Rectovaginal fistulas can be repaired through a multitude of approaches
including transanal, vaginal, perineal, abdominal, and transsacral. A transabdominal approach is usually reserved for high rectovaginal fistulas. The most
important aspect of rectovaginal fistula repair is closing the high-pressure
or rectal side of the defect. Fine, delayed absorbable suture should
be used in the closure of the rectal defect because of prolonged
tensile strength and the greater integrity of knots. Low/Midzone Fistulas Simple fistulotomy may be used in superficial anoperineal fistulas. Here, the tract extends
from an opening in the anal canal to the perineum, and usually, the
sphincter is not involved. The fistulous tract is laid open with a simple
incision. There are reports of women with Crohn’s disease
and anovaginal fistulas successfully treated by fistulotomy.19 More commonly, a transsphincteric approach with layered closure is used for low fistulas because of proximity to
the anal sphincter.28 An episioproctotomy is made and allows for excision of the entire fistulous
tract followed by a repair similar to that of a fresh fourth-degree
laceration at delivery. This technique requires meticulous reapproximation
of both the internal and the external anal sphincters. There
is a risk of fistula recurrence as well as anal incontinence if the healing
is compromised. This approach allows for reconstruction of the perineal
body as well. Episioproctotomy has been favorably described for
patients with quiescent Crohn’s disease without prior fecal diversion.29 Inversion of the fistula into the rectal lumen via a transvaginal approach is also suitable for
small low rectovaginal fistulas30(Fig. 3). The vaginal mucosa is incised around the fistula ostium, and the fistula
is closed with purse-string sutures tied in such a fashion as to
invert the soft tissue into the anorectum. The vaginal mucosa is then
reapproximated. For high rectovaginal fistulas, the anterior and posterior
vaginal walls, once mobilized, can be utilized in the inversion of
the fistula into the rectum (a modification of the Latzko procedure
for vesicovaginal fistulas).30 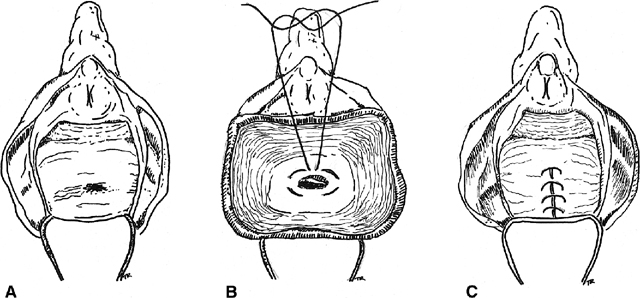 Fig. 3. Inversion technique of rectovaginal fistula repair. A. A low fistula is identified. B. The vaginal mucosa is incised around the defect. A purse-string suture
is placed. C. The purse-string is tied, inverting the defect into the anorectum, and
the vaginal mucosa is closed over the site Fig. 3. Inversion technique of rectovaginal fistula repair. A. A low fistula is identified. B. The vaginal mucosa is incised around the defect. A purse-string suture
is placed. C. The purse-string is tied, inverting the defect into the anorectum, and
the vaginal mucosa is closed over the site
|
Boronow reported excellent results in patients with radiation-induced rectovaginal
fistulas, utilizing a layered closure with interposition of
a modified Martius graft of bulbocavernosis–labial fat pad tissue31 (Fig. 4). A vertical incision is utilized on the labia majora. The fatty tissue
is mobilized sharply with careful attention to preserving the blood
supply of the graft either inferiorly or superiorly. The thumb-sized graft
is tunneled subcutaneously beneath the vaginal mucosa and the labia
minora to overlay the repaired fistula site. The donor site may require
a drain. Boronow emphasized a delay of surgery for 6 months after
radiation exposure, a biopsy of the margins of the tract, a diverting
colostomy in advance of the repair, interposition of vascularized tissue, and
a tension-free closure. Elkins and colleagues have also utilized
the Martius technique to repair a number of rectovaginal fistulas
with satisfactory results.32 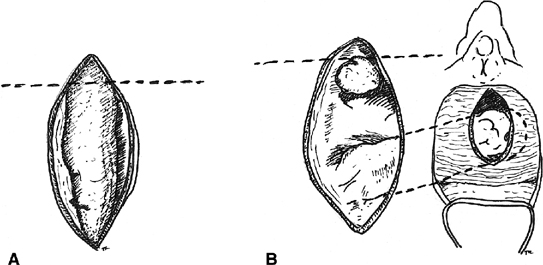 Fig. 4. Martius labial fat pad graft. A. The fibrofatty tissue is mobilized with preservation of either the superior
or the inferior blood supply. B. The graft is tunneled to the site of the repair. Fig. 4. Martius labial fat pad graft. A. The fibrofatty tissue is mobilized with preservation of either the superior
or the inferior blood supply. B. The graft is tunneled to the site of the repair.
|
Hoexter and coworkers, proposed a transanal repair.33 They excise the fistula tract, approximate the rectal musculature, and
advance the rectal mucosa caudad to protect the repair. The vagina mucosa
is left open to heal by secondary intention. The authors found no
recurrence in 35 patients with benign rectovaginal fistulas followed
for 1 to 6 years. Repair from the rectal side has the advantage of correction
of the defect from the high-pressure side, perhaps decreasing
the rate of failure of repair. Goligher has described a transperineal approach, through which the anus and rectum are separated from the vagina
and the fistula tract is divided.34 The mobilization of the vaginal and rectal walls permits a tension-free
closure. In addition, he recommends slight rotation of the rectum and
vagina in opposite directions to protect the suture lines. This approach
to repair allows the surgeon to preserve an intact internal and external
anal sphincter. Wiskind and Thompson devised another transperineal approach akin to Goligher’s.35 Using a transverse incision across the perineal body above the sphincter
complex, dissection is carried out between the anterior rectal wall
and the posterior vaginal wall. The external anal sphincter is preserved, vaginal
and rectal defects are closed separately, the fistula tract
is transected, and attention is directed toward reapproximation of
the endopelvic fascia in the midline and interposing the approximated
puborectalis muscle between the vaginal and the rectal defects. These
defects can be closed transversely or longitudinally. By closing the defects
longitudinally, vaginal and anal length is maintained. In the series
by Wiskind and Thompson, 21 patients with low fistulas, including 7 with
Crohn’s disease, had no recurrence during a follow-up
ranging from 3 months to 8 years (mean, 18 months). Sher and associates
also emphasize the importance of approximating the levator ani muscles
in the midline between the rectal and the vaginal suture lines.36 Laparoscopic upper rectovaginal mobilization facilitating the transvaginal
repair of recurrent rectovaginal fistulas has also been described
by Pelosi and Pelosi.37
Many feel the best method of repairing low and midzone fistulas is the
sliding or advancement flap. Based on the work by Noble,38
there have been many modifications. Stowe and Goldberg describe developing
a broad-based flap of rectal mucosa, submucosa, and circular muscle and
advancing it caudad39 (Fig.
5). Before the flap is sutured over the fistula site, the tract is
excised from the distal end of the flap and the circular muscle is reapproximated
with absorbable suture. The flap is then advanced over the repair and
secured with interrupted absorbable sutures. The vaginal side is left
open to heal by secondary intention and provide drainage of the surgical
site. Several authors report a high rate of success with this method even
when used for the treatment of rectovaginal fistula secondary to Crohn’s
disease.36,40 Some have
described advancing the entire circumference (sleeve) of rectum for lesions
involving more than one third of the circumference of the anus.40,41
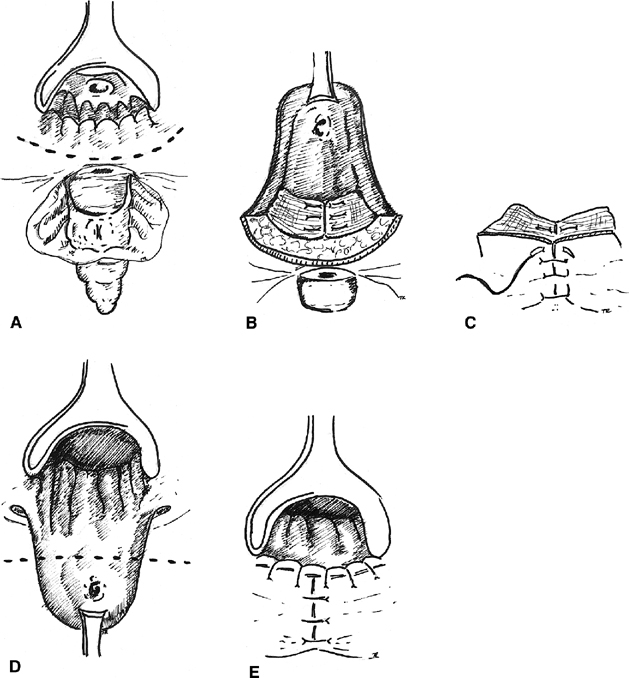 Fig. 5. Rectal advancement flap. A. A circumanal incision is made. B. The rectal flap is elevated, and the sphincter mechanism is repaired, C. The perineal tissue is closed. D. The flap is advanced with excision of the fistulous tract. E. The mucocutaneous junction is restored. Fig. 5. Rectal advancement flap. A. A circumanal incision is made. B. The rectal flap is elevated, and the sphincter mechanism is repaired, C. The perineal tissue is closed. D. The flap is advanced with excision of the fistulous tract. E. The mucocutaneous junction is restored.
|
High Fistula Repair Most often, a transabdominal approach is used for a colovaginal or high
rectovaginal fistula because of coexisting pelvic disease and the inaccessibility
of the fistula through the vagina. However, Lawson has successfully
used a perineal approach to treat fistulas near the cervix.42 The vagina is divided up to the lateral fornix, and the pouch of Douglas
may be opened behind the cervix to improve exposure. The rectum is
drawn downward for a layered repair. Lawson reported success with this
technique in 42 of 53 cases. The Latzko technique of using the anterior
and posterior walls of the vagina in the closure of a vesicovaginal
fistula can also be adapted for high rectovaginal fistulas.30 The etiology of the fistula must be appreciated when anticipating a transabdominal
repair for a high fistula. If the fistula is secondary to
trauma or pelvic drainage of an inflammatory process (e.g. diverticulitis), the
defect can be successfully closed by opening the rectovaginal
septum and closing the rectal and vaginal defects with interposition
of an omental J-flap. Bowel resection or the interposition of nondiseased
tissue is usually required in cases involving radiation injury or
advanced malignancy. Bricker and colleagues described a complex repair for radiation-induced
rectovaginal fistula.43 The procedure involves an onlay patch of well-vascularized intestine to
the damaged tissue and a descending colostomy. The advantages of Bricker’s
technique are that it requires only anterior mobilization
of the rectum, avoids denervation injury to the anorectum and hemorrhage, and
that the patch or flap retains its native blood supply. Timing of Repair The majority of fistulas secondary to trauma or obstetric causes may be
repaired early. Repair should be deferred until the resolution of active
infection, inflammation, induration, or local cellulitis. The authors
agree with Hibbard, who feels that the decision of when to operate
should depend on the condition of the tissue and not on an arbitrary
period of time.44 Diverting Colostomy Diverting colostomy is typically used in the management of radiation-induced
fistulas, very large rectovaginal fistulas, and some fistulas secondary
to inflammatory bowel disease. A repair of the fistula can then
be accomplished after all evidence of cellulitis and inflammation has
resolved (usually 8 to 12 weeks). Colostomy takedown may be scheduled
for 3 to 4 months after the repair. |