persistence or recurrence of the tumor. True tumor diathesis has necrotic cells with nuclear and cytoplasmic fragmentation, hemolyzed, blood, and debris; the mere presence of a “grungy” background is not sufficient to qualify.
Systemic chemotherapy may affect cells exfoliated from the cervicovaginal area. Cellular changes bear some similarities to changes caused by ionizing radiation.39 Occasionally, the cells look highly abnormal, except for the maintenance of the amount of cytoplasm and the absence of mitotic figures.40 After the use of several modalities of immunosuppressive therapy for organ transplantation, there is an increase in the number of neoplasms at several body sites,41 including the vulva and cervix.42 The incidence of cervical neoplasms has been reported to reach a 14-fold increase compared with the incidence in the nonimmunosuppressed population. It is advisable to have a baseline cervicovaginal smear to monitor the development of neoplasms during immunosuppressive therapy.32
Electrocautery of the uterine cervix produces coagulative cellular necrosis and significant inflammatory response represented by polymorphonuclear leukocytes and lymphocytes. The latter has been postulated to represent a host immune response.32 These changes may mimic a tumor diathesis background. In addition, cervicovaginal smears may harbor atypical cells after electrocautery treatment for benign processes. Because these cells may persist for several weeks,43 a waiting period of 4 to 6 weeks should be allowed to permit the necrosis and healing process to occur.32 These changes are not limited to the squamous epithelium; atypia of the endocervical cells is also encountered.32 For these reasons, a history of electrocautery as well as the date of therapy for neoplastic and nonneoplastic processes should be disclosed to the pathologist to avoid misinterpretation of cervicovaginal smears. Laser therapy has been used for years to eradicate dysplasia and CIS of the uterine cervix.44,45 The carbon dioxide laser produces a localized intense heat that inactivates cellular components, interrupting the replication process at the cellular level.45 Laser therapy has several advantages over cryotherapy with respect to cervicovaginal smears. Because of the almost-complete absence of bleeding and necrosis as well as the faster healing process, the inherent difficulties (atypical cells and inflammatory background) in cryotherapy are short-lived with laser treatment, and the cervicovaginal smears return to normal quickly.44,45,46,47 The regenerative process takes place from the epithelial edges of the ulcer that is produced by the laser and fills the gap within 2 weeks. In spite of a positive Schiller’s test result, the cells desquamated from this site are easily recognized as benign.44,48 After approximately 6 weeks, this test result becomes negative as the epithelium matures in the newly re-epithelialized site.46
Benign Surface Reactions Of Squamous Epithelium.
Hyperkeratosis.
Under the influence of a variety of stimuli, the surface of the squamous epithelium may develop a granular layer and several layers of anucleated cells as a protective mechanism. This phenomenon is referred to as hyperkeratosis (Fig. 5). Clinically, this condition is seen as an area of leukoplakia; cytologically, the smears contain single or groups of anucleated squames (see Fig. 5).49 The cells derived from the granular layer show keratohyaline cytoplasmic granules (Fig. 6).
|
Thick patches of those cells with irregular outlines seen consistently throughout the smear are of diagnostic importance. Keratohyaline granules and a few anucleated squames are normally seen at the height of estrogenic stimulation at midcycle and have no clinical significance. However, the presence of many of these cells, associated with patches of anucleated squames, indicates hyperkeratosis and presence of a granular layer in the cervical mucosa. Occasional anucleated cells on the edge of the slides or in the specimen should not be mentioned in the diagnosis because they probably represent contamination from the patient’s vulva or from the fingers of the person handling the slides. It is thus advisable to report only cells with keratohyaline granules associated with anucleated squames that are intermixed with other normal components of the smears. Extensive hyperkeratosis, when appropriately identified, may be associated with underlying abnormal epithelium in up to 42% of patients.31,49
Parakeratosis.
Parakeratosis is another type of surface reaction that is characterized by a proliferation of layers of small nucleated squamous cells with pyknotic nuclei. In smears, miniature keratinized squamous cells are seen, either singly or in sheets (Fig. 7). The nuclei are small and either round or oval. The nuclear-cytoplasmic ratio is low. Parakeratotic cells without nuclear abnormalities can be seen in a variety of situations, such as inflammation, pessary use, and other processes that are irritative to the cervical mucosa. When they are enlarged and irregular, they are classified as atypical squamous cells or atypical parakeratosis (see Fig. 7).31,49 Atypical parakeratosis is frequently (84%) associated with underlying abnormal epithelium. Once a diagnosis of atypical parakeratosis is made, the smears should be repeated with a forceful scrape to obtain cells of deep layers. Some authors36 advise that two smears should be taken, the first to remove the parakeratotic cells and the second to obtain deeper cells, which may be abnormal (see Fig. 7).
Intrauterine Device Effect.
The presence of an intrauterine device in the endocervical and endometrial cavity causes an inflammatory response and subsequent irregular desquamation and shedding of these epithelial cells from these areas may mimic glandular or squamous neoplasm. Squamous metaplasia showing variable degrees of maturation is frequently seen in ectocervical-endocervical smears. The immature metaplastic cells may maintain the mucus-producing ability inherent to endocervical cells because they have a similar histogenesis. This feature mimics the appearance of atypical glandular cells, and may be difficult for the cytologist to identify. Immature metaplasia in single cells with nuclear enlargement only, may mimic high-grade squamous dysplasia. Atypical glandular cells of endocervical32,50 or endometrial origin also may be encountered.51 They can be recognized as reactive or benign if clinical information about intrauterine device use is disclosed, avoiding false-positive results.51 A less common cell type, referred to as indeterminate, has been postulated as being of endometrial origin.52,53 Few of these cells are seen in a cervicovaginal smear, and they have a variety of features that distinguish them from CIS, such as multinucleation, presence of enlarged nucleoli, and absence of other atypical cells in the smear showing a spectrum of dysplastic changes.32 After removal of the device, these cells disappear from cervicovaginal smears after 1 month to 1 year.53
Microglandular Hyperplasia.
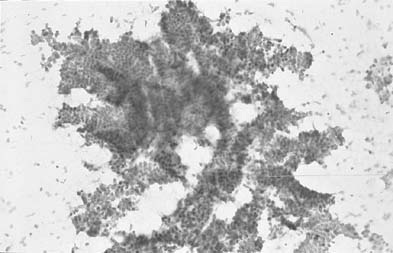
Microglandular hyperplasia has been described in patients taking oral contraceptives, in pregnancy, and postpartum. Therefore, it was considered a reflection of the action of progestrogenic stimulation on the endocervical epithelium. However, cases have also been seen in patients with no history of hormonal intake and occasionally in patients with hyperestrogenism or exogenous estrogenic therapy. It is not considered to be a preneoplastic change. Florid microglandular hyperplasia in histologic sections may architecturally resemble clear cell adenocarcinoma.54 Lack of stromal invasion, rare mitosis, and intracellular glycogen excludes the diagnosis of malignancy. Microglandular hyperplasia is a common finding in cervicovaginal smears. The classic appearance of the smears is of numerous dispersed rounded endocervical cells (Fig. 8). Some show a pseudokeratinized appearance caused by ischemic necrosis; hence, the misnomer pseudoparakeratosis was used in the past.49,54 Cytologically, some cases maybe mistaken for atypical squamous or atypical glandular cells.
|
Squamous Epithelial Lesions
DEVELOPMENT AND CLASSIFICATION.
At the beginning of the century, the concept that intraepithelial neoplasia antedated the development of invasive squamous carcinomas was postulated by Rubin.55 With increased sophistication in the field of cytopathology, it was found that analysis of cellular samples could provide a precise diagnosis that could be correlated with histologic samples. Additional work and increased awareness of the value of cytology led pathologists and gynecologists to realize that even more incipient lesions, such as dysplasias of varying degrees, could be assessed correctly in smears and supported by colposcopic and histologic studies. The terms dysplasia and CIS as well as CIN were introduced to unify the cytologic and corresponding histologic assessment of squamous lesions of the uterine cervix.56,57,58,59,60,61,62,63,64,65 The concept was that intraepithelial lesions represent a continuum, and in some cases, lesions of variable degrees can be found in the same cervix, and can be detected by cytologic and histologic methods. Dysplasia refers to a lesion that shows abnormal cells replacing a portion of the epithelial thickness.
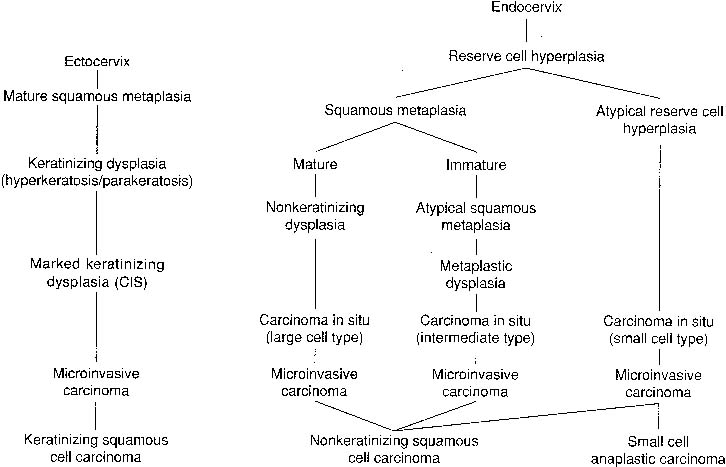
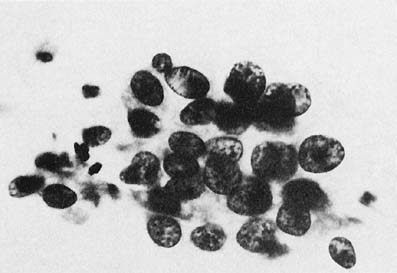
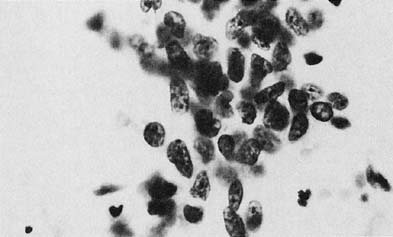
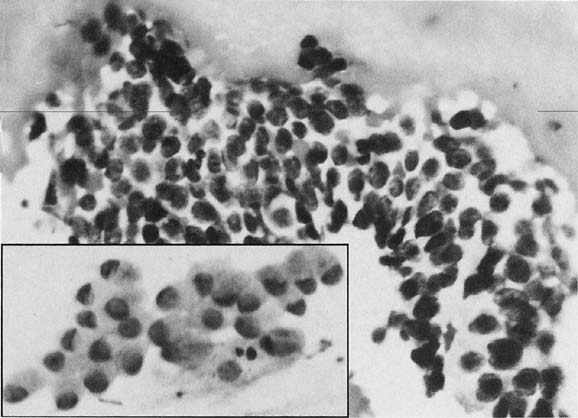
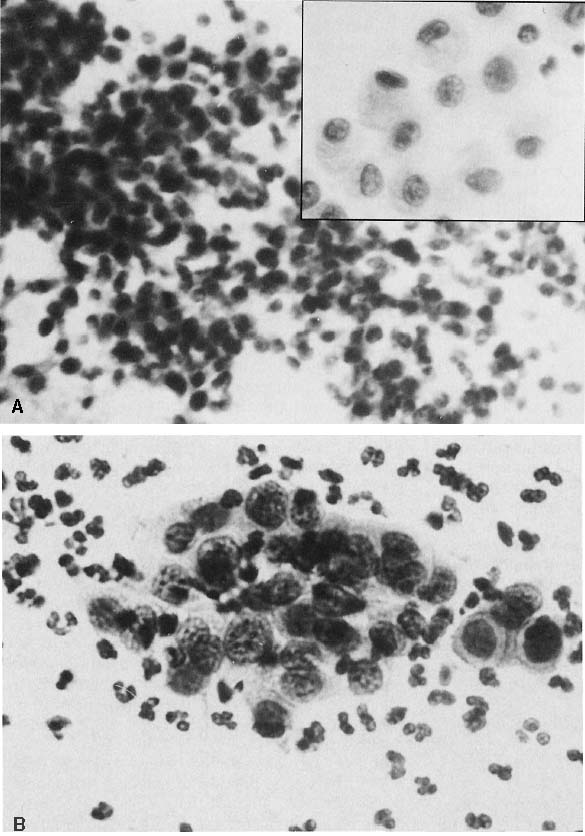
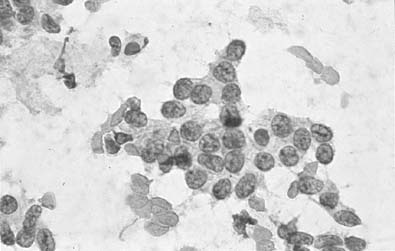
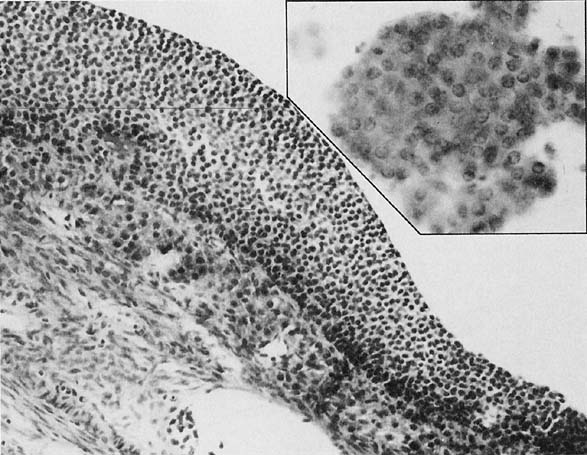
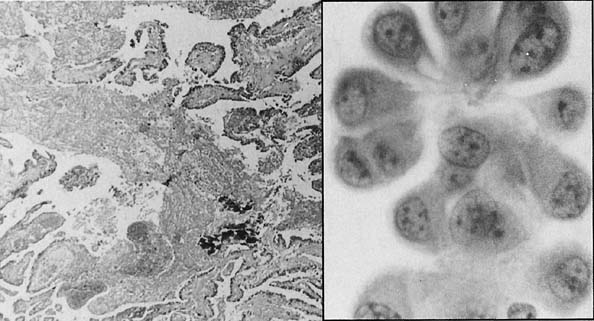
To understand the histogenesis of intraepithelial neoplasia and invasive carcinoma, knowledge of some basic morphologic concepts is necessary (see Fig. 9). The endocervical canal is composed of tall columnar ciliated and nonciliated and mucus-producing cells. Normally, the endocervical epithelium is attached directly to the stroma without a basal layer. Under the mucosa, there are isolated immature totipotential cells that are the source of development of the endocervical cells. Under a variety of hormonal or physical stimuli, these germinative cells may proliferate, giving rise to reserve cell hyperplasia (Fig. 10). Reserve cell hyperplasia may develop in young patients as a result of physical injury and in postmenopausal patients because of hormonal stimuli, or from hormones produced by the adrenal gland that are later metabolized to estrone.66 The cells desquamated from reserve cell hyperplasia have small nuclei, finely granular chromatin, and small amounts of cytoplasm. Caution should be taken not to overdiagnose them as CIS in cytologic and histologic samples. They have a monomorphic appearance, do not display hyperchromasia, and usually are seen in organized small clusters (see Fig. 10). A neoplastic process may develop in those cells as CIS of the small cell type (Fig. 11) and, later, as small cell invasive carcinoma (see Fig. 9 and Fig. 12). Small cell undifferentiated squamous CIS is seen as small cells with hyperchromatic nuclei with limited amounts of cytoplasm. To a certain extent, the features of invasive small cell carcinoma are similar to those of the intraepithelial form. However, tumor diathesis, that is necrosis, hemolyzed blood, and cellular debris, and enlarged nucleoli frequently are associated with the invasive type.
|
|
|
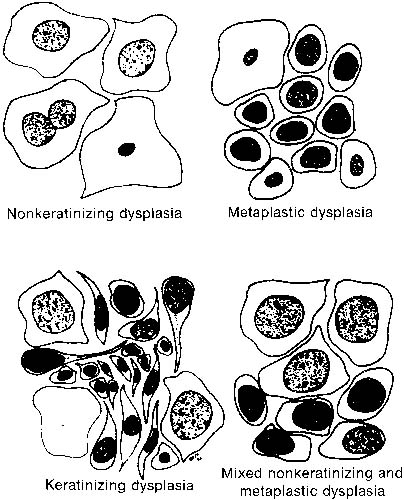
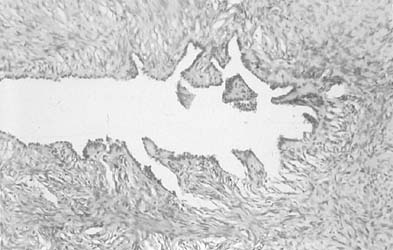
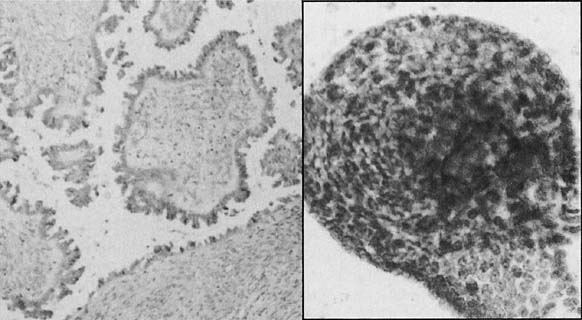
By a process of maturation, the reserve cells may acquire squamous features, and squamous metaplasia will replace the original glandular epithelium (see Fig. 9 and Fig. 10). The stimuli inducing those changes can be physical, inflammatory, or hormonal.36,66 Squamous metaplasia is a benign process found frequently in smears. As a benign component, it should be recognized for adequacy of sampling because its presence indicates adequate sampling of the transformation zone (Fig. 13). However, under the influence of an unknown stimulus, preneoplastic and neoplastic changes may take place57 in a minority of cases. In those instances, metaplastic dysplasia of varying degrees (Fig. 14 and Fig. 15), nonkeratinizing dysplasia or CIS (see Fig. 15 and Fig. 16), or nonkeratinized invasive squamous carcinoma may develop.57,58,59,60,61,62
|
|
By sampling the surface of the abnormal mucosa, it is possible to evaluate the process of maturation from the basal to the uppermost layers.36 The stimulus that induces maturation is related directly to the degree of differentiation of the abnormal basal layers. The less differentiated cells will respond poorly to stimuli inducing maturation, and will emerge to the surface and a higher degree of abnormality will be seen when the surface is sampled.
CURRENT TERMINOLOGY FOR SQUAMOUS INTRAEPITHELIAL LESIONS.
The 1988 TBS,5 introduced a two-tiered terminology, dividing the spectrum of squamous epithelial abnormalities into LSIL and HSIL lesions. Low-grade lesions encompass the cellular changes associated with the cytopathic effect of HPV, also referred to as koilocytotic atypia, and mild dysplasia/CIN I. High-grade lesions encompass moderate dysplasia, severe dysplasia, and CIS/CIN II/III. TBS divides the spectrum of SIL cytologic abnormalities into two categories for the following reasons: (1) studies have reported poor interobserver and intraobserver reproducibility with conventional three- and four-grade classification systems, (2) in the United States, the difference between moderate/severe dysplasia and CIS is not relevant to patient management, (3) data from natural history studies comparing low- and high-grade lesions imply differences between these two lesions, although the biologic behavior of any individual abnormality, whether high or low grade, cannot be predicted.6
The rationale of introducing the term squamous intra-epithelial lesion (SIL) to replace dysplasia/CIN, was based on observations that most mild dysplasia/CIN regress, approximately half of the cases of moderate dysplasia/CIN do not progress, and as a group, high-grade lesions are more likely to progress than low-grade lesions, but the behavior in an individual patient is unpredictable.6 The term lesion rather than neoplasia was chosen to convey the uncertain biologic potential in any individual patient.6
The dichotomous classification of SIL (LSIL/HSIL) was retained in TBS 2001, because it seems to be supported by current virologic, molecular, and clinical evidence that LSIL is generally a transient infection with HPV, and high-grade SIL is more often associated with persistence of HPV and higher incidence of progression.8 In addition, data from the ASCUS/LSIL Triage Study (ALTS), trial has further supported the two-tier terminology by demonstrating that (1) LSIL/HSIL is a fairly reproducible breakpoint, (2) confirmed that subdividing HSIL into moderate and severe dysplasia is not reproducible, and (3) HPV cytopathic effect cannot be reliably separated from mild dysplasia/CIN I.6,8
The 2001 Bethesda System8 suggests reporting squamous epithelial abnormalities as follows: atypical squamous cells of undetermined significance, (ASC-US); atypical squamous cells/rule out HSIL, (ASC-H); LSIL; and HSIL.
Approximately 50 million Pap smears are performed each year in the United States,67 of which approximately 7% (3.5 million) are reported as abnormal.68 Among these, approximately 3 million women have LSIL or ASC smears.69
Atypical Squamous Cells.
Terminology.
Atypical squamous cells of undetermined significance (ASCUS) was introduced into TBS terminology to represent squamous cell abnormalities that are more marked than those attributable to reactive changes but that quantitatively or qualitatively fall short of a definitive diagnosis of SIL.6 The cellular changes in this category may represent a reactive/reparative/benign change or a more serious lesion; however, they cannot be unequivocally classified by the pathologist, and are thus interpreted to be of undetermined significance. The cellular changes include nuclear enlargement to two and half to three times that of a normal intermediate squamous cell nucleus, a slightly increased nuclear-cytoplasmic ratio; with possible variation in nuclear size and shape, and binucleation. The nuclei may also show mild hyperchromasia, but the chromatin remains evenly distributed without granularity and nuclear outlines usually are smooth and regular.6 TBS 1991 encouraged pathologists to qualify ASCUS as to whether a reactive process or SIL was favored,6 however in actual practice, a large number of these cases were reported as atypical squamous cells of undetermined significance, not otherwise specified (ASCUS-NOS).8
A diagnosis of ASCUS can be caused by a variety of etiologic factors6 such as inflammation and repair, and both qualitative (poorly fixed smear) and quantitative (few/rare abnormal cells) reasons may contribute to the pathologist making this diagnosis. Keratotic cells shed singly or in three-dimensional clusters that demonstrate cellular pleomorphism (caudate or elongate shapes) and/or increased nuclear size or chromasia should be categorized as ASCUS (atypical parakeratosis) or SIL depending on the degree of the cellular/nuclear abnormalities.6
Despite efforts to provide specific criteria for the diagnosis of ASCUS, this diagnosis is poorly reproducible.8 In order for laboratories not to overuse this term, it is suggested that the frequency of ASCUS diagnoses should not exceed two to three times the rate of SIL in the same laboratory6 or the ASCUS: SIL ratio should not be greater than 2–3:1. As an example, if the frequency of SIL in a laboratory practice is 1%, the frequency of ASCUS should be less than or equal to 3%.6
TBS 2001 retained this equivocal category of reporting atypical squamous cells because approximately 10% to 20% of women with ASCUS may have underlying CIN II/III, and 1 in 1000 may have invasive carcinoma.69 However, the TBS 2001 classification differs in several ways with regard to reporting equivocal squamous epithelial abnormalities. ASC are now qualified either as being of “undetermined significance” (ASC-US) or as “cannot exclude HSIL” (ASC-H). The “undetermined significance” qualifier was retained to emphasize that some cases of ASCUS maybe associated with an underlying HSIL. Additionally, ASCUS-favor reactive was eliminated; all ASC are considered now to be suggestive of SIL.8
Atypical Squamous Cells of Undetermined Significance.
In the vast majority, 90-95%, of cases, ASC involves nuclear enlargement in squamous cells with mature, superficial/intermediate-type cytoplasm8,69 (Figs. 17A, 18, 19, 20 and 21). The differential diagnosis is most often between a benign change in reaction to a stimulus versus a LSIL.8 This is the category of ASC-US that is now referred to as ASCUS in TBS 2001. Cells with cytologic features diagnostic of HPV cytopathic effect, that it, a well-defined, clear, perinuclear cavity with a peripheral rim of thickened cytoplasm as well as required nuclear alterations, are classified as LSIL6 (Fig 17B). Cells with features that are suggestive but not diagnostic of HPV cytopathic effects, are included in the ASC-US category. Cytoplasmic vacuolization alone, without any nuclear atypia, is considered to be a benign cellular change and should not be classified as LSIL or ASCUS.6
|
|
|
Marked cellular changes involving tissue fragments, or sheets of immature squamous cells, so-called atypical repair, also are included in the ASC category, but present a different cytologic picture. In typical repair, cells occur primarily in monolayer sheets and syncytia and contain prominent nucleoli. However, nuclear piling, significant anisonucleosis, and irregularities in chromatin distribution that exceed changes seen in typical repair were considered to be ASCUS in TBS 1991.6 The differential diagnosis is between an exuberant reparative process versus invasive carcinoma, however, atypical repair lacks both a tumor diathesis and isolated abnormal cells seen in squamous cell carcinoma.6 A variety of reactive cellular changes, such as degeneration, autolysis, etc., may be seen in an atrophic smear and occasionally they can mimic HSIL or squamous carcinoma. A diagnosis of ASCUS associated with atrophy should be considered if cells demonstrate: nuclear enlargement (at least two times normal), significant hyperchromasia, nuclear chromatin or membrane abnormalities, or marked pleomorphism in the form of tadpole or spindle cells.6
The ALTS, a multicenter trial sponsored by the National Cancer Institute, showed that HPV DNA testing for high-risk types is a viable option for management of women with ASC-US, and has a greater sensitivity to detect CIN III or above and specificity comparable to a single additional cytologic diagnosis of ASC-US or above.69 The American Society for Colposcopy and Cervical Pathology (ASCCP) has published consensus guidelines for the management of women with cervical cytologic abnormalities, based on the TBS 2001 terminology.18 The guidelines support DNA testing for high-risk types of HPV for an ASC-US cytologic diagnosis. Women who test positive for HPV DNA should be referred to colposcopy and those who test negative for high-risk HPV-DNA can be followed up with repeat cytologic testing at 12 months. If no lesion is found at colposcopy in a high-risk HPV–positive woman, follow-up cytology at 6 and 12 months or HPV-DNA testing at 12 months are suggested options.18 Reflex test orders from the clinician at the time of obtaining the sample allow the laboratory to send the same specimen collected for the cervical smear for HPV testing, avoiding a return visit, and 40% to 60% women will be spared a colposcopic examination. For ASCUS in postmenopausal women, a course of intravaginal estrogen followed by a repeat cytologic test obtained approximately 1 week after completion of the estrogen test is acceptable if the smear is atrophic and there is no contraindication to using intravaginal estrogen.18 Immunosuppressed women with ASCUS should be referred to colposcopy and pregnant women should be managed in the same manner as nonpregnant patients with ASC-US.18
Atypical Squamous Cells/Rule Out High-Grade Squamous Intraepithelial Lesions.
In a smaller number of ASC cases, 5% to 10% of all ASC, the atypical changes occur in less mature squamous metaplastic cells, previously called atypical metaplasia.8 This category has been reclassified as ASCH or atypical squamous cells/rule out HSIL, in TBS 2001, and reflects a mixture of HSIL and its imitators.8 Nuclear enlargement approximates one and a half to two times the area of a mature squamous metaplastic nucleus, or three times the area of a normal squamous intermediate-cell nucleus (Fig. 22). In these cases, the differential diagnosis is between immature squamous metaplasia and a HSIL and LSIL is not a consideration.6
While there is interpreter variability in making the diagnosis of ASC, even among expert cytopathologists,70 studies suggest that ASC-H has a positive predictive value for histologic CIN II/III that is intermediate between ASC-US and HSIL.8 A woman with ASC-US has a 5% to 17% chance of having CIN II/III confirmed by biopsy, while CIN II/III is identified in 24% to 94% of those with ASC-H.18,69,71 The recommended management for women with ASC-H obtained using conventional or liquid-based cytology is referral for colposcopic evaluation.18 There is no role for HPV testing or repeat cytology. If no lesion is found at colposcopic, a review of cytology, colposcopy, and histology results should be performed. If the cytologic diagnosis of ASCH is upheld and no revised interpretations are found, cytologic follow-up at 6 and 12 months or HPV-DNA testing at 12 months are acceptable.18
Low-Grade Squamous Intraepithelial Lesion.
On histology, mild dysplasia is diagnosed when the upper two thirds of the abnormal squamous epithelium shows an adequate degree of differentiation. In many institutions, koilocytic or HPV change maybe reported on biopsy. Corresponding cytologic samples in both cases show abnormal cells with mature or superficial-type cytoplasm.6 In LSIL, nuclear enlargement is at least three times the size of a normal intermediate cell nucleus.6 Although the nucleus is hyperchromatic, the chromatin is distributed uniformly or it may appear degenerated and smudged if associated with cytopathic changes induced by HPV (see Fig. 17B). Cells with HPV cytopathic effect (koilocytes), are also interpreted as LSIL on cytologic smears. In these cases, the nucleus maynot be enlarged, but is usually hyperchromatic and “wrinkled.”
While most LSIL, especially in young women regress/are self- limited HPV infections,71,72 approximately 15% to 30% of women with LSIL on cervical cytology will have a CIN II/III on a subsequent cervical biopsy. The ALTS trial has shown that HPV DNA testing is not useful in triaging women with LSIL because 83% were positive for HPV.69 ASCCP guidelines18 currently recommend colposcopy as the preferred management for women with LSIL. Subsequent management will depend on whether a lesion is identified, whether the colposcopy is satisfactory, and whether the woman is pregnant. Routine diagnostic excisional procedures such as loop electrosurgical excision procedure (LEEP) are discouraged for initial management of women with LSIL in the absence of biopsy-confirmed CIN. Special recommendations for postmenopausal women, adolescents, and pregnant women are also outlined in the ASCCP consensus publication.18
High-Grade Squamous Intraepithelial Lesion.
In the TBS terminology, HSIL encompasses moderate and severe dysplasia and CIS. In moderate dysplasia, the morphologic appearance of the upper one third of the epithelium is relatively preserved in contrast with abnormal changes of the underlying layers. Cytologically moderate dysplasia shows cells with less cytoplasm, larger nuclei, and occasionally with asymmetrical nuclear outlines. The chromatin is increased and granular. In severe dysplasia or CIS, histology shows an epithelium that has immature cells throughout its thickness. These changes are seen in cytologic smears as immature cells, with scant cytoplasm and a high nuclear-cytoplasmic ratio (Fig. 23). The abnormal cells can be seen singly or in crowded, dark sheets/groups. At times it may not be possible to exclude the possibility of invasive carcinoma and in such cases, the terminology for reporting this finding is “HSIL, with features suspicious for invasion.” The incidence of CIN II/III is estimated to be approximately four times that of invasive carcinoma. All patients with a HSIL cytologic diagnosis should be referred for an immediate colposcopic evaluation.18
|
Borderline Squamous Intraepithelial Lesion.
For practical purposes, the cells from mild dysplasia/HPV cytopathic effect show abundant cytoplasm with enlarged or hyperchromatic, wrinkled nuclei (see Fig. 17B). As the lesion progresses from moderate to severe dysplasia (Fig. 23), the amount of cytoplasm decreases as the nucleus increases in size, increasing the nuclear-cytoplasmic ratio. The pattern of DNA distribution in dysplastic lesions is not a helpful indication of the biologic behavior of the lesion.73 However, an indication of progression can be expected in severely atypical lesions in which aneuploidy is encountered.74,75
At times there maybe difficulty in categorizing cells with intermediate features into low or high grade. Although occasional borderline cases occur, most of these can be classified as either LSIL or HSIL. Features that favor a high-grade lesion include increased numbers of abnormal cells, higher nuclear/cytoplasmic ratios, greater irregularities in the outline of the nuclear envelope, coarsening of nuclear chromatin, and chromatin clumping.6 Cell size, overall is smaller in HSIL as compared to LSIL. The appearance of the cytoplasm is usually different in LSIL and HSIL cases. LSIL typically involves squamous cells with mature, intermediate, or superficial-type cytoplasm with well-defined polygonal cell borders, whereas cells from a HSIL have a more immature type of cytoplasm, that can be lacy and delicate or dense/metaplastic, with rounded cell borders.6 When it is not possible to grade a SIL as low or high grade, a diagnosis of “SIL, grade cannot be determined” or “LSIL with few cells suggestive of HSIL” can be rendered on the cervical smear. These patients should under colposcopy/biopsy.6
Progression and Regression of Squamous Intraepithelial Neoplasia.
Spontaneous regression of LSIL occurs with great frequency (60% or more),36,72 and HSIL regress in approximately 40% of cases.56,57 Most severely abnormal lesions are destroyed by a variety of modalities of treatment. However, some evidence suggests that only a few lesions will invade and most of them, if left unattended, disappear.73,74,75,76 Progression of mildly dysplastic lesions occurs in 15% of cases.77 HSIL persists or progresses to more severe lesions, such as invasive squamous carcinoma, in fewer than 1.5% of cases.78,79,80 Abnormal epithelium has fewer desmosomes,81,82 and the decrease in the number of desmosomes induces decreased cohesiveness among cells, and easier detachment of abnormal epithelium.
Intraepithelial Neoplasia in Pregnancy.
During the first trimester of pregnancy, dysplastic cells are smaller than in nonpregnant patients, thus giving the false impression of a higher grade of abnormality.49 In the late stages of pregnancy, the size of the cells is comparable to the nongravid population. The biologic growth rate is similar to that of the general population. Regression of the lesions was shown in 45% of cases, whereas in 29% the lesions persisted, and in 25% they progressed to a more severe process.78 The possibility of undetected intraepithelial lesions may be higher than in nonpregnant patients because of a larger number of unsatisfactory smears.19
Management of Squamous Intraepithelial Lesions.
It must be emphasized and remembered that cervical cytology is used for screening. The cytologic interpretation/result should be evaluated in conjunction with the clinical findings. ASC denotes an atypical category where HPV testing maybe useful for ASC-US, however, ASC-H cases need colposcopic evaluation. The subsequent management of a cytologic diagnosis of LSIL includes a colposcopically directed biopsy and/or follow-up with smears.18 HSIL should be evaluated by colposcopy and directed biopsy. Management should focus on preventing invasive carcinoma by the most conservative means possible. The ASCCP guidelines provide recommendations for management of TBS 2001 diagnostic categories.8,18
Invasive Squamous Cell Carcinoma.
In areas in which adequate screening programs are available, the incidence of invasive squamous carcinoma of the uterine cervix has decreased considerably.83,84,85 Based on clinical and histologic studies,86,87 a classification was created that specifically indicates the histogenesis of several histologic types of squamous carcinoma. In addition, this classification could predict the biologic behavior of each subtype and its response to therapy.88 It also would correlate with the classification of abnormal cells exfoliated from those tumors (see Fig. 9). This classification of squamous carcinoma of the cervix includes three subtypes: nonkeratinized, keratinized, and small cell undifferentiated carcinoma.89 The 5-year survival rates are 78.6%, 47.8%, and 20%, respectively (see Fig. 9).57 The World Health Organization determined the validity and usefulness of this classification, and later adopted it.90
A well-differentiated neoplasm refers to a tumor that closely resembles the parent tissue. Because the uterine cervix normally is a nonkeratinized epithelium, neoplasms that lack keratinization represent the better-differentiated form of this tumor. The better biologic behavior and response to radiation therapy support this contention.86,88 In some series, the most common type of tumor is nonkeratinizing carcinoma (43.3%), followed by keratinized carcinoma (29.1%), small cell carcinoma (10.9%), and adenosquamous carcinoma (7%). Histogentically, the first two neoplasms arise from the ectocervical squamous mucosa and squamous metaplastic epithelium (see Fig. 9).89 In contrast, small cell undifferentiated carcinomas develop from the endocervical reserve cells (see Fig. 9).57 Although this classification had worldwide acceptance as well as acceptance by the World Health Organization, the histogenesis of small cell carcinoma of the cervix was challenged, and was assigned to a neuroendocrine origin.36 Small cell undifferentiated carcinomas frequently arise in the most proximal portion of the endocervical canal. For this reason, specimens obtained by gentle swabbing of the endocervix may show smears that lack tumor cells. Endocervical brushing circumvents the problem.
Cells from nonkeratinized dysplasia have a delicate cytoplasm with abnormal nuclei (see Fig. 16). Nonkeratinized invasive squamous carcinoma shows clusters and isolated cells with highly abnormal nuclei, with macronucleoli, and abundant cytoplasm with variable degrees of thickness. Tumor diathesis is a common finding. Keratinizing dysplasia and invasive carcinoma arise in most basal cells of the ectocervical mucosa, which is a nonkeratinized squamous epithelium (see Fig. 9, Fig. 24, and Fig. 25). According to Patten,57 keratinizing dysplasia of severe degree (see Fig. 25) may be difficult to differentiate from invasive keratinizing squamous carcinoma (see Fig. 24). In 10% of cases, smears of invasive keratinizing squamous carcinoma lack tumor diathesis, a finding that may lead the cytopathologist to diagnose the lesion in smears as noninvasive (see Fig. 24). It is advisable when diagnosing severe keratinizing dysplasia to add a comment regarding the possibility of invasion.
|
Postradiation Dysplasia and Carcinoma In Situ.
A variable degree of chronic radiation atypia is detected in smears of radiated patients. It may linger from months to several decades. Patten57 described several morphologic differences of dysplasia arising in radiated epithelium in contrast to dysplasia arising in nonradiated mucosa. Maturation of the normal components is greater, and the dysplastic cells are larger than those of conventional dysplasia. The mature appearance of the epithelium seen in association with this entity is reported as preceding the development of dysplasia.57 When postradiation dysplasias arise within 3 years of the last treatment, the prognosis is poorer than when it occurs more than 3 years after the completion of treatment.57
Glandular Epithelial Lesions
ENDOCERVIVAL NEOPLASIA.
The incidence of endocervical neoplasms has been increasing in recent years,91,92 with an absolute as well as a relative increase being noted. These lesions can be seen in situ, in microinvasive, and invasive stages on histology. The introduction of brushing for endocervical sampling has had widespread acceptance, and has greatly improved the yield of endocervical cells in cervical cytology specimens. Endocervical cell samples obtained by swabbing the canal show mature cells that are identifiable as benign, however, when taken by force, less mature forms as well as cells from immature squamous metaplasia and reserve cell hyperplasia may be dislodged and overdiagnosed as squamous or glandular neoplasia.
The revised TBS 20018 reporting format for glandular abnormalities is as described below:
GLANDULAR CELL
Atypical
endocervical cells (NOS or specify in comments)
endometrial cells (NOS or specify in comments)
glandular cells (NOS or specify in comments)
Atypical
endocervical cells, favor neoplastic
glandular cells, favor neoplastic
Endocervical adenocarcinoma in situ
Adenocarcinoma
endocervical
endometrial
extrauterine
not otherwise specified (NOS)
Atypical Glandular Cells.
In TBS 2001, the term atypical glandular cells of undetermined significance (AGUS) has been eliminated to avoid confusion with ASCUS. Glandular abnormalities are classified as “atypical endocervical, endometrial, or glandular cells.” In the majority of cases, morphologic features allow a differentiation of endocervical from endometrial cells,8 and this distinction is clinically significant because the management of patients may vary significantly depending on the cell type.18 The term atypical epithelial cells may be used for cases where a squamous or glandular origin is difficult to ascertain. The diagnosis of atypical glandular cells (AGC) is clinically significant because the percentage of cases associated with an underlying high-grade disease is higher than that for ASC-US8. On follow-up, a high-grade lesion, either squamous or glandular, is seen in 10% to 39% of AGC cases.93,94,95,96 The prior category of “atypical glandular cells, favor-reactive” has also eliminated, and replaced by “not further specified” so as not to give false reassurance to the clinician. In the 1991 TBS, adenocarcinoma in situ (AIS) was included in AGUS, favor neoplastic. Since then the morphologic criteria for recognition of AIS have been refined and are reproducible when properly applied; resulting in AIS being a distinct diagnostic entity in TBS 2001.8,97
Endocervical Adenocarcinoma In Situ.
The histologic features of AIS are seen in tissue samples in which there is abundant stroma that will allow assessment of the depth and location of the neoplastic glands. In cytologic samples, the features may resemble some features of frankly invasive carcinoma.98,99,100 Recent improvements in diagnostic criteria allow cytopathologists to recognize these neoplasms at early as well as advanced invasive stages.96 As is well established for squamous epithelium, it is reasonable to assume that the endocervical epithelium also goes through a series of progressive dysplastic steps. However glandular dysplasia is a debatable and poorly reproducible entity, even on histopathology,101 and it is not diagnosed as such in cytologic smears. Depending on the degree of suspicion these changes are classified as “atypical glandular cells, endocervical (AGEC), favor neoplasia” in order to convey a significant level of concern to the clinician.
Samples of AIS and well-differentiated invasive adenocarcinoma usually are usually seen in sheets, without the classic honeycombing appearance of normal endocervical cells (Fig. 26). The “colonic-type” AIS resembles colonic epithelium. There are well-defined cytologic criteria for making this diagnosis, including: crowded hyperchromatic groups, columnar cells, palisading, nuclear enlargement, coarse granular chromatin, micronucleoli, mitosis/apoptotic bodies and architectural features such as strips, rosettes, and feathering and lack of tumor diathesis. The term microinvasive cervical adenocarcinoma has been introduced into the literature. Histologically, this term indicates that the tumor has invaded less than 5 mm from the surface. Cytologically in addition to features of AIS, cellular dyscohesion, syncytial crowded sheets, papillary groupings, and numerous mitoses indicate that invasion may have occurred.96,100 In invasive endocervical adenocarcinoma, a diathesis is common in the background and there is abundant tumor cellularity.
There are certain benign processes that can lead to atypical cells mimicking a endocervical neoplastic process.96 The most common is tubal metaplasia, in which the cells can show certain features of AIS, and this problem becomes more accentuated the higher the metaplastic cells are in the endocervical canal. In a study from the University of Rochester, tubal metaplasia was identified in the endocervical canal in 100% of hysterectomy specimens.102
Endometrial cells are generally smaller and have a higher nuclear: cytoplasmic ratio than endocervical cells. With direct samping of the lower uterine segment (LUS) and endometrial cavity, large two-dimensional groups of endometrial cells can show columnar morphology and pseudostratified architecture, as opposed to the small three-dimensional tight clusters seen in exfoliated endometrial cells.103 Other differential diagnosis of endocervical adenocarcinoma include cervical endometriosis, and endometrial cells in postcone samples. In postcone samples, the endometrial lining can be reached during sampling, and normal endometrial cells showing lack of honeycombing and mitosis may be mistaken for neoplastic endocervical cells.
On subsequent follow-up, a significant percentage of lesions diagnosed as AGUS, (now AGC), represent squamous lesions mimicking glandular cells. This is particularly a problem when squamous carcinoma in situ involves the endocervical gland necks, with tight aggregates, giving an appearance of palisading or columnar forms. The pathologist should look for the presence of typical squamous features in other abnormal cells and lack of typical features of endocervical neoplasia in the abnormal “glandular” groups.103
There is a frequent association of endocervical AIS with intraepithelial squamous abnormality104 as well as with squamous atypia induced by HPV infection. The presence of HPV-DNA virus type 16 or 18 has been reported in adenocarcinoma and adenosquamous carcinoma of the cervix and a common etiologic agent suggested for squamous and endocervical adenocarcinoma.105 The most significant observation in these tumors is reflected in the degree of differentiation of the nuclei rather than the architectural features seen in histologic sections.106 This observation can be evaluated in cytologic samples and is important because there is a strong correlation between survival and degree of nuclear differentiation. Fu and colleagues107 made another important observation of the biologic behavior of these tumors. They studied the DNA ploidy in tumors, some of which had a comparable degree of nuclear differentiation. Low ploidy and good prognosis were found in well-differentiated tumors. In contrast, high ploidy and poorer prognosis usually were found in poorly differentiated cervical adenocarcinoma. Advanced clinical stage was associated with poor outcome; even in low-ploidy tumors.107 This observation supports the contention that the experience of the cytopathologist plays an important role in detecting incipient neoplasms of endocervical origin.
ENDOMETRIAL HYPERPLASIA AND ADENOCARCINOMA.
The incidence of endometrial neoplasia has been rising.108 This increase has several causes, such as the increase in the elderly population and the increased use of unopposed estrogen, and more recently tamoxifen.
The detection of hyperplasia and adenocarcinoma depends primarily on the type of sampling used. Direct endometrial cell sampling done under sterile conditions is the ideal technique, and it can be performed in the physician’s office or in outpatient clinics. However, this technique is time consuming, causes patient discomfort, and is not cost effective for the detection of endometrial lesions108 Endocervical aspiration is the next most valuable type of sampling because it overcomes most of the clinical difficulties of direct endometrial sampling. Another feature that enhances the diagnosis of endometrial adenocarcinoma with this technique is the availability of well-preserved endometrial cells because of the anatomic closeness of the endocervical and endometrial cavities. Endocervical swabbing and posterior fornix sampling are less valuable because the endometrial cells are less suitable for diagnosis because of degenerative changes and also because of the small number of endometrial cells present in those specimens. It should be remembered that the cervicovaginal smear is not aimed at detection of endometrial neoplasia.
Depending on the sampling technique used, endometrial cells may be seen singly or in clusters, and variable degrees of degeneration may be seen, precluding adequate assessment of these cells. When exfoliated spontaneously, endometrial cells are seen in variable amounts from day 1 to 14 of the menstrual cycle and are rarely seen in cyclic women in the secretory phase of the cycle or in postmenopausal women.108 Normal endometrial cells in smears can be of epithelial or stromal origin (Fig. 27A). In the past, it was well established that in cyclic women, the presence of exfoliated benign endometrial cells during the second half of the cycle indicated abnormal endometrial desquamation108 and was associated with benign or malignant lesions in 0.2% of young women. This incidence rises substantially (22%) in postmenopausal patients.108 This may not apply, however, if endocervical brushing is used, because benign endometrial cells are retrieved forcefully from the lower uterine segment.
Benign endometrial cells can shed normally in a variety of situations such as: the immediate postpartum or post abortion period, during pregnancy, as a result of dysfunctional uterine bleeding, in association with oral contraceptive intake, in patients receiving estrogen therapy, in the presence of intrauterine devices, in patients with an anovulatory cycle, in pyometra, in patients with endometrial polyps, in association with submucosal myoma, in patients with cervical and vaginal endometriosis, and after recent endometrial instrumentation.108 For the reasons stated above, a complete clinical history should be provided to the cytopathologist to allow adequate cytologic interpretation. Numerous histiocytes of putative endometrial origin encountered in cervicovaginal smears of perimenopausal and postmenopausal women may indicate the presence of endometrial abnormality in up to 10% of cases, and the endometrial lesions may range from hyperplasia to primary and metastatic malignancies of variable origin.109 However, histiocytes may be present in many cases in which endometrial disease is absent. For practical purposes, in addition to numerous histiocytes, the presence of hemolysed blood, necrosis, and high estrogenic effect (the latter is seen occasionally with hyperplasia and well-differentiated adenocarcinoma) increase the rate of detection of endometrial adenocarcinoma. Clinical correlation by the gynecologist is important to evaluate the value of the findings. Patients with hyperplasia and adenocarcinoma also shed abnormal cells in progressive numbers, depending on the degree of atypicality of the hyperplasia and the increased dedifferentiation of adenocarcinoma. The cytologic diagnosis of endometrial hyperplasia of various degrees of atypicality and grading of differentiation of adenocarcinoma can be diagnosed by direct sampling of the endometrial cavity or by aspiration of the endocervix. These findings are not duplicated in most laboratories, perhaps because of the other sampling techniques used. The presence of normal and abnormal cells in the range of hyperplasia and well-differentiated adenocarcinoma usually cannot be identified accurately in spontaneously desquamated samples. In these instances, the cells desquamated from these lesions can be classified only as neoplastic. The presence of tumor diathesis points toward a diagnosis of adenocarcinoma, whereas its absence indicates hyperplasia in most cases. Tumor diathesis refers to a granular transudate with or without white blood cells and necrotic cellular debris. This type of background is frequently found (90% of cases) in smears of patients harboring endometrial adenocarcinoma.108,110 There are other causes of tumor-like diathesis, such as pyometra and heavy infection with coccoid bacteria. In the absence of neoplastic cells, the assessment of diathesis should be done with caution. Hyperplasia as well as early invasive well-differentiated adenocarcinoma may lack tumor diathesis (see Fig. 27B).108 Increased maturation of the squamous epithelium, mimicking estrogenic stimulation, can be seen in some cases of hyperplasias and well-differentiated adenocarcinomas.108,110
Cytologically benign-appearing endometrial cells also can desquamate in patients with hyperplasia and adenocarcinoma.108 Many of these patients may be asymptomatic (57% with carcinoma and 70% with hyperplasia).110 The number of cytologically normal-appearing endometrial cells increases progressively in normal status, hyperplasia, and adenocarcinoma when specimens are obtained by aspiration of the endocervical canal.108 However, in exfoliated material, it is difficult to appreciate this increase in the number of benign cells in relation to the increase in the severity of the lesion.108,111,112,113 Given that the laboratory may not be aware of the clinical circumstances and that the last menstrual period (LMP) date and history of hormone therapy may not have been provided or be accurate, TBS 2001 suggests that cytologically benign endometrial cells seen in cervicovaginal smears in women 40 years or older be reported in a “other” general categorization.8 The clinician should then follow the patient as warranted.
Management of Atypical Glandular Cells.
If properly applied, the cytologic diagnosis of AGC is a potentially more serious lesion than ASC. Thus, it is imperative that communication with clinicians is well established and that they understand that screening cytology has a sensitivity of only 50% to 72% for identifying glandular neoplasia, and that CIN usually ≥ CIN II is the most common form of neoplasia identified in women with a cytologic result of AGC.114
Repeat cytology, colposcopy, and endocervical sampling, traditionally used to evaluate women with AGC/AIS, all have limitations, with repeat cytology being less sensitive than colposcopy for detecting CIN II/III and glandular lesions in women with AGC.115 In working up women with AGC, age is a key factor, with a higher risk of CIN II/III and AIS in premenopausal women and a higher incidence of endometrial hyperplasia/carcinoma in postmenopausal women. Approximately 50% of women with biopsy-confirmed AIS have a coexisting squamous abnormality and thus, the presence of a coexisting squamous abnormality detected by cytology does not change the management of women with cytologically detected AGC or AIS116.
If utilizing TBS 2001, the cytopathologist will qualify whether the AGC is endocervical or endometrial in nature in most cases, however in a minority of cases, the distinction may not be possible and the cells are determined to be atypical glandular cells, not other wise specified (AGC-NOS).18,116 Colposcopy with endocervical sampling is recommended for women with all subcategories of AGC, with the exception that women with atypical endometrial cells should initially be evaluated by endometrial sampling. The endometrial sampling should be in done in conjunction with colposcopy in women older than 35 years with AGC and in younger women with AGC who have unexplained vaginal bleeding.18 A repeat cervical cytologic smear for initial management of AGC/AIS is not acceptable under current recommendations. A diagnostic excisional procedure is recommended for women with AGC-favor neoplasia and AIS, if a lesion is not identified on initial workup.18 The role of HPV testing in AGC is not yet well established, however preliminary studies show potential value.117
OTHER MALIGNANT TUMORS.
Tumors present in the pelvis and reaching the uterine cavity, cervix, and vagina through patent fallopian tubes, usually lack tumor diathesis (80%).108 As reported by Ng,108 20% may have tumor diathesis when there is implantation with tissue destruction.
The most common and classic cytologic picture of an extrauterine tumor in smears is a few groups of tumor cells in a clean background.108 Occasionally, when the ovaries are involved by metastatic tumor, there is reactive hyperplasia of the stromal elements, with hormonal production and estrogenic stimulation of the cervicovaginal epithelium.
Metastatic disease can be detected in cervicovaginal samples. It is advisable that any history of previous cancer, other than uterine sites, be included in the requisition form that is sent to the cytology laboratory. In our experience, the most common sites are the ovary, stomach, and breast, but other sites can metastasize to the lower genital tract.108,118,119
Ancillary Testng
HUMAN PAPILLOMA VIRUS.
HPV is now recognized as the major cause of cervical cancer, a disease that kills more than 200,000 women around the world each year. However, HPV is common, infects essentially all vertebrate species, and induces primarily, but not exclusively, squamous epithelial neoplasia. Anogenital HPV infections are the most common sexually transmitted diseases. A conservative estimate of genital HPV prevalence in the United States is approximately 15% to 20%.120 Among more than 100 types of HPV, fewer than 20 are considered high risk for the development of cancer. Some HPV are associated with benign lesions, such as condyloma acuminata, especially types 6 and 11. Other viral types, such as 16, 18, 31, and 45 have been identified in 90% of squamous invasive carcinomas121,122 as well as in LSIL and flat condylomas.123,124,125 HPV 18 is associated more consistently with adenocarcinomas and small-cell neuroendocrine carcinomas than invasive squamous carcinomas of the cervix.
Histologically, these lesions have in common the presence of koilocytes126 in the intermediate and superficial layers of the squamous epithelium, in addition to other nonpathognomonic features.123,127,128,129,130,131,132,133 Three histologic types of condylomatous lesions are known.123 In the flat acanthotic condyloma lesions, the lower layers appear normal and the upper portion of the epithelium contains koilocytes. The papillary lesions show an exuberant exophytic growth, with the same architectural characteristics of the flat condyloma and atypia at the cellular level.123 The inverted and rare type grows inward into the endocervical glands.123 Regardless of the histologic type, the cells desquamated from these lesions are similar.
Human papillomavirus infection is recognized in cervicovaginal smears in an array of squamous cells that are quite characteristic of this virus’ cytopathic effect, namely koilocytes and dyskeratocytes.134 Cytologically, a koilocyte is a mature squamous cell with a well-defined large halo surrounding one or more atypical nuclei (see Fig. 17B). These cells were first observed by Ayre,135 who by clinical and cytologic correlation, described these balloon cells, as he called them, as related to a viral infection. Koss and Durfee126 named these cells koilocytes. Many years passed before a specific etiologic agent was encountered. Meisels and Fortin,123,134 using ancillary methods such as immunohistochemistry and electron microscopy, identified the intranuclear virus particles. The other cells described by Meisels and Morin,123 and currently accepted as a clue to HPV infection, are dyskeratocytes. Dyskeratocytes are keratinized squamous cells that are organized in three-dimensional clusters and characterized by vesicular nuclei with indistinct chromatin detail. They also are referred to as parakeratotic cells.123 Although some authors123 consider these cells an indication of HPV infection, others36 believe that they can be found in infectious processes of other etiologies. A variety of other nonclassic cells indicating HPV infection were recently introduced into the cytology literature,136,137,138,139 in addition to the classic koilocytes and dyskeratocytes. The newer nonclassic additions of cellular forms that are diagnostic of HPV infection include mild koilocytosis (koilocytes without nuclear atypia) (see Fig. 17A), mild dyskeratocytes (sheets of keratinized superficial squamous cells with normal nuclei), cells with keratohyaline-like granules (see Fig. 18), spindle squamous cells, nuclear hyperchromasia in otherwise benign cells, binucleation or multinucleation and perinuclear clearing (see Fig. 19),136 and spindled nuclei (see Fig. 20).139 There is evidence that, at the research level, nonclassic cytologic features are proof of HPV infection. This contention is supported by the detection of HPV-DNA sequences, by molecular biology techniques, in cells with minimal alterations.136 According to Schneider,136 when the diagnosis of HPV infection was made using the classic koilocytes and dyskeratocytes, only 15% of HPV-DNA–positive patients were identified correctly. However, when a panel of nonclassic cellular features was used, statistically discriminating analysis identified 84% of HPV-DNA–positive patients.136,140
The suspicion raised by zur Hausen122 of an association between the presence of HPV in the cellular genome and the development of cervical neoplasia was confirmed. For many years, the culture of these viruses was not feasible by traditional methods. A mature keratinized cell (permissive cell) is necessary for particle replication, and the difficulties involved in culturing this virus arose from the fact that this degree of maturation cannot be achieved in tissue culture.123 Subsequently, it was confirmed that patients with no evidence of morphologic abnormality at the cellular level harbored HPV-DNA sequences in their cervical epithelium.141,142 It has been shown that HPV-related lesions are more common in sexually active patients of younger age groups than in those older than 30 years, and that HPV infection is more common in the second half of pregnancy.143 In low-grade lesions, all viral genes are expressed as a manifestation of vegetative replication. In contrast, in HSILs and invasive carcinomas, there is a restricted pattern of viral gene expression involving primarily E6 and E7. In benign HPV lesions, the viral DNAs exist as extrachromosomal plasmids, mostly in monomeric circular molecules.144 Viral integration disrupts E2 open reading frame (ORF), which encodes the transcription regulatory proteins. Loss of these regulatory proteins is thought to be the basis for potential dysregulation of the expression of the transforming E6 and E7 ORFs.144,145 The only cells in the cervical epithelium that are capable of divison are the parabasal and basal cells. HPV gene expression is permitted only in cells that have begun squamous differentiation, and which concurrently have lost the ability to divide. The eventual outcome of coordination between the host and the virus is the production of a histologic LSIL, the only demonstrated source of infectious virions. Such lesions can regress or maintain themselves for extended periods of time.146
The nuclear enlargement and hyperchromasia seen microscopically is a result of the activation of host DNA synthesis mediated by E6 or E7.146 If the cells also have the correct amount and form of the cytokeratin binding protein, HPV E4, expressed, then they appear as koilocytes. Progression to HSIL may include viral integration or mutation in E2 such that E2 controlled regulation of E6 and E7 is lost. In such cases, E6 and E7 viral oncogenes are inappropriately expressed in cells that have the capacity to divide, hence cell proliferation and progression is initiated.146
Laboratory Techniques for Detection of HPV Infection and Reporting of Results.
Several laboratory techniques have been used to identify HPV in cells and tissue preparations, including immunohistochemistry and electron microscopy. These techniques can miss 50% of HPV-associated lesions.123 Subsequently, laboratory DNA hybridization tests were introduced to identify virus genomes in histologic and cytologic preparations. These tests involve variable technology and include in situ, filter in situ, hybridization, dot blot, Southern blot, and PCR analysis.123 Commercial kits are now available for in situ hybridization (Ventana), and a Hybrid Capture II assay from Digene Corporation is FDA approved for testing low-risk and high-risk HPV types.
The TBS does not endorse or promote the use of ancillary testing in conjunction with cervical cytology, however, specific ancillary tests may be used to complement the cytology test.8 Currently, many laboratories perform human papillomavirus (HPV) DNA testing as an adjunct to the cervicovaginal smear, especially in cases of ASCUS, based on the ALTS trial data69 and ASCCP guidelines.18 The Bethesda 2001 workshop developed examples of how ancillary testing may be reported in conjunction with cervical cytology results.
Description of Testing Method and Results.
The results of all ancillary tests should specify laboratory methods. With respect to HPV testing, several methods such as PCR, in situ hybridization, hybrid capture, etc., can be used. Each method has specific test characteristics and variable sensitivity and specificity based on the threshold that has been set for a positive result. TBS 2001 recommends that the method used should be stated and the results should be reported so they are easily understood by the clinician. For HPV testing, the results should be reported as positive or negative for HPV DNA of a certain type or class, and specific HPV types included in the assay can be listed, if the laboratory desires to do so.
Reporting of Molecular and Cytologic Results.
Integrated reporting of cytologic and molecular results together is not practical in all clinical settings. TBS 2001 endorses integrated reporting when possible; when this is not possible, the molecular report should refer to the other cytology report. (Bethesda System 2001 Workshop, Raab S, et al, unpublished data).
Gonorrhea and Chlamydia.
The FDA approved “out of vial testing” for gonorrhea and chlamydia, by the Roche Amplicor technique in 2002.
Computerized/Automated Screening
It is recommended that if computerized screening is used, a report title or a test methodology heading to indicate this as a part of all cervical cytology reports. The methodology for specimen preparation should be reported in addition to the use of instrumentation employed for arriving at the final interpretation/result.8 The result of automated slide examination should be stated in the report. The name of any individual who examines the cervical cytology slide and gives an opinion for the final report should be documented in the report with the role of the person clearly stated. If the slide is not manually screened (i.e., automated primary screening only), then there should be no name on the report that can be misconstrued as a person who examined the slide. The results generated by the instrument should be reviewed and verified by laboratory personnel with appropriate training and authorization and an internal laboratory record maintained according to regulations stipulated in the Clinical Laboratory Improvement Act of 1988 (Prey M, et al. Bethesda 2001 Workshop Consensus, unpublished data).
Various technologies are available and/or under development for primary screening and location guided screening of cervical cytology (Tripath Imaging, Cytyc Health Corporation). TriPath’s FocalPoint slide profiler, formerly AutoPap, is currently FDA approved for primary screening on both conventional and PrepStain processed slides.
Educational Notes and Recommendations on Reports
Continued communication between laboratories and clinical providers is crucial if cervical cytology is to maintain its high level of efficacy as a cancer screening test.147 Written comments regarding the validity and significance of cytology results are the responsibility of the pathologist generating the report and are directed to the clinician who ordered the test.8 The use of educational notes and/or suggestions is considered optional in TBS 2001,8 and if they are appended to cytology reports, they should be clearly, carefully, and concisely worded, and evidence-based. The use of notes that emphasize the limitations of cervical cytology as a screening test can also be used. If written suggestions are used, the format and style may vary depending on the preferences of the laboratory and the provider.8 Alerting clinicians to references containing consensus guidelines published by different professional organizations, such as The American Society for Colposcopy and Cervical Pathology (ASCCP), The American College of Obstetricians and Gynecologists (ACOG) and The National Comprehensive Cancer Network (NCCN) may also be helpful.8
Laboratory Quality Assurance of Cervical Cytology Reporting
To ensure accurate and reliable test results, the laboratory must monitor and assess the quality of all phases of the testing process from specimen collection, accessioning, preparation, microscopic evaluation, reporting, and record retention. To achieve this goal, laboratories have quality assessment parameters to evaluate that include preanalytic, analytic, and postanalytic processes and personnel involved. While specific components of the quality assurance program are established by the laboratory director, federal, and in many cases, state regulations define mandatory standards for laboratory practice.
With respect to cervicovaginal cytology, cytotechnologists screen all cases and can diagnose smears that have no evidence of intraepithelial lesion and those without any changes requiring confirmation by a pathologist. Pathologists review and diagnose all gynecologic cases that have any changes that are reactive, atypical, or have any intraepithelial or malignant lesion and all nongynecologic cases. Several quality control measures are in place for gynecologic cytology. These include, but are not limited to the following: (1) quality control rescreening: 10% of gynecologic smears not requiring a pathologist review, are rescreened by a second, senior cytotechnologists as a quality control measure; (2) monitoring of diagnostic categories: laboratories monitor the breakup to various diagnostic reporting categories and monitor rates of ASC, AGC, and ASC/SIL ratios; (3) cytologic-histologic correlation is done as follow-up and any significant discrepancies should ideally be discussed by the clinician and pathologist(s) involved to ensure appropriate follow-up; and (4) follow-up letters on HSIL and malignant cases are sent by the laboratory to the clinician if no follow-up histology/smear is seen following a diagnosis of a significant lesion (HSIL, AGUS-neoplasia/AIS/cancer).
Vaginal Lesions
Inflammatory diseases of the vagina are similar to those described in this chapter in the Organisms section. Benign processes in this area include inclusion cysts and cysts of müllerian origin, which are lined by squamous and columnar or mucosecreting epithelium, respectively.148 These benign processes are adequately sampled by fine-needle aspiration.
Vaginal adenosis is found most frequently in the upper anterior vaginal wall. A variety of cells can be obtained by scraping these lesions. The endocervical cell type is the most common. Squamous metaplasia, microglandular hyperplasia, and epithelium resembling endometrial and oviduct epithelium148 also may be seen. Clear cell adenocarcinoma is rare in patients exposed to diethylstilbestrol (DES) in utero, occurring in approximately 0.1%. It coexisted with adenosis in 95% of the cases reported by Robboy and associates.149,150 Most commonly, malignant neoplasms of the vagina are of a metastatic nature. Because of its close proximity to other organs, the vagina may be the site of metastasis or direct extension of malignant neoplasms (80% to 90% of cases).148 These neoplasms can easily be identified by cytologic methods.
Hormonal Evaluation
The analysis of cellular vaginal samples for assessment of hormonal status has some limitations, but offers an easy, efficient, and rapid method to evaluate the hormonal status of the patient.151 The ideal specimen is obtained from the upper lateral vaginal wall151 by gentle scraping. The presence of inflammation and inflammatory changes of the squamous cells precludes the assessment of hormonal evaluation. In the interpretation of hormonal cytologic findings, it is essential that certain clinical facts be available, particularly patient age, recent menstrual history, and pertinent clinical endocrine findings. As a screening test, the assessment of the hormonal status of a patient can be an adjunct to other clinical procedures for the diagnosis of several endocrinologic abnormalities (Table 3).
TABLE 3. Hormonal Cytologic Findings in Various Endocrinopathies
Primary Amenorrhea
Gonadal dysgenesis: In Turner’s syndrome, the smear is atrophic, and in 80% of
cases, findings are negative for the sex chromatin body. Occasionally, there
may be two or more sex chromatin bodies, or variety, suggesting
mosaicism. In some patients with atypical dysgenesis, the smear may
show slight proliferation.
Pituitary infantilism: The smear varies from atrophic to intermediately proliferative. Sex chromatin
findings are positive.
Ovarian eunuchoidism: The smear is atrophic. Sex chromatin findings are positive.
Testicular femininization syndrome: The smear shows a good estrogen effect, but sex chromatin findings are
negative.
Simple congenital absence of uterus: The smear shows a normal cyclic pattern. Sex chromatin findings are positive.
Congenital adrenal hyperplasia: Sex chromatin findings are positive. The degree of intermediate proliferation
varies, and the smear may be atrophic.
Precocious puberty syndrome: Constitutional or idiopathic: The smear is proliferative and may show
cycling.
Lesions of central nervous system: The degree of proliferation varies.
Granulosa and theca cell tumors of the ovary: The smear is highly proliferative.
Secondary Amenorrhea of Hypothalamic Origin
Psychogenic: Smears vary from atrophic to moderately proliferative. The degree of estrogen
effect is useful guide to the prognosis.
Environmental: Smears generally show varying degrees of proliferation.
Syndromes of oligomenorrhea and hirsutism
Stein-Leventhal syndrome: The proliferation of the smear varies from intermediate to good. Large
parabasal and intermediate cells of the navicular type may be present. The
picture may be one of mixed hormonal effect.
Adrenogenital syndrome: The smear may vary from atrophic to small intermediate cell proliferation. Some
mixed hormonal patterns are present.
Cushing’s syndrome: Generally, cell proliferation is intermediate.
Masculinizing tumors of the ovary: Generally, the smear is atrophic. Some with mixed hormonal effect.
Genetic: The smear is proliferative and may be cyclic.
Syndromes of amenorrhea and galactorrhea
Chiari-Frommel syndrome: Typically, the smear is markedly atrophic.
Pituitary tumors and central nervous system lesions (Forbes-Albright syndrome): The smear varies. Generally, cells are parabasal and small intermediate
or occasionally good proliferation is present.
Del Castillo’s syndrome (nonpuerperal, dysfunctional): Usually, small intermediate cells predominate.
Pseudocyesis: Proliferation often with progestational characteristics.
Menopausal syndrome: Great individual variation. Early in the climacteric, the smear may show
high proliferation, but no cycling. In some cases, atrophic changes
may occur abruptly, whereas in others, varying degrees of proliferation
may be evident for years, with a gradual shift in the maturation index
to the left.
Rakoff AD: Hormonal cytology of endocrinopathies. In Wied GL, Keebler cM, Koss LG, Reagan JW (eds: Compendium of Diagnostic Cytology, pp. 49–50. 6th ed. Chicago: Tutorials of Cytology, 1990.)
For practical purposes, the Papanicolaou stain is used when the smears are screened for preneoplastic or neoplastic processes and for hormonal evaluation. However, the rapid Shorr method, used specifically for hormonal evaluation, can give excellent results, but is not used routinely in the United States.151 Other rapid supravital stains with practical application for immediate in-office evaluation also offer excellent results. Phase microscopy is a rapid, reliable technique that uses fresh, unfixed material.151 It offers the additional advantage of evaluating microbiologic flora.
Several indexes are used to report the hormonal status of patients. The most useful is the maturation index, which uses three cell types (parabasal, intermediate, and superficial cells) (Fig. 28). Superficial cells have pyknotic nuclei and are seen when the vaginal epithelium is stimulated by estrogenic substances (Fig. 29). Intermediate cells usually have cyanophilic cytoplasm and vesicular viable nuclei (see Fig. 29), and their presence most frequently indicates the progestogenic effect usually seen during the second half of the cycle, in pregnant patients, and in some postmenopausal patients who never achieve a parabasal atrophic pattern.151
This lack of atrophy depends on several parameters, including the adrenal production of pregnenolone,151 which is metabolized to estrone in the peripheral fat. This substance is an estrogenic stimulator that maintains the vaginal epithelium of some postmenopausal women, with resultant maturation to an intermediate or superficial cell type.
Persisting hypermaturation of the cells exfoliated from the vaginal mucosa can result from the presence of a functioning ovarian tumor, but an unexplained pseudoestrogenic effect is occasionally encountered in endometrial, ovarian, and breast carcinoma.151
The parabasal pattern is also found in smears of newborn girls after the effects of the mother’s hormones disappear, in postpartum and postmenopausal women, and in women with anorexia nervosa. The last effect is because of the decrease in the level of pituitary gonadotropin that occurs with secondary ovarian failure (see Fig. 29).151
Nonhormonal factors may induce changes in vaginal mucosa that do not necessarily indicate similar hormonal status. Several bacterial types, trichomonads, and fungal infection may cause pseudoeosinophilia that precludes adequate cytohormonal evaluation.151
Döderlein’s bacilli destroy the cytoplasm of the intermediate cells by a process of cytolysis, preventing the maturation of the cells to a superficial level and rendering the assessment of hormonal status erroneous.
Irradiation affects the mitotic apparatus of the cells from the basal layers, and exfoliation after this modality of treatment yields cells that show an atrophic pattern that is resistant to exogenous estrogenic stimulation.152 Avitaminosis A and C can induce changes that mimic estrogenic stimulation caused by generalized hyperkeratosis of the mucosa.153 Nonhormonal influences may cause changes in the vaginal epithelium that may be misleading to the cytopathologists. For instance, large doses of digitalis induce increased maturation mimicking increased levels of estrogenic substances in patients with decreased liver function.153
ESTROGEN STIMULATION TEST.
Diagnostic problems in atrophic smears can be overcome by the use of short-term low dosages of estrogen. This estrogenic test is indicated when it is doubtful whether the atypical cells are due to severe benign atrophic changes or neoplasia. The induced growth and maturation of the cells will be complete in benign or atypical changes caused by atrophy, and the atypical cells will remain in cases of neoplasia.151,152 The test is also indicated in radiation-treated patients to exclude severe radiation changes or recurrent tumor.11 The estrogenic test is not used to clarify the nature of glandular atypia. The accepted procedure is either topical administration of vaginal cream daily for 3 days, with smears repeated on the fifth day, or parenteral use of 20 mg long-acting estrogenic substance, with smears repeated by the seventh day.151
Vulvar Lesions
The success of cytologic diagnosis of vulvar lesions is dependent on the technique used to obtain the specimen.148 The best technique is a forceful scrape of the lesion, because a thick keratinized layer is part of the normal vulvar mucosa. If a gentle swab of the lesion is used, most of the cells obtained will be anucleated squames. A warm, moist gauze pad can be soaked in saline solution and placed over the thick keratin layer overlying the lesion. The first specimen may be discarded, and a second taken to obtain cells from the deeper layers, which would be diagnostic of the lesion.
Unless they are ulcerated, biopsy should be performed on pigmented lesions. For ulcerated lesions, a touch preparation should be obtained with a glass slide held against the ulcerated surface.148
The most common infectious lesions diagnosed in the vulvar area are of viral etiology: herpes genitalis and HPV infection. Topical treatment of some infectious vulval lesions with podophyllin may induce cellular changes mimicking dysplasia or CIS.148 Adequate clinical history would avoid false-positive diagnosis of treated lesions.
Mycotic infections usually are caused by several species of Candida and Torulopsis glabrata.148 The epithelial changes induced are inflammatory and are easily identifiable cytologically.
DYSTROPHY.
Several types of dystrophy affect the vulvar area: hyperplastic, atrophic, and mixed. They are overlayered by keratotic layers. Because keratosis is a normal component of the vulvar area, the presence of anucleated cells is not diagnostic of dystrophic lesions.148 The same holds true for lichen sclerosis. A precise clinical diagnosis of lichen sclerosis associated with smears containing a large number of anucleated squames is suggestive of this entity, but this finding does not exclude other diseases that may underlie the thick keratotic layers. The clinician must recognize the possibility of the association of underlying dysplasia in 16% and 10% of mixed and hyperplastic dystrophies, respectively. Underlying dysplasia rarely exists in lichen sclerosis.148,154,155 There also is the potential for the development of malignant processes from dysplastic dystrophy that would go undetected by cytology if the surface keratotic layers are not disrupted and the deeper layers sampled.
MALIGNANT DISEASE.
Malignant neoplasms of the vulva account for 5% of the malignancies that affect the female genitalia. The most common lesion is invasive squamous carcinoma (51%) followed by in situ squamous lesions (25%), Paget’s disease (8%), malignant melanoma (5%), adenocarcinoma (2%), basal cell carcinoma (2%), and sarcoma (1%).156,157 Metastatic disease to the vulvar area accounts for 8% of these lesions.156,157 Squamous carcinoma is classified histologically as keratinized, nonkeratinized, and small cell. The biologic behavior is variable depending on the histologic type that can be assessed in cytologic samples.155 Verrucous carcinomas refer to tumors with minimal nuclear atypia forming exophytic papillary growths overlayered by hyperkeratosis or parakeratosis. Verrucous carcinomas exfoliate squamous cells with minimal nuclear atypia, in addition to hyperkeratotic and parakeratotic cells.148 The cellular components are identical to those of cells exfoliated from several hyperplastic processes.148 A biopsy should be obtained that includes the base of the lesion for adequate histologic diagnosis.148 If adequately sampled, basal cell carcinoma can be diagnosed cytologically. Most frequently, these lesions are found in the labia majora148 and the perirectal area. They should be diagnosed carefully and adequately with cytologic or histologic methods, because in this area the differential diagnosis is cloacogenic carcinoma of the rectum.148
Paget’s disease also is frequently found in the labia majora in older women.148 There are several well-documented theories about the histogenesis of Paget’s disease of the vulva from the basal layer of the epithelium and its appendages or underlying glands.148 Cytologic techniques cannot differentiate between Paget’s disease, other primaries, and metastatic invasive adenocarcinomas occurring in the vulva.148,155
Anal Cytology
The use of anal-rectal cytology in the evaluation of HPV-related lesions is a relatively new tool. Its usefulness is still being investigated and described.158,159 The target of sampling is the entire anal canal, including the keratinized and nonkeratinized portions and the anal transformation zone. There are parallels between cervicovaginal and anal-rectal cytology, and it is suggested that terminology, criteria, and guidelines for evaluation of anal-rectal specimens should parallel those used for gynecologic cytology.8
Cytologic samples are often collected without direct visualization of the canal, although some clinicians may use an anoscope to introduce the collection device. Collection devices used include Dacron-fiber swabs, cytobrush; the former is reported to be better tolerated by patients. Both conventional and liquid-based cytology maybe used; there have been reports that liquid sampling decreases obscuring fecal material, air-drying, and mechanical artifacts.160,161 Criteria for adequacy have not been well established, however the topic was addressed at the 2001 Bethesda Workshop. Cell types seen in these preparations include anucleate squames, nucleated squamous cells, metaplastic squamous cells, and columnar glandular cells from the rectum.
Ovarian Cytology
Approximately 10% of ovarian cancers are thought to have a hereditary basis. The strongest risk factor for the development of disease is a family history of ovarian cancer. Germline mutations in the BRCA1 and BRCA2 breast-ovarian cancer susceptibility genes appear to account for most hereditary ovarian cancers. A much smaller fraction occur as a result of DNA repair gene mutations.162 BRCA1 is a phosphoprotein located in the cell nucleus that functions as a negative regulator of the cell cycle.163,164,165 It is considered an active inhibitor for development of neoplasia, a tumor-suppressor gene, and is located in chromosome 17q21. Gene mutations of BRCA1 have been implicated in 80% of familial breast and ovarian cancers166,167 and these mutations are inherited through the germ cell line.167 The risk of a BRCA1 carrier developing breast carcinoma is between 33% and 50% in contrast to 2% in young patients without inherited mutations.168 The risk for the development of ovarian carcinoma in these women is also increased and estimated to be between 28% and 44%.168,169 Germline mutations in the BRCA 2 gene, which is located on chromosome 13q12, may account for as much as 10% to 35% of ovarian cancers.
The cells scraped from ovarian surface of patients with BRCA1 and BRCA2 mutations show a complex arrangement of cells with florid papillary fronds and large sheets (Fig. 30). Some clusters may contain cells with slightly enlarged nuclei and infoldings of the nuclear envelopes are often noticeable. These findings contrast with the cytologic features of cells from normal ovaries, which show small monolayers of cuboidal cells with minimal cytoplasm, and occasionally clusters of columnar cells with maintained polarity (Fig. 31). The cytologic features seen in BRAC1 and BRCA2 ovaries correlate well with the histological findings, in which complex micropapillary excrescences are seen in foci of the ovarian surface epithelium with multiple clefts deep in the ovarian stroma (Figs. 32 and 33).
|
|
|
|
Surveillance and management of young patients with the propensity to develop ovarian carcinoma may include prophylactic surgery, preventive chemotherapy,168 and most recently, a new approach of studying cells obtained by scraping the ovarian surface during laparoscopy.170 Several laboratory techniques may be employed to identify patients that carry this type of abnormality.170 In the surgical pathology setting, immunohistochemical staining using avidin-biotin-peroxidase complex method is feasible. The immunoreactivity identified is graded by the proportion of malignant cells showing distinctive nuclear staining as well as the aberrant cytoplasmic staining in these cells,171 however, utilization of this methodology is still controversial.172