Conservative surgical treatment of ectopic pregnancy is well established, and laparoscopic salpingostomy is the preferred operative method in unruptured cases. Nonsurgical therapy for ectopic pregnancy, however, may prevent undesired postoperative adhesions that often result from surgical manipulation of the fallopian tubes.21 Despite the theoretical benefits of medical management, the notion that early tubal pregnancy disruption without surgery will ultimately increase fertility potential remains speculative.1 This section considers the current roles of expectant, medical, and surgical management of ectopic pregnancy.
Observation
Some patients with ectopic pregnancies undergo spontaneous absorption and require no therapy.22 In 1955, Lund23 reported on 119 women with unruptured tubal pregnancies who were treated with bed rest and frequent observation. Of these 119 patients, 68 (57%) were eventually discharged from the hospital without operation, but 60% required hospitalization for more than 1 month. The remainder required operative intervention for tubal rupture or worsening clinical course. Fernandez and Rainhorn24 reported that, of 14 women with small, unruptured, laparoscopically diagnosed ectopic pregnancies who were managed expectantly, four required subsequent reoperation. Whether a rigid and expensive hospital protocol that includes serial ultrasound examinations and hCG measurements results in better subsequent pregnancy rates than removing the ectopic pregnancy by salpingostomy at first laparoscopy awaits further study. At present, it is considered better to remove an unruptured ectopic pregnancy at the time of first laparoscopy to avoid the additional expense of hospitalization, serial hCG assays, and a second laparoscopy.25
Medical Management
Nonsurgical therapy for ectopic pregnancy may prevent the undesired postoperative adhesions that often result from surgical manipulation of the fallopian tubes. The first case report describing the use of medical therapy for tubal pregnancy appeared in 1982.26 Observational studies in the mid-1980s used, with varying success, methotrexate, prostaglandins, dactinomycin, etoposide, hyperosmolar glucose, anti-hCG antibodies, potassium chloride, and mifepristone (RU 486).19 Although treatments given systemically have proved practical, several of these agents also have been injected locally into the ectopic gestational sac under laparoscopic or ultrasound guidance or by hysteroscopic intratubal cannulation.
METHOTREXATE.
Methotrexate, a folic acid antagonist, inhibits dihydrofolate reductase, an enzyme necessary for nucleic acid synthesis, and thereby interferes with DNA synthesis and cell multiplication in actively dividing tissue. The efficacy of methotrexate in the treatment of gestational trophoblastic disease made it an attractive candidate for chemotherapeutic use in ectopic pregnancy. Patients treated with methotrexate should be counseled to refrain from alcohol consumption and intercourse and to avoid use of vitamin preparations containing folic acid until complete resolution of the ectopic pregnancy.
Systemic Methotrexate.
The efficacy of methotrexate in the treatment of unruptured ectopic pregnancy using multiple-dose regimens had been well documented.27,28,29 In 1989, Stovall and associates30 reported on the use of an individualized, multidose regimen in which 1 mg/kg of methotrexate was given intramuscularly on alternating days, with 0.1 mg/kg of leucovorin given on the intervening days until the β-hCG level had dropped by at least 15% in 48 hours or until four doses of methotrexate had been given. Using this regimen, 15% to 20% of patients required only a single methotrexate dose. In 1991, Stovall and colleagues18 reported on the efficacy of single-dose methotrexate in the treatment of ectopic pregnancy. Of 30 hemodynamically stable patients diagnosed with an unruptured ectopic pregnancy less than 3.5 cm in greatest dimension who desired future fertility, 29 (96.7%) were successfully treated with a single dose of methotrexate (50 mg/m2 given intramuscularly) without leucovorin rescue (Table 5). Mean time to resolution of ectopic pregnancy was 36 days. No patient experienced any chemotherapeutic side effects. Six of 30 (20%) reported an increase in lower abdominal pain between days 5 and 10, and 2 required hospitalization overnight for observation. Five of 6 (83%) ectopic pregnancies with cardiac activity were successfully treated with this protocol. The one treatment failure, a patient whose ectopic pregnancy had cardiac activity, required surgical intervention on day 6 because of tubal rupture. Cardiac activity can be identified much earlier in ectopic gestation because of recent advances in transvaginal ultrasonography; hence, the finding of adnexal cardiac activity is now considered a relative contraindication to medical therapy. Caution should be exercised when medically managing ectopic pregnancies with cardiac activity.
TABLE 5. Protocol For Single-Dose Methotrexate For Treatment of Unruptured
Ectopic Pregnancy
Day | Therapy and Blood Tests |
0 | hCG, CBC, SGOT, blood type and Rh |
1 | MTX, hCG |
4 | hCG |
7 | hCG, CBC, SGOT |
If <15% decline in hCG between days 4 and 7, give second dose of MTX. | |
If | |
hCG, quantitative serum β-human chorionic gonadotropin level; CBC, complete blood count; SGOT, serum glutamate-oxaloacetate transaminase; MTX, intramuscular methotrexate, 50 mg/m2.
(Modified from Stovall TG, Ling FW, Gray LA: Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol 1991;77:754)
Critics of medical therapy for ectopic pregnancy point to the need for prolonged follow-up. The follow-up described by Stovall and colleagues, however, is similar to the follow-up hCG monitoring required after any conservative surgical procedure for ectopic pregnancy.
Methotrexate by Direct Injection.
The advantages cited for the direct instillation of methotrexate, laparoscopically or transvaginally under ultrasound guidance, include higher local drug concentrations, less systemic distribution, a smaller therapeutic dose, and less toxicity. Despite the theoretical advantages of direct injection of methotrexate, success rates in practice appear unacceptably low, whereas other outcomes (tubal patency and subsequent fertility) are similar to those seen with systemic methotrexate19 (Table 6).
|
|
| Tubal Patency |
| Subsequent Fertility |
| |
|
|
|
|
|
| Intrauterine | Ectopic |
| Treated | Successful (%) | HSG | Patent (% | Included | Pregnancy | Pregnancy |
Directly injected | 295 | 244 (83) | 65 | 57 (88) | 40 | 31 | 2 |
Systemic | 306 | 286 (94) | 132 | 107 (81) | 77 | 49 | 6 |
Wolf and Witt21 reported on the outpatient use of laparoscopically directed injection of methotrexate in nine patients with unruptured tubal pregnancies measuring less than 4 cm. Fifteen milligrams (0.6 mL of 25 mg/mL solution) of methotrexate was injected through a 15-cm, 25-gauge spinal needle, using a three-puncture technique to disperse the drug. Daily telephone contact was made with each patient. Eight patients (89%) had complete resolution of the pregnancy in 10 to 33 days; the ninth patient required surgical intervention. Of the five patients with initial hCG levels greater than 1000 mIU/mL, four were treated successfully. The failure among the study patients had an initial hCG level of 17,000 mIU/mL. Wolf and Witt,21 and other authorities,31 have suggested that ectopic pregnancies with higher hCG values may be less likely to respond to this treatment, although a patient has been reported who had a serum hCG level of 122,000 mIU/mL and a fetus with cardiac activity who responded to vaginal ultrasound-guided intraamniotic methotrexate injection.32
Methotrexate by Tubal Cannulation.
In a multicenter trial, Risquez and colleagues33 reported resolution of 27 of 31 cases by the instillation of methotrexate using hysteroscopically directed tubal cannulation. The remaining four patients ultimately required surgery. Although encouraging, this approach appears to have no major advantage over other methods, except that methotrexate can be instilled into very small ectopic sacs without actually visualizing them.
PROSTAGLANDIN BY DIRECT INJECTION.
Prostaglandins cause strong tubal muscular contractions and local vasoconstriction. In 1987, Lindblom and associates34 reported treating nine women with small unruptured tubal pregnancies with 0.5 to 1.5 mg of prostaglandin F2α injected into the affected tube and corpus luteum. The preliminary experience was promising, but a larger study later failed to confirm the results. Moreover, cardiac arrhythmia, cardiopulmonary edema, and gastrointestinal symptoms have been reported after intratubal injection of prostaglandin.35
ELIGIBILITY FOR MEDICAL MANAGEMENT.
Methotrexate is contraindicated in patients who are hemodynamically unstable and in those who have severe anemia, renal insufficiency, active liver disease, leukemia, bone marrow abnormalities, or allergy to methotrexate. Patients with white blood cell counts of less than 4000/mL, hematocrit less than 26%, bilirubin greater than 1.2 mg/dL, aspartate aminotransferase or alanine aminotransferase greater than 70 IU/dL, and serum creatinine levels of 1.4 mg/dL or greater should be excluded from such management.
As mentioned previously, caution should be exercised when medically managing ectopic pregnancies greater than 4 cm or with cardiac activity. Some authorities advise that methotrexate be used only in patients who desire future fertility.
Surgical Management
HISTORICAL.
Before the development of safe and effective surgical management, ectopic pregnancy was managed expectantly, with a mortality rate of 69%.36 In 1884, Tait37 reported on a series of five patients who underwent salpingectomy for the treatment of tubal pregnancy. In 1897, Martin38 reported removing a tubal pregnancy by opening the tube and shelling out its contents, with closure of the incision. No follow-up was provided. Prochownick,39 in 1894, described a similar operation in a residual tube; 2 years later, this patient conceived and delivered at term.
Despite these case reports, extirpative surgery remained the preferred procedure in most cases of ectopic pregnancy. In 1962, Grant40 advocated the use of salpingotomy in the treatment of tubal pregnancy only when the contralateral tube was “absent or blocked.” In his study of 141 patients who had undergone conservative surgery for a pregnancy in the remaining tube, 27 (19%) achieved intrauterine pregnancies, and 9 (6%) developed subsequent tubal pregnancies. At the time, this represented an unacceptably high risk of ectopic pregnancy; in 1962, the risk of ectopic pregnancy was 0.03% in the general population and 2.4% among infertility patients.
In 1967, Timonen and Nieminen41 studied 1085 patients with ectopic pregnancies and noted a slightly higher term pregnancy rate after nonconservative surgery (27.2% versus 23.8%) as well as a decreased risk of recurrent ectopic pregnancy (11.5% versus 15.7%). These investigators concluded that nonconservative therapy was justified in all cases except in “childless women with the contralateral tube severely damaged or completely destroyed.” A high tubal occlusion rate was thought to occur after conservative therapy.
CONSERVATIVE VERSUS NONCONSERVATIVE SURGERY.
A shift in attitude was slow until improved results of conservative surgery were well documented. In 1979, DeCherney and Kase42 compared conservative surgery and nonconservative surgery and found no difference in the term viable pregnancy rate (40%). Conservative therapy, however, did not increase the repeat ectopic pregnancy rate (8%). Although there was no improvement in outcome, these investigators strongly urged conservative therapy whenever possible. This lack of apparent benefit is particularly true in the presence of a normal contralateral tube. Langer and coworkers43 correlated pregnancy outcome with contralateral tubal condition. Although the ratio of intrauterine to ectopic pregnancy after conservative surgery is about 6:1, it rises to more than 10:1 in patients with normal contralateral tubes. Sherman and associates44 performed a retrospective study and noted that postoperative infertility was associated significantly with coexistent periadnexal or tubal disease as well as with a history of infertility, advanced maternal age, and tubal rupture. These investigators demonstrated that among those patients with the previously listed risks factors, conservative surgery significantly increased the chances of achieving intrauterine pregnancy (76% versus 44%) compared with extirpative surgery. Among those patients with an otherwise noncontributory reproductive history, however, the surgical approach did not alter subsequent pregnancy potential.
By the 1980s, conservative surgery had become the preferred procedure for unruptured ectopic pregnancies. As larger series became available for scrutiny, however, it became apparent that the type of procedure selected for tubal pregnancies, whether conservative or extirpative, had little correlation with fertility outcome. Thorburn and colleagues45 reported on a series of 148 patients treated for ectopic pregnancy. Although the overall subsequent birth rate was 53.6%, a history of infertility, previous abdominal surgery, previous curettage, previous ectopic pregnancy, and the presence of adnexal adhesions at surgery significantly reduced the subsequent live birth rate. Choice of surgical management appeared to have no impact on subsequent outcome. Tuomivaara and Kauppila46 published results on a series of 323 patients desiring pregnancy after an ectopic pregnancy. Nulliparous patients had a significantly lower conception rate than parous women (74% versus 86%). Nulliparous women also had a higher incidence of repeat ectopic pregnancy than multiparous patients (22% versus 9%). Again, it was concluded that surgical management did not influence subsequent fertility. Querleu and Boutteville47 stratified 473 patients on the basis of parity and history of infertility. They found that the intrauterine pregnancy rates were higher among conservatively treated patients, but the difference was dramatic when the infertile nulliparous patients were compared with nulliparous patients without a history of infertility (24.1% versus 41.4%). Again, nulliparous patients had a higher incidence of repeat ectopic pregnancy than multiparous patients, irrespective of the surgical approach (22% versus 9%). Pouly and associates48 studied 223 patients treated laparoscopically and found that a history of infertility, ipsilateral adhesions, and poor contralateral tubal status reduced the subsequent conception rates and were associated with a higher incidence of recurrence. When a scoring system was applied (Table 7), the subsequent intrauterine pregnancy rate varied inversely with the score, whereas the risk of recurrence varied directly. Based on this scoring analysis, treatment recommendations at the time of initial ectopic pregnancy favored a conservative approach when risk factor analysis was deemed low and favored extirpative surgery with contralateral sterilization followed by in vitro fertilization (IVF) when risk factor analysis was considered high.
TABLE 7. Statistical Weight of the Risk Factors and Therapeutic Score of
the Ectopic Pregnancy
Score Data | Score | Statistical Weight |
One previous ectopic pregnancy | 2 | 0.434 |
For each additional ectopic pregnancy* | 1 | 0.261 |
Previous laparoscopic adhesiolysis† | 1 | 0.258 |
Previous tubal microsurgery† | 2 | 0.351 |
Solitary tube | 2 | 0.472 |
Previous salpingitis | 1 | 0.242 |
Ipsilateral adhesions | 1 | 0.207 |
Contralateral adhesions‡ | 1 | 0.198 |
Score 0 to 3: laparoscopic conservative treatment
Score 4: laparoscopic salpingectomy
Score 5 or more: laparoscopic salpingectomy and contralateral sterilization
*If the ectopic pregnancy occurred in both tubes, just count “solitary tube.”
†Only one is taken in count.
‡If the tube is blocked or absent, count “solitary tube.”
(Modified from Pouly JL, Chapron C, Mannes H, et al: Multifactorial analysis of fertility after conservative laparoscopic treatment of ectopic pregnancy in a series of 223 patients. Fertil Steril 1991;56:453)
The conservative technique described in many early reports was salpingotomy, which requires closure of the tubal incision. The currently preferred surgical approach is salpingostomy, first described by Tompkins in 1956,49 whereby the linear incision is left open. McComb and Gomel50 demonstrated in rabbits that healing by secondary intention decreases the risk of secondary tubal obstruction and facilitates better approximation of the mucosal folds.
CONCOMITANT OOPHORECTOMY.
Jeffcoate51 advocated salpingo-oophorectomy instead of salpingectomy alone, reasoning that if ovulation occurs on the side of a previous ectopic pregnancy, transmigration is more apt to result in recurrent ectopic pregnancy. In light of current IVF availability and the potential for a higher yield of oocytes at retrieval with two ovaries present, concomitant oophorectomy for ectopic pregnancy cannot be supported.
PREOPERATIVE CONSIDERATIONS.
With the availability of sensitive and rapid serum hCG determinations and high-resolution ultrasonography, the initial presentation of ectopic pregnancy more often has shifted from an acute emergency to early diagnosis during the prodrome. Nevertheless, when a patient presents in the emergency room with an acute abdomen and hemodynamic instability, she must be expeditiously managed, limiting therapeutic options and the opportunity to review them with the patient.
Preoperative Discussion.
In discussing surgical options during the preoperative discussion, patient expectations and the desire for future fertility should be addressed. If future child bearing is not desired, extirpative surgery should be employed. Additionally, if the patient is concerned about minimizing the risk of a subsequent ectopic pregnancy, a nonconservative approach may be indicated, with consideration given to contralateral tubal ligation. If the contralateral tube is abnormal, salpingectomy followed by IVF yields higher and earlier probability of term pregnancy than attempting spontaneous conception (30.5% versus 25.6% within 18 months).52 In addition to discussing the risks of the procedure, such as bleeding, infection, and potential damage to the viscera, it is important to discuss the possible need for laparotomy in the event of visceral injury, to achieve hemostasis, or if the size of the specimen exceeds the limits for laparoscopic removal.
Laparoscopy Versus Laparotomy.
The opportunity to treat ectopic pregnancy definitively at laparoscopy has expanded the role of the laparoscope from a purely diagnostic tool. Shapiro and Adler53 described the first case of laparoscopic excision of an ectopic pregnancy, and Bruhat and coworkers54 first described laparoscopic linear salpingotomy for ectopic pregnancy. If a conservative procedure by laparoscopy is planned, necessary equipment and personnel must be available. A high-flow insufflator (8 L/min CO2), multiple operative ports, an instrument for incising the tube (laser, needle-tip electrosurgical device, or scissors), instruments for hemostasis (unipolar and bipolar electrosurgical devices), and an irrigation-aspiration instrument capable of removing large volumes of fluid with clot from the pelvis should be available at the outset. Hypovolemic shock and intestinal distention are absolute contraindications to laparoscopy, but tubal rupture in the absence of hypovolemic shock is not. Other considerations include obesity, multiple previous abdominal procedures, and a history of a bleeding diathesis.
The advantages of operative laparoscopy include less postoperative discomfort, better cosmetic results, shorter hospitalization, faster recuperation, earlier return to work, and reduced costs when compared with laparotomy. In experienced hands, the laparoscopic approach should not prolong anesthetic exposure significantly. Reproductive outcome is not significantly different between the two approaches,55 although a conservative approach using the laparoscope has been associated with a higher risk of persistent ectopic pregnancy (9%) than seen in those managed by laparotomy (3%).56 The surgeon should not attempt at laparoscopy what he or she would not perform at laparotomy.
OPERATIVE CONSIDERATIONS.
The selection of operation is guided by (1) the location of the pregnancy within the fallopian tube, (2) tubal integrity, (3) patient wishes with regard to future fertility, (4) the capabilities of the surgeon, and (5) the availability of special instruments. The ultimate goal in patients desiring future pregnancy is the preservation of a maximal length of functioning tube. Subsequent tubal function is affected by the condition of both tubes. Adhesion formation and tubal damage can be minimized by ensuring hemostasis, atraumatic tissue handling, prevention of serosal drying, and the use of fine, nonreactive sutures.1
Ampullary Pregnancy.
Ampullary pregnancies are readily managed by linear salpingostomy. Before the incision, desiccation of the serosal vessels along the anticipated incisional line is performed with an electrosurgical device or defocused CO2 laser beam. Dilute vasopressin solution (10 U in 100 mL) can be injected into the antimesenteric border to decrease bleeding. Using scissors, laser, or electrosurgery, a linear incision is made over the gestational tissue at the area of maximal distention. Myosalpingeal contraction may occur after incision, resulting in spontaneous expression of the products of conception. If spontaneous extrusion does not occur after 3 minutes, gentle external compression may be performed with the aid of forceps (Video Illustration 1). Hydrodissection also may be helpful in dislodging the gestational tissue. The surgeon must resist vigorous evacuation to avoid disruption of the underlying vascular bed. Often, the implantation bed is not immediately hemostatic. Useful measures to restore hemostasis include pressure, injection of vasopressin, needle-tip or bipolar electrocoagulation, and ultimately, suture placement in the mesosalpinx. Alternatively, mesosalpingeal ligation can be accomplished with bipolar electrocoagulation. Once hemostasis is achieved, the incision is allowed to close by secondary intention. Closing the incision may increase the risk of persistent ectopic pregnancy and subsequent adhesion formation.57
Isthmic Tubal Pregnancy.
Segmental resection (partial salpingectomy) has been advocated in the setting of an isthmic ectopic pregnancy because salpingostomy at this site was thought to result in tubal occlusion and recurrent ectopic pregnancy rates of up to 50%.58 It has been suggested that, in most isthmic pregnancies, implantation is extraluminal, the mucosa is generally not preserved, and the muscularis is destroyed.59 Pauerstein and colleagues,60 however, reviewed 25 cases of tubal pregnancy and concluded that trophoblastic spread and tubal hemorrhage were predominantly extraluminal in only 6 cases, 5 of which had ruptured. In 1986, Pouly and coworkers61 reported on the use of salpingostomy to treat isthmic pregnancies. Of 22 patients with isthmic pregnancies treated with salpingostomy, 12 (54.5%) achieved intrauterine pregnancies. The repeat ectopic pregnancy rate was 36.4% (8 patients).
Immediate tubal reanastomosis has been performed with success,62 although most surgeons prefer to perform an interval anastomosis after the resolution of tubal edema and the development of normal tissue planes. DeCherney and Boyers63 reported on four conceptions in six patients who underwent delayed anastomosis after segmental resection, including one ectopic pregnancy in the conserved tube.
Distal Tubal Pregnancy.
Distal tubal pregnancies already undergoing spontaneous tubal abortion may be atraumatically removed from the distal end of the fallopian tube with gentle traction. Fimbrial expression, however, particularly when performed by laparoscopy, is associated with a high risk of persistent ectopic pregnancy (10% to 12%)54,56 and with greater tissue trauma because a portion of the conceptus commonly is located within the fallopian tube wall.
Tubal Rupture.
In the face of tubal rupture and overt hemorrhage, salpingectomy may be necessary to provide rapid treatment and to prevent serious morbidity and mortality. Alternatively, segmental resection may be performed in a hemodynamically stable patient. Ipsilateral oophorectomy, as proposed by Jeffcoate,51 is not recommended unless the ovary is closely involved with the ectopic pregnancy, vascularity is compromised, or the ovary is otherwise grossly abnormal.64
Partial salpingectomy at laparotomy is performed by desiccating or applying ligatures across the tube, both proximal and distal to the extrauterine gestation. The mesosalpinx is ligated along this portion of the tube, and the specimen excised. Laparoscopic salpingectomy is performed in a similar fashion with bipolar electrosurgery and scissors, stapling devices, or endoscopic loop ligatures.
Repeat Ectopic Pregnancy.
Repeat ectopic pregnancy may be an indication for more aggressive therapy (see Table 7). DeCherney and colleagues,65 however, reported that 4 of 13 patients managed conservatively delivered at term after two previous ectopic pregnancies.
Tissue Removal.
Often, tissue removal poses special challenges. If small enough, the specimen can be drawn up through an expanded accessory port (10, 12, or 15 mm) with grasping forceps. If the specimen exceeds 5 cm, consideration should be made for morcellation, which can be performed after the pregnancy has been placed into an endoscopic pouch. Care must be taken to evacuate all gestational tissue from the pelvis.
POSTOPERATIVE CONSIDERATIONS
Persistent Ectopic Pregnancy.
To identify persistent ectopic pregnancy, all patients who undergo linear salpingostomy should be followed at least weekly by quantitative serum hCG levels until nonpregnant levels are attained. Residual trophoblastic tissue may continue to proliferate and lead to life-threatening hemorrhage resulting from tubal rupture. The diagnosis of persistent ectopic pregnancy is made when serum hCG levels rise or decline by less than 20% between two consecutive measurements taken 3 days apart.56 In most cases, plateauing serum hCG levels do not require further therapy.24 If serum hCG levels continue to rise, however, three treatment options are available:
- An additional conservative procedure may be considered, but there is a
strong possibility that the remaining trophoblastic tissue will be extraluminal
and a second operation may again be ineffective in removing
all of the trophoblastic tissue.
- A salpingectomy may be performed, but the potential fertility-sparing effects
of the first procedure will be lost.
- Systemic methotrexate is effective in treating persistent trophoblastic
tissue and, despite the absence of large series confirming its efficacy, it
may represent the best clinical option.
Adjuvant Methotrexate Therapy.
Graczykowski and Mishell66 reported on a nonblinded, randomized controlled series on the use of adjuvant methotrexate therapy in 116 patients undergoing conservative treatment of an unruptured ectopic pregnancy. The patients in the treatment arm received 1 mg/kg of methotrexate by intramuscular injection within 24 hours of the operative procedure. One of 54 patients (1.9%) in the methotrexate prophylaxis group and 9 of 62 (14.5%) in the control group had persistent ectopic pregnancies. The results were statistically significant (P < 0.05). The relative risk of developing persistent ectopic pregnancy after prophylactic methotrexate was 0.13 (95% confidence interval: 0.02 to 0.97). Three patients in the prophylaxis group reported side effects; one complained of mild stomatitis and two of mild gastroenteritis lasting 1 to 2 days. In all three, the side effects resolved spontaneously and did not require medical intervention.
Patients undergoing salpingectomy rarely have persistent trophoblastic activity. Routine postoperative measurement of serum hCG levels is not required.
Rh Factor.
All Rh-negative, unsensitized women with ectopic pregnancies should receive Rh immunoglobulin at a dose of 50 μg if the gestation is of less than 12 weeks' duration and 300 μg otherwise. Grimes and associates67 reported that, because hospitalization typically is unscheduled and not preceded by Rh screening, most Rh-negative women with ectopic pregnancies in the United States do not receive Rh(D) immunoglobulin. The magnitude of the risk of sensitization is unknown but is estimated to vary from no risk at 1 month to about 9% at 3 months' gestation. Because of the potential benefits and lack of risk, administration of Rh immunoglobulin should be undertaken in all Rh-negative unsensitized women with ectopic pregnancy.25
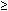 15% decline in hCG between days 4 and 7, follow until <5 mlU/mL
15% decline in hCG between days 4 and 7, follow until <5 mlU/mL