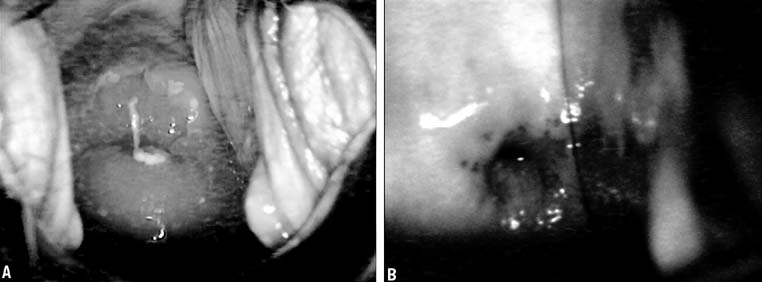
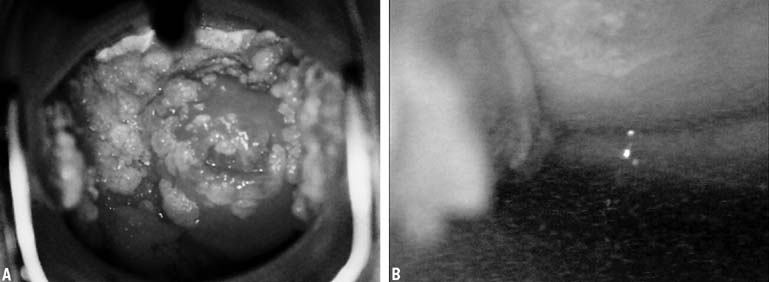
In 1968, Richart and Sciarra3 reported effective treatment of CIN diagnosed by Pap smear and biopsy
with cauterization of the cervix, portio, and endocervix. In 1970, Crisp
and colleagues4 reported on the effectiveness of cryosurgery in the treatment of CIN. Both
methods were conservative and efficacious in the management of CIN
and a significant alternative to cervical cone biopsy with hysterectomy
for carcinoma in situ, which was the only other available approach. Multiple other means have
been developed since that time, and cryotherapy is the primary thermal
method in use at the present time. Cryotherapy may be used for treatment
of condylomata or for ablation of CIN. Again, satisfactory pretreatment
evaluation and individualization of therapy based on age, disease, and
gravidity is important. Cryosurgery is an office procedure that
usually can be performed without anesthesia or analgesia. Occasionally
patients will experience discomfort, but it is seldom of a severity
to require discontinuation of the treatment. Self-limiting vasomotor
reactions characterized by light-headedness and flushing
are common. After cryosurgery, patients will usually have 10 to 14 days
of watery discharge requiring four or five sanitary napkins daily. Coitus
and intravaginal tampons are not recommended during that time. Technique Most cryosurgical instruments use either nitrous oxide (freezing point
of −89°) or carbon dioxide (freezing point of −65°). Proper freezing requires attention to the pressure
within the tanks because a decrease in partial pressure changes the
freezing rate of the probe. By changing the rate of freezing, the extent
of cryonecrosis can be modified. The larger (D) tank is
preferable to the narrower (E) tank for circumstances in which
a number of sequential patients are treated. The pressure within the
tanks must be at least 40 kg/cm2 before and at the completion of the freeze. If there is a pressure decrease
during cryosurgery, the procedure should be discontinued and repeated
with adequate pressure levels. Probe tips of various configurations are available and should be tailored
to individual cervical anatomy. The various flat and cone tips and
the 8-mm rod tip are appropriate for most cryotherapy procedures. Areas
to be treated should be outlined by a visible lesion or by a
colposcopic map, and direct connection of the probe tip to the area to
be treated must be possible with the tip chosen. Patients should be treated within 1 week of cessation of menstrual periods. A
thin layer of water-soluble lubricant applied to the tip
of the probe allows for better heat transfer between the probe and the
cervix and fills any potential air gaps in the irregular surface of
the cervix to provide a more uniform freeze. Freezing of a large ectocervical
lesion should begin at the periphery and use overlapping fields
of necessary. The ice ball should extend at 4 to 6 mm beyond the edge
of the abnormal epithelium. The depth of cryonecrosis will be approximately 4 to 5 mm
and theoretically should destroy any intraepithelial
neoplastic process extending into endocervical glands on the portio. The
extent of the ice ball beyond the confines of the lesion is more critical
than the length of the freeze. This will usually occur within 2 minutes, so
most clinicians use a freeze technique with 3 minutes on, thaw, and
repeat. If constant tank pressure is maintained, tank warmers
are used, and careful attention is paid to detail, then a single freeze
may be effective. Cryonecrosis and Surveillance Cellular death occurs at a temperature of approximately −20 °C. This
temperature is within 2 °C of the eutectic point of a sodium
chloride solution. Cryosurgery produces severe biochemical and biophysical
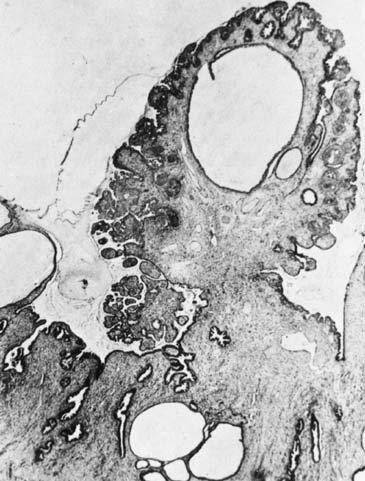
changes resulting in coagulation of the affected tissues. Rupture
of the cell wall occurs with the formation of intracellular and extracellular
ice crystals. Avascular necrosis is produced by circulatory
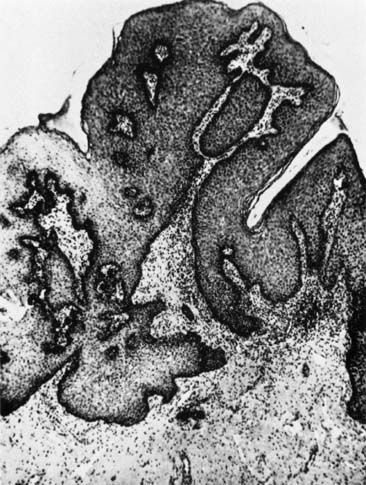
compromise because of capillary obstruction and stasis.5 Regeneration of the epithelium involves the production of initially immature
squamous epithelium, which over time will mature into a stratified
squamous layer that replaces the neoplastic process. The entire reparative
process requires approximately 3 to 4 months. Pap smear surveillance
should not be reinitiated before 3 to 4 months, and even then a
Pap smear may show a high number of cells in the reparative phase. If
a normal Pap smear is obtained, two additional Pap smears during the
first year after cryosurgery should be planned and then should be performed
every 6 months until 2 years have elapsed. Subsequent follow-up
should be decided on the basis of individual risk parameters. Efficacy Since the introduction of thermal ablation, there has been skepticism about
its efficacy in the conservative therapy of CIN. Two concerns need
to be addressed. First, factors must be identified that are associated
with primary failure of the technique. Second, the ability of the neoplastic
process to recur must be considered along with the potential
benefits or deficits of a procedure in expediting the long-term
follow-up. This second concern is directly impacted by ongoing
exposure to other papillomavirus infections, smoking, and the presence
of other viral infections, particularly HIV. Attempts to describe the recurrence rate of any technique are heavily confounded by the action of these c-carcinogenic
influences in individual patients. Concerns continue
to be raised about long-term follow-up issues after thermal
ablation of the cervix. In one study of a small group of patients (27), the
colposcopic appearance underestimated the actual
disease (11% stromal invasion) in follow-up
after cryotherapy.6 Failures with cryotherapy may reflect many factors. The size of the lesion
appears to be critical, with larger lesions more likely to recur.7,8 Failure rates also vary based on grade, ranging form 8.8% for CIN
I to 14.5% for CIN III.9,10,11 A positive ECC before treatment is particularly worrisome, as failure
rates of up to 20.8% have been reported. Inadequate freezing has
also been postulated as a factor, but this is unlikely given the changes
in the equipment ensuring predictable freeze levels. The majority
of failures occur in the first 2 years (93%), supporting
the practice of closer observation during this period.12 Immediate side effects are few.13 Long-term fertility is not appreciably altered,14 and pregnancy outcome does not appear to be affected by cryotherapy.15 An additional concern is the loss of the transformation zone, which may
lead to a need for more invasive procedures in the future for young
women who have a continuing need for observation and who may have additional
episodes of preinvasive disease. |