Once the incontinence workup is completed and the decision to proceed with a sling procedure has been made, options remain. First is the choice of graft material. Organic grafts require a harvesting procedure or can be obtained from a tissue bank. Autologous grafts include vaginal epithelium, rectus fascia, or fascia lata. Allografts include cadaveric dermis, fascia lata, dura, and pericardium. Heterologous grafts include porcine dermis and small intestine submucosa. Multiple inorganic grafts are readily available. Second is the choice of surgical approach. The various sling procedures performed today are technically somewhat similar. Most sling procedures are performed using a combined abdominal and vaginal approach, with most of the dissection performed vaginally. As reported in the early 1900s, a complete abdominal approach is possible but may be associated with increased urethral injury. Early avoidance of a combined procedure was common because of concern about vaginal contamination and increased postoperative infection. With today's improved surgical techniques and antibiotic coverage, combined abdominal and vaginal procedures are safe. Sling variations include a full-length sling, a patch sling, or a TVT sling.
As part of the preoperative preparation, the risks and benefits of the procedure, along with postoperative restrictions, are reviewed with the patient. Many surgeons teach all patients clean intermittent self-catheterization before surgery. On the day of surgery, the patient should receive appropriate antibiotic prophylaxis, according to patient allergy and medical history, 60 minutes before surgery. The patient is positioned in the dorsal lithotomy position and prepared and draped in a sterile fashion for abdominal and vaginal surgery. A Foley catheter is placed transurethrally.
Full-Length Sling
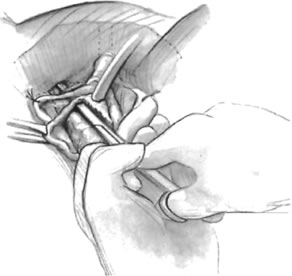
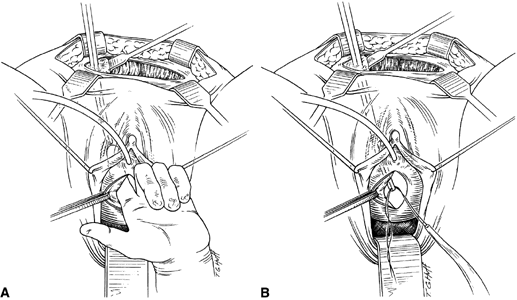
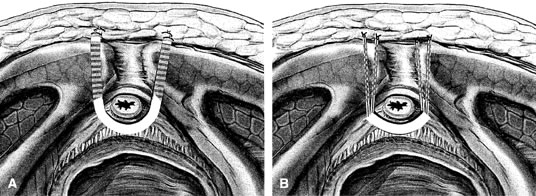
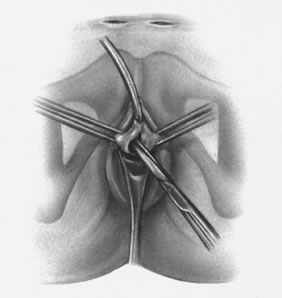
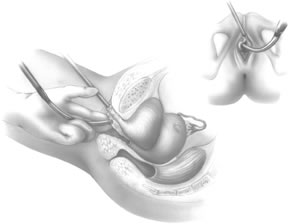
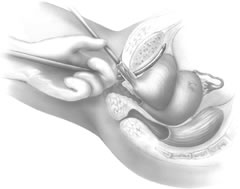
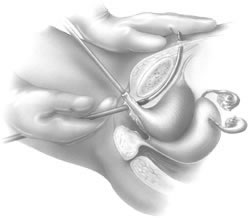
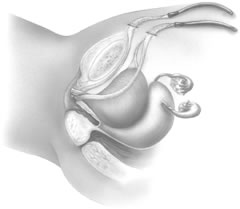
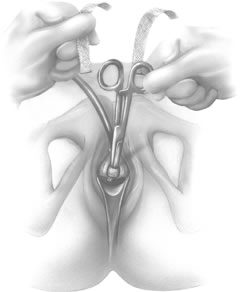
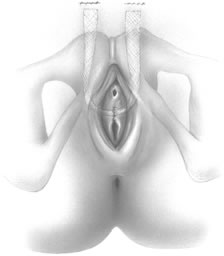
If a full-length sling is planned, the graft of choice is prepared and soaked in antibiotic solution. If an autologous fascial graft is planned, a harvesting procedure for either fascia lata or rectus fascia is completed using a Wilson fascial stripper or a vein stripper. Similarly, if an allograft is used, this too should be hydrated in an antibiotic solution. Generally, the graft should measure approximately 2 ×12 to 18 cm for a full-length sling, according to patient habitus. A transverse suprapubic incision is made 2 fingerbreadths superior to the symphysis pubis, measuring approximately 4 cm. The underlying rectus fascia should be exposed with either sharp or blunt dissection. The anterior vaginal epithelium is then infiltrated with either sterile saline or dilute anesthetic/epinephrine solution. A midline anterior vaginal incision is made, measuring about 3 cm, at the level of the urethrovesical junction. The vaginal epithelium is sharply dissected away from the periurethral and perivesical tissue. The dissection should be completed laterally to the inferior pubic rami. Gentle traction is applied to the Foley catheter to allow identification and palpation of the balloon, indicating the urethrovesical junction. The bladder is decompressed. With the surgeon protecting the urethra with the nondominant hand, the perineal membrane is perforated bilaterally, allowing entrance into the space of Retzius, using either Metzenbaum or curved Mayo scissors at a 45° angle, always lateral to the urethra and directly behind the symphysis pubis (Fig. 1). The midportion of the sling is secured using delayed, absorbable suture along the proximal urethra, with the most proximal edge at the bladder base to prevent folding or kinking of the graft material. As is done with a needle urethropexy, packing forceps are passed with guidance from a vaginal finger, from the abdominal incision through the space of Retzius to the vaginal incision. The graft arm is grasped and carried back through to the abdominal incision. This is repeated on the opposite side (Fig. 2). When passing through the space of Retzius, the surgeon takes care to start medial to the pubic tubercle to avoid ilioinguinal nerve injury. Once both sling arms are passed, urethrocystoscopy is performed to rule out urethral or vesical injury. Ureteral function is also confirmed by intravenous indigo carmine injection. A suprapubic catheter is placed under cystoscopic guidance for postoperative bladder drainage and voiding trials. The sling arms are then sutured to the rectus fascia using permanent suture to allow a urethrovesical junction angle of 0° with the horizontal plane. The incisions are irrigated with antibiotic solution. The vaginal and abdominal incisions are reapproximated using delayed, absorbable suture (Fig. 3).
Patch Sling
A variation of the full-length sling is the patch sling. This procedure can be performed with either a patch of in situ vagina, as described by Raz and associates in 1989,2 or other organic or inorganic grafts. The patch sling has been described as a variation of a Pereyra or Raz procedure.22 The patch should measure approximately 2 × 5 cm. Surgical preparation, the abdominal and vaginal incisions, and dissections are completed as described for the full-length sling. Entrance into the space of Retzius may or may not be accomplished, according to the surgeon's preference. As in the full-length sling procedure, the midportion of the patch graft is secured suburethrally using delayed, absorbable suture to prevent folding or kinking of the graft. In a helical fashion, permanent suture is passed along the long axis of the patch graft. A Stamey ligature carrier is passed with guidance from a vaginal finger, from the abdominal incision through the space of Retzius to the vaginal incision on each side for suture transfer. Once both suture arms have been transferred, urethrocystoscopy is performed after intravenous indigo carmine administration to rule out urethral, vesical, or ureteral injury. A suprapubic catheter is placed under cystoscopic guidance for postoperative bladder drainage and voiding trials. The suture arms are tied down to a urethrovesical junction angle of 0° with the horizontal plane. The incisions are irrigated with antibiotic solution. The vaginal and abdominal incisions are reapproximated using delayed, absorbable suture (see Fig. 3).
The full-length and patch sling procedures are most often performed under general anesthesia; however, if medically indicated, they may be completed under regional anesthesia.
Tension-Free Vaginal Tape Sling
The TVT system consists of a reusable stainless-steel introducer handle, a reusable rigid catheter guide, and the single-use device. The TVT device consists of a 1 × 40-cm ribbon of polypropylene mesh covered by a plastic sheath attached onto two curved stainless-steel needles (Fig. 4).
The TVT procedure is most often performed under local anesthesia with intravenous sedation. The procedure is performed with the patient in the dorsal lithotomy position with her lower extremities supported in Allen-type stirrups. An 18F Foley catheter is inserted to the urethra and the bladder is emptied. Local anesthetic is applied suprapubically at two points, 1 to 2 cm above the pubic symphysis and 2 to 3 cm lateral to the midline. The abdominal skin, underlying rectus muscle, and fascia as well as the posterior aspect of the pubic bone is infiltrated bilaterally. Two small abdominal skin incisions (0.5 to 1.0 cm) are then made at these points. No further abdominal dissection is necessary.
A Sims speculum is then inserted into the vagina to allow visualization of the anterior vaginal wall. The indwelling Foley bulb is used to identify the location of the internal urethral egress, while the external meatus is easily visualized. Using these two points as landmarks, the region of the midurethra is identified. The local anesthetic solution is injected into the vaginal submucosa in the midline and slightly lateral on each side of the urethra. Allis clamps are placed bilaterally for countertraction as a small sagittal incision (1.5 cm) is made in the midline at the level of midurethra. The incision should begin approximately 1 cm from the external urethral meatus. The Metzenbaum scissors are then used to minimally dissect the vaginal wall, freeing it from the underlying periurethral tissue and developing a small tunnel bilaterally. This dissection should be limited to a depth of 1 to 1.5 cm. Care should be taken not to puncture the pubocervical fascia or injure the urethra (Fig. 5). Additional local anesthetic solution should be injected bilaterally using a long spinal needle, placing the solution along the inferior and posterior aspects of the pubic symphysis.
The rigid catheter guide is then inserted into the Foley catheter. The handle of the rigid guide is then moved to the ipsilateral side of the anticipated passage of the forthcoming TVT needle. With the aid of the introducer handle, the surgeon places the tip of the needle into the previously developed periurethral tunnel (Fig. 6). Two hands are required to pass the needle safely. The needle is directed slightly laterally, most often in direct alignment with the patient's ipsilateral axilla. The TVT needle is gradually advanced by applying gentle pressure with the palm of the vaginal hand, with continued vaginal finger guidance and slight pressure from the second hand on the introducer handle. As the needle tip passes through the endopelvic fascia, a distinct drop in resistance is appreciated. At that point, with downward deflexion of the introducer handle, the surgeon guides the needle superiorly through the space of Retzius, with the needle being immediately opposed to the back side of the pubic symphysis (Fig. 7). As the needle opposes the underside of the rectus muscle and fascial sheath, resistance is again appreciated. At this point, the introducer handle is used solely to direct pressure anteriorly, advancing the needle tip through the previously made small abdominal incisions. The surgeon's nondominant hand is used suprapubically to help guide the needle tip (Fig. 8).
The rigid catheter guide and Foley catheter are removed and diagnostic urethrocystoscopy is performed to evaluate for any unintentional injury of the urethra or bladder. Once correct needle placement is confirmed, the needles pass completely through the abdominal incision. The steps of the procedure are then repeated on the opposite side. Care should be taken to ensure the tape is not twisted under the urethra (Fig. 9).
After correct placement of the TVT device and before removal of the protective plastic sheath, a cough test is performed to identify the correct positioning of the tape. The cough test is conducted with a full bladder (250 to 300 mL saline) (Fig. 10). Once the proper positioning of the tape is obtained, the plastic sheath is removed and the prolene mesh is left in place without tension under the midurethra. The abdominal ends of the tape are cut just below the skin surface. The procedure is completed with closure of the abdominal and vaginal incisions (Fig. 11).