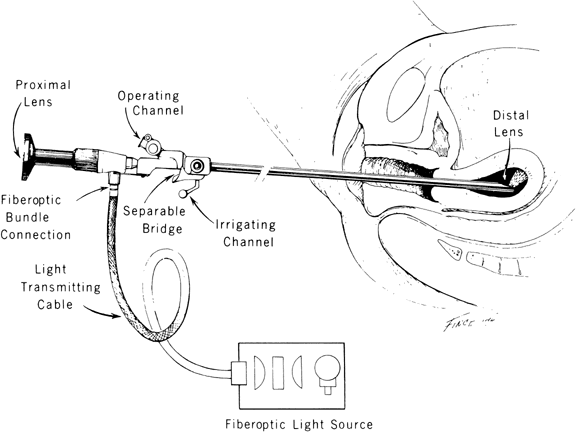
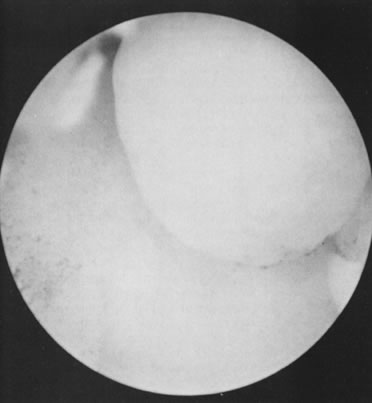
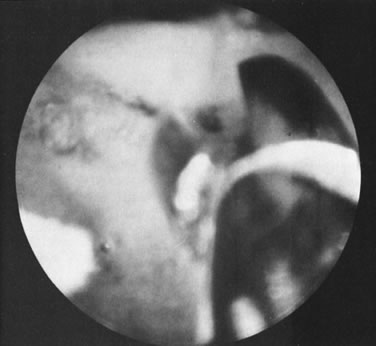
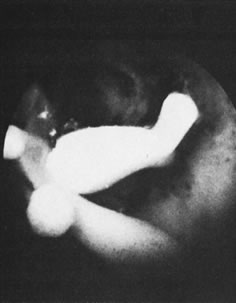
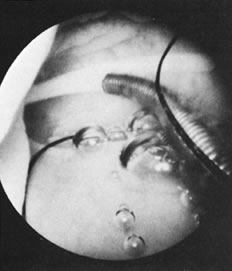
With the advent of medical fiberoptics, diagnostic tools for gynecologic endoscopy were augmented not only in intraperitoneal visualization by laparoscopy and culdoscopy, but also in the visualization of the interior of the uterus and tubal ostia. Hysteroscopy is the technique of visualization of the cervical canal and uterine cavity by means of an instrument that includes a metallic sheath and a telescope receiving light through a fiberoptic bundle from an external illuminating source. A solution or gas is used to distend the uterine cavity during the procedure.
Hysteroscopy as a procedure dates from 1869, but only recently has it become a widely used technique. Maintaining distention of the uterine cavity has been difficult, and endometrial bleeding has obscured visualization. Since the work of Edstrom and Fernstrom in Sweden1 using dextran-70 (32% w/V) in dextrose or Hyskon (10% w/V) as a medium to distend the uterus, however, application of the improved technique has proved broad and promising.
Development
In 1869, Pantaleoni2 first performed hysteroscopy in a living patient when he used his “endoscope” for visualization of the uterine cavity, and chemically cauterized a polypoid growth in a 60-year-old woman with postmenopausal bleeding. The technique was largely forgotten. Interest was renewed by several investigators, especially Rubin,3 who in a 1925 study used carbon dioxide (CO2) for distention of the uterine cavity. In 1927, Von Mikulicz-Radecki and Freund4 introduced electrocoagulation of the intramural portion of the tubes. In the United States, Norment5 worked for more than 30 years with several models of hysteroscopes, including one with a water-rinsing system and one with a rubber balloon attached to its distal part and inflated with air. His results were not satisfactory, however.
In 1962, Silander6 introduced a hysteroscope that had a latex rubber balloon attached to the distal portion for distention of the uterine cavity with normal saline instead of air. This provided acceptable visualization, but the uterine cavity remained inaccessible for biopsy or any other surgical intervention. In 1970, Edstrom and Fernstrom1 published their results with 32% dextran (molecular weight, 70,000) for distention of the uterine cavity. The use of 32% dextran allowed both excellent visualization and biopsy of lesions and surgical manipulation within the uterine cavity. Since then, several media have been used successfully for distention of the uterine cavity, such as 32% dextran, 5% dextrose (D5W), CO2, Ringer's lactate, and normal saline.
Instruments and Equipment
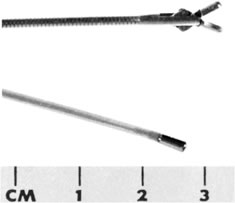
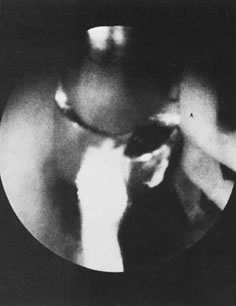
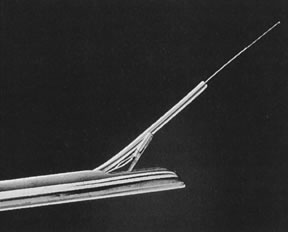
Hysteroscopic instrumentation consists of a telescope 2 to 4 mm in diameter, with Foroblique vision; a metallic sheath for the telescope and accessory channels to deliver the distending medium and introduce operating instruments; a connecting bridge with special channels to introduce manipulating instruments; a cold light fiberoptic bundle to transmit the light; an external light source for illumination; and when electrocoagulation is to be used, an appropriate electrosurgical source (Figs. 1 and 2).
|
|
Hysteroscopes are made commercially by various companies: Storz (Karl Storz Endoscopy-America, Culver City, CA), Wolf (Richard Wolf Medical Instruments, Rosemont, IL) (Fig. 3), Olympus (Melville, NY), and Circon (Stamford, CT).
|
Hysteroscopy is evolving rapidly. Accordingly, new instrumentation has been necessary to parallel this progress, especially in three areas:
- As an office procedure, smaller endoscopes (3 to 5 mm diameter) are used; they
can be introduced atraumatically without previous cervical dilatation
and with little or no anesthesia (Figs. 4 and 5).
 Fig. 4. Small-caliber hysteroscope. Lu-mina-SL telescope, 2.7-mm OD, encased in
a 10.5 French sheath, 3.3-mm OD ( top ). 11.5 French sheath, 3.7-mm OD ( bottom ).
Fig. 4. Small-caliber hysteroscope. Lu-mina-SL telescope, 2.7-mm OD, encased in
a 10.5 French sheath, 3.3-mm OD ( top ). 11.5 French sheath, 3.7-mm OD ( bottom ).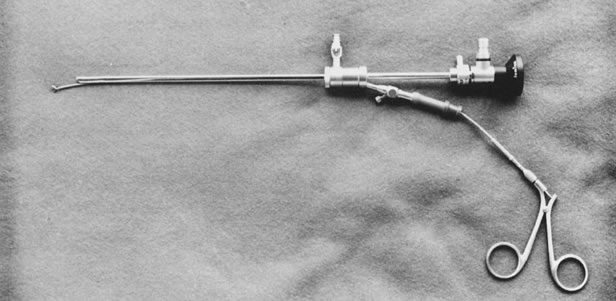 Fig. 5. Assembled hysteroscope with operating sheath: 3-French, 4.3-mm OD, with
a 5-French, 2.7-mm-OD operating channel. Flexible biopsy forceps in place (Wolf).
Fig. 5. Assembled hysteroscope with operating sheath: 3-French, 4.3-mm OD, with
a 5-French, 2.7-mm-OD operating channel. Flexible biopsy forceps in place (Wolf). - For intrauterine surgery, which has expanded its applications, operative
hysteroscopes are used; their channels permit manipulation of sturdy,
rigid, and semirigid operating instruments (Figs.
6, 7, 8,
and 9).
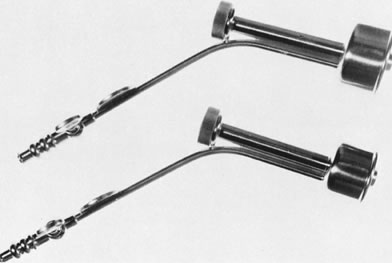
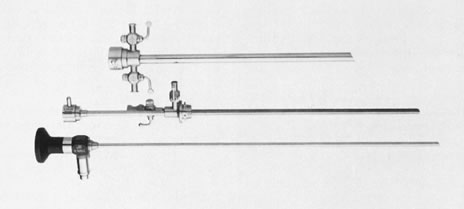 Fig. 6. Unassembled operating hysteroscope. Telescope 4-mm OD ( bottom ); telescope bridge with channel for semirigid instruments ( middle ); operating sheath, 7-mm OD ( top ).
Fig. 6. Unassembled operating hysteroscope. Telescope 4-mm OD ( bottom ); telescope bridge with channel for semirigid instruments ( middle ); operating sheath, 7-mm OD ( top ).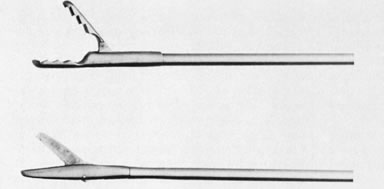 Fig. 9. Close view of distal end of semirigid grasping forceps ( top) and scissors ( bottom ).
Fig. 9. Close view of distal end of semirigid grasping forceps ( top) and scissors ( bottom ). - A resectoscope can also be used for intrauterine surgery.
The urologic resectoscope and the techniques for treating the urinary bladder (particularly for resection of prostatic enlargement) have been adapted for hysteroscopy. Applications include removal of polyps and submucous leiomyomas, resection of uterine septa, division of thick connective tissue adhesions, and ablation of the endometrium to treat abnormal bleeding.
A resectoscope includes a straight (0°) telescope with an outer diameter (OD) of 3.5 to 4 mm, which can be encased in different-sized sheaths (OD, 8 or 9 mm). The instrument has a built-in system that provides motion by means of a spring that moves the distal cutting loop forward and backward to shave and resect lesions by cutting. The outer sheath provides an outflow stopcock for continuous irrigation. The inner sheath permits inflow and introduction of an optical system and injection of solutions and other electrodes replacing the cutting loop. Although the cutting loop can be manipulated directly and moved approximately 4 cm within the visual field of the objective lens, a clear and unobstructed panoramic view of the lesion and its surrounding normal endometrium is essential to avoid accidental perforation. The instruments, which are manufactured by several companies (Storz, Wolf, Olympus, Circon-ACMI, and others), have similar features with minor modifications (Figs. 10, 11, and 12).The cutting loop is activated by connection with a high-frequency electrosurgical unit; therefore, patients must be properly grounded. Furthermore, an electrolyte-free solution should be used for distention of the uterine cavity to avoid conduction and electrical dispersion.
|
|
|
The hysteroscope is a modified cystoscope with a more circular sheath, in most instances, and with distal fenestration and a separable bridge. The procedure requires less illumination compared to laparoscopy because it has a viewing distance of less than 2 cm.
Among the several previously mentioned media for distention of the uterine cavity, 32% dextran (molecular weight, 70,000; viscosity, 225 centipoise [cP] at 37°C) has the desirable properties of being viscid, optically clear, and not readily miscible with blood. It offers a high refractory index as well, and because its flow around the sheath is minimal, a smaller quantity is needed during the procedure; however, a practical and adequate delivery system is desirable. The instruments must be thoroughly washed in hot water immediately after the procedure is terminated to avoid possible damage to the lens system from hardened dextran.
Normal saline (NS) requires specific pressure for distending the uterine cavity in each patient. It is simple to deliver, allows washing of the uterine cavity, produces little or no bleeding, and is readily available in any hospital or office setting.
CO2 insufflation requires a special hysterosufflator to produce a pneumometra and to record intrauterine pressure and gas flow, which are necessary for safety and effectiveness. The hysterosufflator designed by Lindemann (Hysteroflator 1000, F.M. Weist K.G., 1 Berlin 30, Germany) electrically controls a continuous CO2 insufflation with a flow of 30 to 100 mL/min and an intrauterine pressure of 100 mmHg.7,8 Other machines digitally display those features (Storz, Wolf, Olympus).
We use CO2 as a distending medium for office diagnostic procedures. D5W or D5NS and, in some instances, 32% dextran are used as distending media for operative procedures.
When hysteroscopy is performed as an outpatient procedure, it may require a paracervical block tray, particularly for operative procedures. Paracervical block anesthesia is produced according to the individual practitioner's choice.
A tray for proper preparation and draping of the patient and an external light source giving an illumination of 100 to 1000 W are needed. Additional instruments needed if intrauterine surgical intervention is foreseen are scissors, biopsy forceps, and electrosurgical probes (Figs. 13 and 14). Polyethylene catheters for intrauterine washings, particularly when blood or mucus obscures the view, are other ancillary equipment needed. These are particularly important when liquid media are used for uterine distention and an operative hysteroscope without continuous flow system is used. Further studies of intrauterine pressure, tubal motility, or chemical studies of the intratubal milieu require atraumatic catheters. In a patient with a patulous cervix, a special cervical vacuum adapter may be used to avoid reflux of the fluid used for distention of the uterine cavity (Fig. 15).
|
|
Finally, if photography is desired, an electronic flash with an individual external light source is useful. Video systems and digital photography have largely replaced still cameras for documentation.
Technique
The technique of hysteroscopy using NS is simple, and the following summary can be adapted to the available methods.9,10
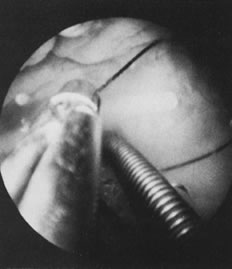
Once a patient has been selected for hysteroscopy, a Papanicolaou smear, cervical/vaginal smear, and cultures are used to rule out any active infection. The patient is asked to void before the procedure. The technique for hysteroscopy varies according to the hysteroscope used, whether operative (7 mm OD) or diagnostic of a small-caliber (3 to 5 mm OD). (For office procedures, see section on Office Hysteroscopy.) On the examination table, the patient is placed in the lithotomy position, and her vulva and vagina are cleansed with an appropriate antiseptic solution. Once the cervix is visualized, a paracervical block is performed using 8 to 10 mL of chloroprocaine hydrochloride 1% (Nesacaine) solution on each side when cervical dilatation is needed. Ten minutes are allowed for the anesthetic to take effect. The cervical canal is dilated up to (or until) a number 7 Hegar's dilator. The hysteroscope, attached to a light source and to tubing that delivers a selected solution to distend the uterus, is introduced and the proper pressure for the individual patient is achieved. Approximately 3 to 5 minutes are sufficient for good visualization of the uterine cavity, including both ostia. Any unusual tissue, polyps, or submucous leiomyomas may be sampled or removed with special biopsy forceps (Fig. 16).
|
In some instances, when adequate visualization is impossible because of undue bleeding, clots, or excessive mucus and endometrial debris, it is advisable to irrigate the endometrial cavity under a low-gravity pressure system, collecting this material through a polyethylene tube large enough to avoid plugging Figs. 17 and 18).Uterine distention thereafter offers a better view. This is almost mandatory when foreign bodies are investigated or removed by this method.
|
|
When hysteroscopy is performed with a patient under general anesthesia, the same steps are followed. In this instance, hysteroscopy is usually followed by additional surgical procedures, such as laparoscopy. We prefer general anesthesia only when additional surgical intervention is required; otherwise, paracervical block suffices.
Learning the technique of diagnostic hysteroscopy offers only minor difficulties. Minor intrauterine abnormalities in a distended uterus may not be readily recognizable, and experience is required for proper interpretation.
Intrauterine manipulation and instrumentation require experience, especially when catheterization of the fallopian tubes is attempted. However, experience is achieved and refined easily as direct observation of the intrauterine cavity leads to familiarity with its structures.
In our experience in more than 300 hysteroscopic examinations, the technique was performed for various indications, such as abnormal uterine bleeding, postmenopausal bleeding, “lost” intrauterine contraceptive devices (IUDs), infertility, and many other conditions in which intrauterine pathology was suspected (Table 1).
Table 1. Preoperative Diagnoses in 318 Patients
Preoperative Diagnosis | No. of Patients | % |
Abnormal uterine bleeding | 104 | 32.7 |
Foreign body | 94 | 29.6 |
Postmenopausal bleeding | 36 | 11.3 |
Primary infertility | 24 | 7.5 |
Myomatous uterus | 18 | 5.7 |
Secondary infertility | 12 | 3.8 |
Defect on hysterosalpingography | 11 | 3.5 |
Habitual abortion | 8 | 2.5 |
Suspected uterine anomaly | 4 | 1.3 |
Tubal catheterization before tubal | 3 | 0.9 |
reanastomosis |
|
|
Suspected uterine perforation | 2 | 0.6 |
Possible incomplete removal of | 2 | 0.6 |
products of conception |
|
|
A 71.4% abnormality rate was found, and the technique successfully detected several previously unrecognized uterine pathologic conditions, such as submucous leiomyomas, endometrial polyps, intrauterine adhesions, septate uteri, foreign bodies, carcinoma of the endometrium, and endometrial adenomatous hyperplasia (Table 2).
Table 2. Hysteroscopic Findings in 318 Patients
| No. of |
|
Finding | Patients | % |
Normal | 93 | 29.5 |
Foreign bodies* | 81 | 25.5 |
Endometrial polyps | 64 | 20.1 |
Submucous leiomyoma | 30 | 9.5 |
Septate uterus | 11 | 3.5 |
Intrauterine adhesions | 11 | 3.5 |
Atrophic endometrium | 8 | 2.5 |
Cesarean section scar defects | 6 | 1.9 |
Endometrial adenomatous hyperplasia | 5 | 1.6 |
Unilateral uterine horn | 3 | 0.9 |
Tubal catheterization before tubal | 3 | 0.9 |
Reanastomosis |
|
|
Uterine perforation | 2 | 0.6 |
Incomplete removal of products of conception | 1 | 0.3 |
*In 78 patients, IUDs were found within the uterine cavity. In 2 patients, a migrated laminaria tent was found. In 1 patient, a fragmented cotton-tipped applicator was observed and removed.
In these patients, focal lesions were found and suspected of being carcinoma. Direct biopsy of these lesions proved them to be endometrial adenomatous hyperplasia
Indications
Indications for hysteroscopy are numerous. As the technique improves, more applications will undoubtedly be found. The most common uses are as follows:
In abnormal uterine bleeding, to rule out an organic cause When submucous
leiomyomas or polyps are suspected
If an IUD filament cannot be seen at the cervix, or if foreign bodies are
suspected within the uterine cavity
In postmenopausal bleeding, to rule out an organic cause
In primary or secondary infertility, especially if anomalies or defects
are seen by hysterosalpingography
In lysing of intrauterine adhesions by direct visualization
In tubal catheterization, to record tubal motility and for biochemical
study of the tubal milieu
In patients with habitual abortion, to explore the cervical canal and uterine
cavity
For sterilization by hysteroscopic occlusion of the fallopian tubes
As a diagnostic method for staging adenocarcinoma of the endometrium and for delineating the possible extent of the tumor in the lower uterine segment or on the borders of the endocervical canal, thus aiding in establishing the proper therapy12
Contraindications
There are three major contraindications to hysteroscopy: acute pelvic inflammatory disease, pregnancy, and profuse bleeding. Previous infection must be ruled out before hysteroscopy is undertaken. The technique may be modified and used as amnioscopy in pregnancy, especially in diagnosing premature rupture of the membranes or in detecting chronic fetal distress by changes in the amniotic fluid.13
Complications
Any surgical instrumentation within the uterine cavity requires great care, especially operative endoscopy. Unnecessary manipulation must be avoided to prevent damage to the endometrial lining. Adherence to the protocol of indications and techniques will prevent exacerbation of an already present infection or seeding of a new infection.
Significant complications of hysteroscopy that have been reported include bleeding, uterine perforation, and infection. These are rare, however.
Advantages
Hysteroscopy offers several advantages over present methods of diagnosis and, occasionally, permits surgical therapy for pathologic lesions within the uterine cavity. The following are the most relevant advantages:
Direct visualization of the endometrial cavity avoids diagnostic errors
associated with dilatation and curettage (D & C) or blind endometrial
biopsy procedures.
As an outpatient procedure, it requires only local anesthesia.
It is acceptable to patients because discomfort is minimal.
Its risks are minor.
The average duration of the procedure is 5 minutes.
Compared to surgery as a means of sterilization, hysteroscopy promises reduced hospitalization, pain, and discomfort, and rapid return to normal activities. The technique of hysteroscopic sterilization is still undergoing clinical evaluation, however, and results are not yet satisfactory.
Although the technique does not solve all problems and certainly cannot replace other procedures used for diagnosis and therapy, it complements them. Hysteroscopy is a new and valuable tool for gynecologic diagnosis.
Diagnostic Applications
With direct visualization of the uterine cavity and endometrial lining, gynecologists no longer must rely on blind curettage or on abnormal hysterographic shadows. Pathologic conditions can be observed in vivo and in situ, giving the technique an advantage over other modes of gynecologic diagnosis.
D & C has been the most frequently used diagnostic method in patients with uterine bleeding, but many small lesions, polyps, and submucous leiomyomas may be overlooked.
In 1957, Englund and coworkers14 reported on a series of patients in whom hysteroscopy subsequent to D & C demonstrated abnormal tissue, polyps, and submucous leiomyomas in 65%. Several others have reassessed these findings. According to Englund and coworkers,14 hysterosalpingography is also subject to a 50% error in diagnostic interpretation. Subsequent hysteroscopy was able to detect the error. The addition of hysteroscopy to fractional D & C and hysterography in the staging of adenocarcinoma of the endometrium when the lesion is intrauterine has increased the accuracy and effectiveness in delineating this disease, offering an improvement over some of the misleading results found with the traditional methods.15 Although still experimental, with this approach, hysteroscopy seems to offer a distinct advantage over fractional D & C and hysterography; as shown by Sugimoto12 and by Joelsson and associates.16
Possible abnormalities or lesions in the cervical canal and internal cervical os that may lead to cervical incompetence have been difficult to observe and detect. Direct visualization by hysteroscopy provides an accurate view of this previously inaccessible area. Embryologic developmental anomalies of the uterus, including a broad variety of septate uteri, can be seen clearly by hysteroscopy. Intrauterine adhesions can be observed. Unusual hysterographic shadows, which may present difficulties of interpretation, can easily be clarified by direct visualization. A modification in the technique makes hysteroscopy possible in a pregnant patient to confirm or rule out complicating factors, such as premature rupture of the membranes or chronic fetal distress by direct observation, as described by Aguero and Aure.13
HYSTEROSCOPY FOR ABNORMAL UTERINE BLEEDING
Abnormal uterine bleeding is a common gynecologic problem afflicting women at various stages of their lives; the condition has psychological, physiologic, and pathologic implications. Traditionally, D & C, endometrial biopsy specimens, and hysterosalpingography have been the most accurate diagnostic methods used in adult women at risk of uterine pathology and in younger patients with persistent or recurrent abnormal uterine bleeding that has failed to respond to hormonal therapy. Because the appropriate diagnosis has not always been obtained with these methods, and because the symptoms can be prolonged or obscured with unnecessary hormonal treatment, additional methods of evaluation are sought. One useful diagnostic and therapeutic adjunct is hysteroscopy. The major advantage of hysteroscopy over other gynecologic diagnostic methods is that it permits observation of the entire cavity of the uterus. The visual dimension provided by hysteroscopy increases the accuracy of diagnosing suspected intrauterine pathology, which ranges in various studies from 28.9% to 70%, depending on the criteria used in selection of patients.17
HYSTEROGRAPHY VERSUS HYSTEROSCOPY
Hysterography is a useful method of evaluating the uterine cavity; its sensitivity and specificity have been challenged, however, because transient distortion of the uterine cavity by blood, mucus, debris, and air bubbles may produce false-positive results. Errors in technique, selection of dye, and interpretation may also contribute to failure. The ability to observe the inside of the uterus directly can confirm an abnormal shadow seen in the hysterogram and allows biopsy of pathologic lesions. Confirmation of abnormal hysterographic findings by subsequent hysteroscopy varies from 43% to 68% in different studies comparing these two techniques.17
DILATATION AND CURETTAGE VERSUS HYSTEROSCOPY
Although D & C is the most frequently performed gynecologic surgical procedure for the diagnosis and treatment of abnormal uterine bleeding, pathologic findings are scarce and on many occasions the true pathology may be missed. This inadequacy has been demonstrated in studies comparing the diagnostic accuracy of D & C with hysteroscopy, as reported by Edstrom and Fernström,1 in Norment,5 Englund and associates,14 and others. When focal lesions of the endometrium are to be investigated, particularly when these conditions are located at the uterotubal cones, they may be easily missed by D & C. Even relatively large pathologic lesions in the endometrial cavity, such as submucous leiomyomas and endometrial polyps, may be missed by curettage because of the blind approach. In patients with recurrent or persistent abnormal uterine bleeding, the visualization provided by hysteroscopy aids in selecting patients who may require curettage and in determining where the curette is to he guided.
Although biopsy of the endometrium by suction curettage using a Vabra aspirator increases the accuracy and quantity of the tissue sample, it remains inadequate for the diagnosis of endometrial polyps, subcutaneous leiomyomas, and focal pathologic lesions of the endometrium. Endometrial biopsy is an excellent method of diagnosing the overall endometrial response in relation to ovulation and for supplying material for study when a lesion affects a large portion of the endometrium.
Abnormal uterine bleeding has been one of the leading indications for hysteroscopy since the technique was established as a clinical method of evaluation. Because of its additional advantage of visualizing the uterine cavity, the method can indeed ensure adequate curettage or complete removal of pathologic lesions. When hysteroscopy has been used as an adjunct in the evaluation of patients with abnormal uterine bleeding, a significant rate of detection of abnormal findings has been achieved, ranging from 40% to 85% in published studies.17
The records were reviewed of 553 patients who complained of abnormal uterine bleeding as their only symptom and who had hysteroscopy before curettage (419 premenopausal and 134 postmenopausal); 352 (63.6%) had a hysteroscopically detected abnormality. Of the 419 premenopausal patients, 277 (66.1%) had intrauterine abnormalities that could explain their symptoms. Among the 134 postmenopausal patients, 75 (55.9%) were found to have pathologic lesions.
When the hysteroscopic diagnosis was compared with the histologic diagnosis made by hysteroscopic biopsy, a 100% correlation could be made in patients with a diagnosis of focal adenocarcinoma of the endometrium (3 patients). Hysteroscopic diagnosis was confirmed on hysteroscopic biopsy in 15 of 22 patients with endometrial hyperplasia and in 179 of 202 patients with endometrial polyps; submucous leiomyomas were confirmed only when recovered surgically, because the samples taken through the hysteroscope were unpredictable and positive in only 10 of 80 patients, since adequate tissue was difficult to obtain with the biopsy forceps available.
When the hysteroscopic biopsy specimens of uterine lesions were compared with material taken at curettage in patients with focal pathologic lesions, the hysteroscopic detection and biopsy were far more accurate than random curettage. Only when the endometrial lesions were extensive did curettage adequately reveal pathologic areas.18
Although hysteroscopy increases diagnostic precision in patients with intrauterine lesions, using it does not exclude other methods and evaluation. Rather, its use complements them and improves the accuracy of diagnosis and therapy, particularly in situations not amenable to diagnosis by traditional methods alone. Because hysteroscopic visualization of the endometrium may not permit an accurate diagnosis, a hysteroscopic specimen of suspicious lesions should be obtained for further pathologic study (Figs. 19, 20, and 21).
|
|
The application of hysteroscopy for evaluating abnormal uterine bleeding follows guidelines similar to those for endometrial biopsy and curettage. In young adolescent women, a complete history and physical examination and appropriate laboratory studies are usually sufficient for a diagnosis. Even in young women, however, when bleeding persists or recurs, additional examinations may be necessary to rule out organic conditions within the uterine cavity. In adult women, particularly those older than 35 and those in the perimenopausal and postmenopausal period, a complete evaluation of the endometrial cavity is mandatory before therapy is instituted.
OFFICE HYSTEROSCOPY
One of the most significant advances in the use of hysteroscopy occurred in the early 1980s with the introduction of small-caliber endoscopes (OD less than 4 mm) that do not require cervical dilatation for their introduction in the uterus, therefore facilitating the use of hysteroscopy in the office setting.19–21 The small-caliber endoscope simplifies the examination and permits practical, safe, and efficient investigation of the uterine cavity. The examination can be performed in a simpler, faster, and cleaner way, decreasing the need for anesthesia and/or analgesia. Nonetheless, it has inherent drawbacks. Owing to the relatively small diameter, no instrument can be passed through the small hysteroscope, as it is not usually fitted with an operating channel. With these hysteroscopes, only CO2 gas can be used as a practical medium to distend the uterine cavity adequately.
Newer endoscopes have a 4- to 5-mm OD, permitting continuous flow of a low-viscosity fluid via a small, built-in channel as well as the performance of minor operative procedures. These endoscopes can also be used in the office, permitting minor surgical procedures.
The hysteroscopes used in the office setting, OD usually less than 4 mm, are used more commonly for diagnostic purposes. To deliver the CO2 gas as a distending medium, an electronic hysterosufflator is necessary to maintain the appropriate flow rate at a constant 40 to 60 mL/min and an intrauterine pressure of no more than 100 mmHg if the fallopian tubes are open. As the flow rate decreases, the intrauterine pressure may increase, and vice versa.
For office hysteroscopy, in general, no systemic sedatives or medications are required, as the procedure is practically painless. Nonetheless, it is advantageous to perform a paracervical block with 4 to 8 mL of a local anesthetic of an ester type. Chloroprocaine is most useful because of its low toxicity and rapid action. The anesthetic is injected superficially at the base of each uterosacral ligament close to the cervix, and a small amount (0.5 mL) is injected at the site where the tenaculum is going to be placed. Once this anesthetic block is accomplished, a hysteroscope (attached to its light source and flowing CO2 gas) is introduced under direct vision at the level of the ectocervix. The hysteroscopic examination begins, slowly and systematically, following the small microcavity that the CO2 gas produces in front of the endoscope. Once the endocervical canal is completely explored, the endoscope is advanced across the internal cervical os and the panoramic view of the uterine cavity is then evaluated. After the uterine cavity is systematically examined, the endoscope is withdrawn under direct vision for reevaluation of the uterine cavity and endocervical canal at the conclusion of the examination.
A similar technique is used when a small-caliber hysteroscope with a continuous flow system is used. The endoscope is introduced at the external os atraumatically and advanced until the uterine cavity is visualized. The advantage of this method is a continuous flow that washes away any debris, blood clots or mucus that may impair visualization.22
CLINICAL CONSIDERATIONS
In general, office hysteroscopic examinations are only diagnostic. If pathology is found that requires biopsy or hysteroscopic removal, a larger instrument (OD, 7 mm) may be used and operative hysteroscopy performed. The new continuous flow hysteroscopes, nonetheless, allow performance of biopsies with 5 French instruments. However, the decision whether to perform an operative procedure in the office or in an ambulatory surgical center will be determined by the pathology found. For operative hysteroscopy local, regional, or general anesthesia may be required, depending on the individual circumstances.
Patients who have normal hysteroscopic findings are spared other manipulation or biopsy. In patients with abnormal uterine bleeding with a normal uterine cavity, but with irregular endometrial desquamation (typical in patients with abnormal uterine bleeding) found at the conclusion of the hysteroscopic examination, a 4-mm soft plastic cannula is introduced and a vacuum suction curettage is performed to obtain tissue for histologic evaluation.
Hysteroscopy has been facilitated as a simple office procedure with the addition of small-caliber endoscopes. Because of its relative simplicity and ease, the procedure is applicable as a screening method for those patients with abnormal uterine bleeding and/or questionable hysterograms, and for patients with suspected intrauterine pathology.
Although hysteroscopy can be performed in the office with a 7-mm-OD operative hysteroscope, the required cervical dilatation and manipulation involves additional instrumentation and personnel, as well as safety measures in anticipation of side effects such as bradycardia and hypotension, which may occur after cervical dilatation. A procedure similar to the one detailed above is followed, except that the endocervical canal is gradually dilated with a number 7 Hegar or to the exact diameter of the specific operative hysteroscope used. Despite the feasibility of using an operative hysteroscope under paracervical block anesthesia, operative procedures that can be performed in the office setting are limited to procedures such as removal of IUDs, biopsy of focal lesions, removal of some polyps, and tubal cannulation or occlusion. Extended manipulations and surgical interventions, such as the division of extensive intrauterine adhesions, division of uterine septa, and removal of submucous leiomyomas, are more effectively and safely performed in an ambulatory surgical center or in the operating suite, either under regional or general anesthesia, and in some instances, with concomitant laparoscopy.23
Surgical Applications
Intrauterine manipulation under direct visualization facilitates surgical intervention within the uterine cavity. Specially designed instruments, such as forceps, scissors, electrodes, and electrosurgical probes, can be easily manipulated and selectively directed to the area chosen by the endoscopist. Targeted biopsy of lesions, removal of small polyps or submucous leiomyomas, and sampling of the endometrial lining for histologic review are accomplished easily, with minimal or no trauma to the rest of the endometrial cavity. Surgical intervention under these circumstances has proved far superior to blind methods, especially in the lysis of intrauterine synechiae and the removal of a small septum dividing the uterine cavity.24,25
Foreign bodies in the uterine cavity, especially IUDs, no longer need to be localized radiographically when filaments are not visible at the cervix. By means of hysteroscopy, definite diagnosis can be made, and the filaments can be retrieved selectively at the cervical os, avoiding blind removal and unnecessary trauma to the endometrium.
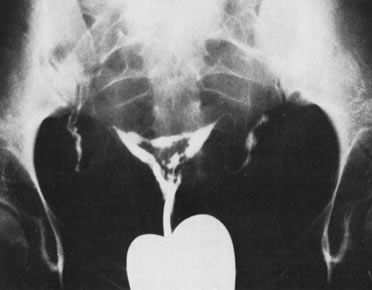
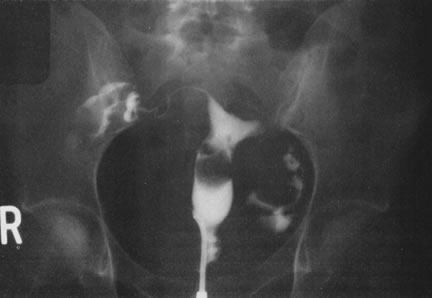
We find hysteroscopy to be of great value in the management of patients with lost IUDs (Figs. 22, 23, 24, and 25). In our experience with 91 hysteroscopic examinations of patients wearing IUDs that they could not feel or that a clinician could not see on examination, successful location of the lost IUD confirmed the validity, safety, and effectiveness of the procedure, as well as its advantage in terms of avoiding unnecessary radiographs and blind manipulations (Tables 3 and 4).26,27
|
|
|
Table 3. IUD in the Uterine Cavity Found at Hysteroscopy (78 Patients)
| No. of |
Condition Found at Hysteroscopy | IUDs |
IUD in situ with curled filaments | 67 |
IUD in situ without filaments | 6 |
Fragmented IUD without filaments | 4 |
IUD superficially embedded with curled filaments | 2 |
Retrograde cervical perforation | 2 |
Total | 81* |
*Three patients each had two IUDs found within the uterine cavity.
Table 4. Type of Intrauterine Device Found at Hysteroscopy (78 Patients)
Type | No. of IUDs |
Lippes loop | 45 |
Copper-7 | 26 |
Dalkon shield | 5 |
Saf-T-Coil | 3 |
Pleated membrane | 1 |
Ota ring | 1 |
Total | 81* |
*Three patients each had two IUDs found within the uterine cavity.
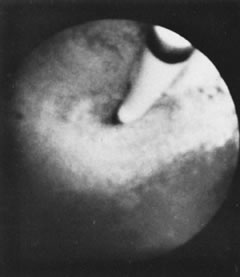
Valuable studies of tubal motility can be accomplished with open-ended catheters introduced into the tubal lumen (Fig. 26). Biochemical determination of the tubal milieu is of value in the investigation of human reproduction.11
The technique provides direct access to the uterotubal junction without invasion of the peritoneal cavity. Undoubtedly, the most promising application of hysteroscopy is tubal occlusion for sterilization purposes. However, surgical intervention within the uterine cavity must not be attempted until the clinician is experienced in intrauterine visualization and observation, especially at the uterotubal junction. The small area of manipulation and the fragility of the endometrial lining make extreme delicacy mandatory for the procedure to be successful and free of possible complications (Figs. 27 and 28).
|
|
ASHERMAN'S SYNDROME
Since Asherman described the syndrome that bears his name more than three decades ago, the treatment of intrauterine adhesions had not changed significantly until the advent of hysteroscopy. In general, the results of blind division of adhesions by curettage have been unsatisfactory. Hysteroscopy has added a new dimension to the evaluation and treatment of these patients. Asherman's syndrome has become recognized as a frequent cause of pregnancy wastage and infertility in patients with delayed curettage after abortion or delivery of a child.
Although a hysterosalpingogram is perhaps the most accurate screening method to detect this condition, the distortion seen on the hysterosalpingogram may on many occasions not accurately represent the true condition; visual appraisal of the uterine cavity is most valuable for ascertaining the presence of adhesions and their extent, quality, and components and at the same time providing their direct treatment by division under hysteroscopic guidance. Depending on the extent of adhesions to be divided, hysteroscopic lysis, with or without concomitant laparoscopy, is the preferred method of surgically dividing intrauterine adhesions.
Although the immediate results of restoring the endometrial cavity architecture and symmetry are excellent, the reproductive outcome correlates well with disease severity: the more advanced and extensive the disease, the poorer the prognosis, the milder and less extensive the cavity occlusion, the better the prognosis. In a review of 61 patients who had hysteroscopic treatment for intrauterine adhesions, the reproductive outcome was as follows: 33 conceptions, including 22 term pregnancies; 5 patients pregnant but undelivered; 5 spontaneous abortions; and 1 ectopic pregnancy. When mild intrauterine adhesions were treated in 16 patients, 10 women (62.5%) achieved a term pregnancy. The majority of patients treated were classified as having moderate adhesions (i.e., fibromuscular adhesions partially or totally occluding the uterine cavity). Of the 33 patients with moderate adhesions, 11 (33.3%) achieved a term pregnancy. In 12 patients with severe intrauterine adhesions, only 2 term pregnancies occurred (16.6%).28
It is undeniable that by adding visualization of the uterine cavity in patients with intrauterine adhesions, the adhesions can be lysed selectively without danger to the adjacent endometrium and uterine wall. Also, the results seem to correlate well with the severity of the adhesions. Three stages of intrauterine adhesions are defined according to Sugimoto's criteria29:
Mild: Filmy adhesions (endometrial) producing partial or complete uterine
cavity occlusion
Moderate: Fibromuscular adhesions, characteristically thick, still covered
with endometrium that bleeds on division; partially or totally occlude
the uterine cavity
Severe: Adhesions composed only of connective tissue, lacking any endometrial
lining, and likely not to bleed on division; may partially or totally
occlude the uterine cavity
The extent of the occlusion, and the type of adhesions involved, is predictive of the outcome in these patients (Figs. 29, 30, and 31).
|
|
UTERINE SEPTA
Uterine malformations resulting from partial or complete failure of the müllerian ducts to fuse or canalize may interfere with normal reproduction in 20% to 25% of patients afflicted with these conditions. Of these uterine abnormalities, a septate uterus is most often associated with reproductive wastage.30 The classic clinical picture is of repeated early-midtrimester pregnancy wastage, with signs of labor, including bleeding. This condition has traditionally been treated with abdominal metroplasty, with excision of the septum through a wedge uterine excision,31 or incision of the septum without excision to prevent reduction in size of the uterine cavity.32 Both operations require a laparotomy and division of the uterine corpus. There has been a revival of operative hysteroscopy for the division of uterine septa. Although this operation was originally reported by Edstrom33 in 1974, it was used only sporadically until recently. Daly and associates34 reported success with hysteroscopic metroplasty in 25 patients, with promising results. The operation is performed with concomitant laparoscopy to assess the external shape of the uterus and monitor the operation; recovery is relatively quick. Cyclic estrogens and progesterones are prescribed for 1 to 2 months, and patients are then allowed to attempt pregnancy. Delivery is vaginal, and cesarean section is reserved for obstetric indications.
We have reviewed our experience during a 6-year period with 12 patients who had a symptomatic septate uterus and underwent hysteroscopic division of the uterine septum monitored by concomitant laparoscopy. The preoperative reproductive history included 41 pregnancies with only 3 viable deliveries. Perioperative prophylactic antibiotics were used, and estrogen and progesterone were also prescribed postoperatively. Of the 12 patients, 10 conceived within 1 year after therapy; 7 of these patients delivered a live infant at term. Two patients had a spontaneous abortion at 6 and 8 weeks after therapy, but their subsequent pregnancies were carried to term. Three patients are currently pregnant. A second hysteroscopic operation was performed in 2 patients because of a remaining septum observed at the posttreatment hysterogram.
Conventional metroplasty requires major surgery and may jeopardize fertility because of subsequent pelvic adhesions. A longer postoperative interval is required before pregnancy can be established; moreover, cesarean section is needed. For these reasons, hysteroscopic division of the uterine septum is more appealing to patients and physicians; the low morbidity, simplicity, and encouraging pregnancy outcome with this operation make it the treatment of choice for a symptomatic septate uterus (Figs. 32, 33, and 34).35
|
|
HYSTEROSCOPIC MYOMECTOMY
Leiomyomas and endometrial polyps are the most common solid uterine neoplasms affecting women, frequently causing abnormal uterine bleeding, pelvic pain, and occasionally reproductive failure. At present, hysteroscopy is the most accurate method for diagnosing endometrial polyps and submucous leiomyomas; furthermore, it offers the best surgical alternative for controlling their transcervical removal.
Uterine leiomyomas are common neoplastic disorders, occurring most frequently in the third and fourth decades of life. They usually require treatment for indications such as excessive bleeding, pelvic pain, rapid growth of the leiomyoma, or infertility. Approximately 20% of women past the age of 30 are afflicted with this problem. When women in their reproductive years are affected with leiomyomas, diagnosis may be difficult, and the treatment should preserve reproductive capability.
As mentioned, in some instances leiomyomas cause infertility, particularly because of their location and size. Leiomyomas located at the uterotubal junctions may mechanically interfere with tubal patency. Large broad-ligament leiomyomas may also interfere with tubal function and disrupt the tubo-ovarian relationship. Large intramural and submucous leiomyomas may distort the uterine cavity and the endometrial lining to an extent that prevents sperm migration, nidation, and implantation of the blastocyst. By far the most common symptom associated with uterine leiomyomas, particularly the submucous type, is abnormal uterine bleeding.
Patients presenting with pregnancy wastage or infertility and associated leiomyomas should undergo a thorough evaluation. A hysterosalpingogram will outline the symmetry and presence of submucous leiomyomas, distortion of the tubal intramural portion, or tubal occlusion. Direct visualization of the uterine cavity allows for selective biopsy of abnormal endometrium and a definite diagnosis of submucous leiomyomas. For a patient who is symptomatic (i.e., has bleeding, pain, or rapid growth of the leiomyoma), myomectomy is indicated to enhance reproductive capability and correct menstrual dysfunction, regardless of the size or growth of the tumor.36
Although symptoms may suggest submucous leiomyomas, currently used methods of diagnosis have not been satisfactory. Uterine sounding, endometrial biopsy, D & C, and hysterosalpingography do not permit consistently accurate diagnosis, particularly if the lesion does not significantly distort the uterine cavity. Hysteroscopy permits direct visualization of the topography and symmetry of the uterine cavity, providing accuracy in the diagnosis of intrauterine lesions. Furthermore, hysteroscopy offers the possibility of their direct treatment.
Even though the classic principles of performing an abdominal myomectomy, such as meticulous surgical technique, hemostasis, and perfect uterine reconstruction, may be followed, surgical treatment of submucous leiomyomas by laparotomy and bisection of the uterine corpus may predispose patients to possible secondary pelvic adhesions, infection, and tubal damage. When conservative treatment of submucous leiomyomas is required, hysteroscopic treatment offers a less invasive alternative and therefore a more acceptable and safer procedure than abdominal myomectomy.
Many conditions are amenable to surgical therapy under hysteroscopy, and certain criteria are useful when considering this technique; any abnormal lesion found in the uterine cavity during hysteroscopy, particularly if it is focal, can be sampled under direct visual control. In women who are of reproductive age and desirous of children, removal of submucous leiomyomas can be attempted when they are pedunculated and relatively small (less than 4 cm). Although sessile submucous leiomyomas can be shaved directly through the resectoscope using a cutting loop, this approach should be reserved for selected patients in whom most of the leiomyoma is intracavitary.37 Because it is estimated that 10% to 15% of patients with uterine leiomyomas may have future recurrences, follow-up for early discovery of possible recurrences is important.
To remove pedunculated submucous leiomyomas, a 7-mm-OD operative hysteroscope is necessary, and use of semirigid scissors to transect the pedicle is most advantageous. When no electrosurgery is used, the distending medium most commonly chosen is one of the low-viscosity fluids with electrolytes, such as lactated Ringer's solution, or normal saline. The resectoscope has become the preferred method for submucous myomectomy requiring fluids devoid of electrolytes for uterine distention, such as glycine 1%, sorbitol 3%, and mannitol 5%. To avoid early signs of water overload and hyponatremia, it is important to provide intravenous electrolytes to patients while performing this operation.
A leiomyoma is transected at its pedicle and then removed after adequate dilatation of the cervix is achieved. When leiomyomas are larger than 3 cm, they may require morcellation with a resectoscope or using a neodymium:yttrium-aluminum-garnet (ND:YAG) laser through a bare quartz fiber or an extruded, noncoaxial, sculptured fiber. Leiomyomas larger than 3 cm are then segmentally morcellated and removed transcervically. The combination of resectoscope, Nd:YAG laser, and hysteroscopic scissors is useful to remove myomas with broad pedicles. Segmental resection of sessile submucous leiomyomas has been attempted with additional procedures performed later, once the remaining portion of the leiomyoma left behind has been extruded in the uterine cavity by contractions of the uterine wall. Nonetheless, this approach is under investigation and should be performed only in selected patients and with appropriate follow-up (Figs. 35, 36, 37, and 38).38,39
|
|
|
The use of gonadotropin-releasing hormone analogs to reduce the size of leiomyomas, particularly in patients with excessive abnormal uterine bleeding and large leiomyomas, has been most helpful in preparing these patients for surgery, allowing for removal of larger leiomyomas that previously could not be approached hysteroscopically. Also, transvaginal ultrasonography with high-resolution probes has been successfully used in evaluating the specific size, location, and penetration of these submucous leiomyomas in the uterine wall to plan appropriate therapy.40–42
ENDOMETRIAL ABLATION
A significant number of patients undergo hysterectomy for treatment of abnormal uterine bleeding of nonmalignant origin that fails to respond to curettage and medical treatment. Although hysterectomy relieves the symptoms, it is a radical approach, involving surgical invasion of the peritoneal cavity, several days of hospitalization, temporary disability, considerable expense, and possible morbidity.
Several chemical, physical, and mechanical methods have been used in an attempt to destroy the endometrial lining to treat abnormal bleeding, such as quinacrine, methyl cyanoacrylate, oxalic acid, cryosurgery, ionizing radiation, silicone rubber, cytotoxic agents, and enriched collagen solutions. Most of these methods never passed the experimental stage, and at present, only two new methods are being used in clinical settings: Nd:YAG laser ablation of the endometrium, and electrocoagulation of the endometrium using a modified resectoscope.43
Laser Ablation of the Endometrium
In 1981, Goldrath and colleagues44 published the preliminary results on the use of Nd:YAG laser energy delivered through hysteroscopy to destroy the endometrial lining of patients suffering from severe menorrhagia. Careful in vitro studies were previously performed to assess the penetrability and depth of tissue effect of the Nd:YAG laser in extirpated uteri by placing copper thermocouples 1 cm away from the endometrial surface and delivering the Nd:YAG laser energy at 55 W of 1.06-nm laser energy through a 0.6-mm fiber for 5 seconds. Although the temperature rose to an average of 46°C, with a pick of 48.6°C, it never exceeded 52°C, and protein denaturation and necrosis were avoided.
Of the lasers available today, only the Nd:YAG laser has been used for endometrial destruction. This laser produces its energy beam from photoexcitation of neodymium ions in yttrium-aluminum oxide that is held in a garnet crystal. This photoexcitation is initiated by ordinary electrical power. The Nd:YAG laser has a wavelength of 1060 nm, which is in the near-infrared (invisible) portion of the light spectrum. Therefore, a special helium-neon aiming beam is used to direct and focus the laser on the area to be impacted. The penetration of this laser is 4 to 5 mm; its effect is achieved by coagulating protein. It is readily absorbed by reddish purple tissue, passes through fluid, and is hemostatic; it cannot cut. Because fluids do not block it, it can be used in organs that are distended with fluids, such as the bladder, stomach, and uterus. Because of its backscatter, the operator and surrounding personnel must wear protective glasses to avoid retinal injuries when the laser is used.
The main tissue effect of the Nd:YAG laser is coagulation. This laser is especially suited for endometrial destruction when not only the endometrium is to be coagulated, but also the superficial portion of the myometrium with its accompanying vessels. The uterus also appears to be an ideal organ for this procedure, because the inactive endometrium is approximately 1 mm thick and the myometrium is approximately 20 mm thick.
Patient Selection
Although hysteroscopic laser ablation of the endometrium is a relatively noninvasive method that can accomplish the treatment without removal of the uterus and can reduce days of hospitalization and disability, it should be reserved for patients who cannot be treated by simpler methods, such as medical treatment. Most such patients have dysfunctional uterine bleeding and are therefore amenable to hormonal treatment.
Patients with obvious anatomic and pathologic causes of bleeding are not appropriate candidates for endometrial ablation. The main indication for laser ablation of the endometrium is intractable menorrhagia when a hysterectomy is contraindicated, is not advisable for medical reasons, or is refused by a patient. All patients should have a screening hysteroscopy and biopsy to rule out other anatomic causes of their bleeding, such as myomas, polyps, endometrial hyperplasia, or even carcinoma.
It is advisable to use adjuvant preoperative treatment with danazol (Danocrine), 400 mg twice daily for the month preceding the laser ablation, to atrophy the endometrium and enhance its destruction. An alternative is leuprolide acetate (Lupron), 3.75 mg monthly for 2 months; the procedure is performed 2 weeks after the second injection.
Equipment and Technique
Hysteroscopic laser ablation of the endometrium requires knowledge and experience in the use of operative hysteroscopy. The required instrumentation does not vary much from that for other operative hysteroscopic procedures, except for the quartz fiber (0.6 mm in diameter) and the Nd:YAG unit. The new operative hysteroscopic sheaths, which permit inflow and outflow irrigation, are valuable for these procedures, and if it is available, a double channel hysteroscopic sheath may also be of great value.
The distending media most frequently used are low-viscosity fluids; it is important to use fluids with electrolytes, particularly those containing sodium. A combination of D5NS (0.9% NaC1) or D51/2NS (0.45% NaCl) is adequate. A lactated Ringer's solution could also be used; however, when several liters of this solution is used, the lactate may be excessive. Dextran 70 (32%) (Hyskon) has also been used successfully in ablation of the endometrium; nonetheless, a double-channel hysteroscope is essential to clear from the field of view the large bubbles produced by the dextran 70. Because dextran 70 does not contain electrolytes, care must be taken to monitor the amount of solution absorbed by the patient and to administer electrolytes intravenously, as required. Whatever solution is chosen, the amount used and recovered from irrigation should be meticulously monitored to calculate the exact amount of fluid absorbed by the patient. This is best achieved by using a special plastic hood draped on the patient's buttocks to collect this fluid through tubing connected to a receptacle.
Adequate uterine cavity distention may be maintained simply, without the use of mechanical or electronic pumps or tourniquets, using plastic bags containing 3 L of D5NS connected to a Y tubing and elevated sufficiently to deliver the fluid at constant pressure. This method is particularly useful during endometrial ablation, which requires about 1 hour of constant irrigation. The procedure is best performed with the patient under epidural or general anesthesia and with an intravenous line placed for administration of medications.
Hysteroscopy is performed in the usual manner by dilating the endocervical canal to the size of the hysteroscopic sheath to avoid reflux, should the hysteroscopic sheath have inflow and outflow channels. If there are no such channels, then the cervical canal is dilated 1 or 2 mm more than the diameter of the hysteroscopic sheath to permit this washing effect. Once visualization of the uterine cavity is completed and debris and mucus or blood clots are removed using a polyethylene catheter, the “bare” quartz fiber (0.6 mm in diameter) is introduced after it has been tested for spot size and functioning. The ablation then begins and is performed systematically, both cornua first and subsequently the anterior wall, to avoid bubbles that usually become attached to the anterior wall when the laser is activated. Both lateral walls are addressed next, and finally, the posterior wall. Care is taken at the conclusion of the endometrial ablation not to invade the cervical canal, but to stop at the level of the internal os. The most difficult portion of the uterus to treat is the lower segment, because it is not easy to align the fiber perpendicular to its surface. This orientation is achieved by dragging the fiber around on this surface and destroying the endometrium on contact.
Three different techniques are used for endometrial ablation: blanching (nontouch), dragging (touch), and a combination of both. The dragging technique (touch), originally proposed by Goldrath and colleagues,44 consists of destroying the endometrium by producing furrows, or sulci, while the bare fiber touches and indents its tip in the endometrium, thereby destroying the area Fig. 39. This is performed in a systematic manner until all endometrium is destroyed. The visual effect is clear, and the destruction can be easily seen. The advantage of this technique is that the visual effect can immediately identify which areas have been destroyed and which ones have not. The disadvantages are that bleeding may occasionally occur at the completion of the procedure because the superficial vessels of the myometrium are destroyed; also, water overload can be a problem because the vessels are opened and the fluid under pressure may penetrate the open vessels.
|
In the blanching technique (nontouch), the fiber does not touch the tissue. Therefore, less debris is accumulated in the bare fiber, and there is practically no need to interchange fibers, which maintain a working capacity at all times. The fiber is maintained 1, 2, or 3 mm away from the surface, and blanching of the area can be clearly seen. The advantages of this technique are that it is much faster than the touch technique and not much debris is accumulated. Because there is no penetration of the surface, less bleeding is produced, and the chances of fluid overload decrease because the vessels are not disrupted and less fluid is intravasated. This technique can also be performed faster than the touch technique (see Fig. 39 ; Fig. 40). The disadvantages are that the uterine cavity must be divided into quadrants to avoid leaving areas untreated; the treated areas may be difficult to differentiate from untreated areas; and no end point of ablation is visible as compared with the touch technique.
|
Most physicians now use a combined technique. The fundal and cornual areas are treated with the nontouch technique, but the lower segment, which is difficult to treat with a nontouch technique, is treated by dragging the fiber in this area, thus completing the procedure. Some physicians perform the dragging technique in several areas of the uterus and complete the procedure with a nontouch technique in areas that have not been completely destroyed. This is the best approach to endometrial ablation with laser, using the combination of these two techniques.
More than 400 cases of Nd:YAG laser ablation have thus far been reported in the literature, and the cumulative results show 88% to 90% success with low failure rates and low complication rates.45,46 Nonetheless, with wider use of the technique, serious complications have occurred. These include uterine perforation with bowel injury, death after gas embolism due to improper use of a coaxial gas-cooled fiber tip, and near-death, probably caused by gas embolism.47 In expert hands, this procedure requires at least 40 to 60 minutes, with team cooperation to monitor the amount of fluid instilled and returned and to operate the laser machine.
Ablation of the Endometrium by Electrocoagulation
Borrowed from urologists, the resectoscope has been modified for use in the uterus, particularly for resection of submucous leiomyomas, endometrial polyps, uterine septa, and intrauterine adhesions. The modified resectoscope also has been used for resection of the endometrium with electrocoagulation, using the wire loop of the resectoscope, controlled by its handle mechanism.48 Unipolar current is used at a coagulation current setting of 40 to 50 W, or at 100 W of pure cutting (nonmodulated) current, and the uterus is distended with Cytal, glycine, sorbitol, or D5W, fluids devoid of electrolytes. It is important not to include electrolytes in these fluids to prevent conduction of electricity; therefore, because the amount of solution used may exceed 1000 mL, it is important to supply electrolytes intravenously to prevent possible water intoxication. Dextran 70 (32%) can also be used for this technique because it is nonconductive and provides excellent visualization and distention of the uterine cavity (Fig. 41).
The technique of ablation of the endometrium by coagulation-resection with a resectoscope is simple and can be completed in 15 to 30 minutes. Instrumentation and power units required are less expensive than those for laser ablation; nonetheless, the technique has some disadvantages. The electrical current used is unipolar, so the patient must be grounded to complete the circuit. No electrolytes can be used with a distending medium, and the possibility of fluid overload and water intoxication must be carefully monitored. There is a possibility of damage to adjacent structures, should coagulation penetrate the uterine wall, because the depth of the coagulated area is less predictable than the penetration provided by the laser.
To destroy the endometrium, the wire loop of the resectoscope can be replaced by the ball-end or roller-ball electrode, which is used to coagulate and destroy the endometrial surface by coagulation, rather than shaving and resecting.49,50 Urologists use a ball-end electrode to coagulate the areas of the prostate that bleed while prostatic resection is performed. Compared to the wire loop, a ball-end electrode has the advantage of expediting the procedure, permitting a more complete and uniform treatment of the total surface of the endometrium. It provides better control for the operator, and because no cutting of superficial myometrial vessels occurs, postoperative bleeding and water overload are lessened.
Ablation of the endometrium is a viable alternative in the treatment of patients with severe menorrhagia that does not respond to medical therapy, or patients who are not acceptable candidates for hysterectomy. The success of these procedures, whether performed with laser energy or the electrical coagulating current using a resectoscope, depends mainly on the selection of patients for appropriate indications, as well as specific training in the appropriate use of each technique. Both techniques are optimally performed by trained practitioners with proper background in diagnostic and therapeutic hysteroscopy. Because there has been no long-term follow-up of these patients, women requesting these procedures should be properly counseled and monitored, particularly if they are young and have borderline indications.
|
Tubal Cannulation
Fallopian tube obstruction is a significant factor in infertility in approximately 30% of infertile women. Proximal obstruction of the fallopian tubes is present in about 10% to 20% of women having hysterosalpingography as part of their infertility evaluation. For this reason, laparoscopy is necessary to rule out physiologic spasms and evaluate other fallopian tube or pelvic pathology. When proximal fallopian tube obstruction is confirmed by laparoscopy, the patient becomes a candidate for surgical reconstruction, particularly when the fallopian tubes are found to be normal externally on laparoscopic examination.
Clinical experience has demonstrated that women subjected to microsurgical treatment of proximal fallopian tube obstruction did not consistently show fibrosis, but did show simple obstruction of the tubal lumen by debris or other proteinaceous material plugging the tubal lumen. Zulak and colleagues51 described amorphous material present in the tubal lumen on histologic examination in 6 of 18 (33%) patients who were operated on for proximal fallopian tube occlusion. Three patients (17%) had tubes without occlusion and with normal anatomy. Finally, 7 of 18 patients (39%) had tubal fibrotic occlusion or salpingitis isthmica nodosa (SIN) on histopathologic evaluation. Therefore, of the patients who had surgery and underwent histopathologic evaluation of the tubal segments removed, more than 50% demonstrated pseudo-occlusion or obstruction produced by debris or thick mucous tissue.
Attempts at tubal cannulation date back over a century, but the major initial attempts at tubal cannulation began with tubal sterilization from the 1930s to the 1970s.52 Early in the 1960s Menken52 introduced the technique of tubal cannulation that was later improved by Quinones in the early 1970s.11,25 Nonetheless, because of the caliber of the catheters and the materials with which they were made, tubal cannulation was not easily accomplished in patients with tubal occlusion. With the introduction of much softer and thinner catheters, derived from experience with angiographic techniques using coaxial catheters, tubal cannulation was made easier, safer, and more reproducible.
Although tubal cannulation with the new coaxial catheters began with the use of fluoroscopy, hysteroscopy soon was found to have an advantage in facilitating the cannulation technique. In conjunction with laparoscopy, it permitted not only cannulation, but also evaluation of the pelvis and treatment of additional problems affecting the fallopian tube, ovaries, and particularly peritubal adhesions. It also helped in monitoring cannulation and direct assessment of tubal patency.
While osteal selective chromopertubation were performed early in the 1970s with the introduction of modern hysteroscopy, this technique was extended and adapted to fluoroscopy. Nonetheless, there are drawbacks with fluoroscopy. With this technique it is difficult to rule out tubal spasm, which is better assessed under general anesthesia and by laparoscopy. In the absence of laparoscopy, many patients undergo tubal cannulation only for a temporary physiologic spasm, which is why gynecologists have preferred a hysteroscopic approach using laparoscopic control. Some patients may benefit from fluoroscopic cannulation, particularly when laparoscopy is contraindicated or has been recently performed and normal findings obtained. Even in this latter case, however, if fluoroscopic cannulation fails, the patient should undergo a hysteroscopic cannulation attempt before a decision is made to perform laparotomy and microsurgical reconstruction or, in some patients, in vitro fertilization and embryo transfer attempt.
Tubal cannulation has variations, particularly in the use of straight coaxial catheters versus catheters with distal balloons to distend the tubal cornual regions. It seems clear from the published reports, however, that there is no difference in the final outcome, and the simplicity with which simple coaxial catheters cannulate the fallopian tubes makes them more appealing to the practitioner. The cumbersome steps in dilating the tubal intramural portion as performed in angiography may not be necessary, particularly because the fallopian tube, with its special compliance after being distended, recovers its normal anatomy quickly. Although the technique of balloon distention is most useful to flatten an atheroma and distend a vessel, in dealing with the intramural portion of the fallopian tubes in which the obstruction must be released, dilatation of this area is not necessary (Figs. 42 and 43).
|
The results of tubal cannulation are promising. The rate of successful visualization of the fallopian tubes with fluoroscopic cannulation has ranged from 76% to 92%. The pregnancy rate with a relatively short follow-up has ranged from 35% to 39%, with an ectopic pregnancy rate of 1% to t3%. Tubal cannulation by hysteroscopy has also demonstrated a 72% to 92% patency rate. The intrauterine pregnancy rate has been 42% to 27%, with an ectopic pregnancy rate of 8%.52–54
Although the number of patients reported in different series is relatively small, it is clear that tubal cannulation for fallopian tube obstruction is the first line of evaluation and treatment for patients presenting with this problem; only those who fail tubal cannulation should be subjected to microsurgical tubal reconstruction. Hysteroscopy affords direct observation of the uterotubal junctions and proximal openings of the fallopian tubes, making it an excellent platform for tubal cannulation.
Although most tubal cannulations can be performed with a rigid operative hysteroscope, flexible 4.9-mm-OD operative hysteroscopes offer an additional useful alternative to accomplish tubal cannulation, particularly when the tubal openings are angulated and difficult to localize with the rigid endoscopes, and when anatomic variations in the uterine configuration make localization of tubal openings difficult. The maneuverability of these endoscopes greatly facilitates tubal cannulation; the endoscope can be aligned in direct apposition with the proximal tubal ostium, simplifying the tubal cannulation procedure and reducing failures (Figs. 44 and 45).
|
Tubal Sterilization
With increased emphasis on simplified and effective methods of female sterilization that are safe, are acceptable to patients, and require no general anesthesia, hysteroscopy has presented a new and valuable dimension in sterilization for fertility control. Attempts to occlude the uterotubal junction by electrocoagulation date back almost a century. Many clinical trials were attempted in the early 1950s, especially in Japan. Even with specially designed instruments, however, it proved so difficult to ensure identification of the uterotubal junction that the success rate was poor. The procedure was abandoned.
With modern hysteroscopy offering direct visualization of the tubal orifices, interest in uterotubal occlusion has revived. It is possible to introduce an insulated electrode into the intramural portion of the tube, and to deliver a specific current that destroys the lining of the lumen and the surrounding muscular layer, resulting in complete occlusion of this area (Fig. 46). After the initial clinical trials were extended, however, serious complications occurred, particularly thermal injuries to the bowel and associated organs. These problems, and the high failure rate, made such electrocoagulation of the intramural portion of the tubes clinically unacceptable.55–58 Other methods were devised using the hysteroscopic approach, such as the use of plugs. However, although the hysteroscope was technically easy to insert, it was difficult to keep in place, and most important, these methods had unacceptable failure rates. Furthermore, problems of bleeding, cramping, and expulsion slowed the extension of clinical trials.58 The most promising method at present is the use of formed-in-place silicone rubber plugs, as described and clinically applied by Reed and Erb.59 As experience with clinical applications has been gained in several centers, drawbacks have been addressed and earlier problems corrected. Although the procedure itself is relatively easy, the preparation, mixing of substances, and additional instrumentation need to be simplified. Regarding sterilization reversibility, further studies are required to ensure this possibility, because the preservation of normal tubal epithelium is essential for proper function. A new method of hysteroscopic tubal sterilization has been introduced using new and safe microcoils deployed in the intramural portion of the fallopian tubes by hysteroscopy. The microcoils expand upon deployment and anchor acutely in the segment of the fallopian tubes. Furthermore, its Dacron mesh component inside the coils promotes ingrowth of tissue inside the device to achieve complete tubal occlusion. Clinical trials are underway now that preliminary studies have demonstrated good anchoring and complete occlusion of the fallopian tubes.60
|
Charting, Photography, and Cinematography
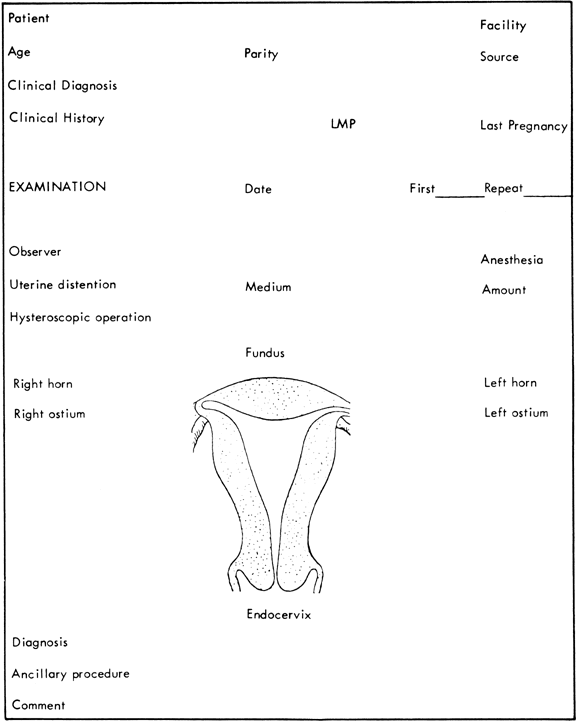
The proper report of findings at hysteroscopy, as in any type of endoscopy, is most important for further reference and review. Drawings, photographs, and motion pictures fulfill these goals. Systematic recording of findings can be accomplished with the form established by Siegler (Fig. 47) 61 Visual concepts can be reported most accurately by photography. Hysteroscopic photographs of normal and abnormal findings are valuable for further interpretation as well as for teaching purposes.
Adapters and lenses for photographic hysteroscopy are available, and cameras used for any endoscopic photography may be adapted to this technique. A high-intensity light source is needed. A flash attachment is desirable for still pictures. At present, most video systems and digital photography have practically replaced the cameras previously used for documentation. Most units comprise a complete video system for routine use in all endoscopic procedures. Moving pictures best reproduce the observer's view and findings and are especially helpful for teaching. A 1000-W light source may be used with high-speed Ektachrome film. The optimal combination of film and exposure time will vary according to the intensity of light used. Initially, several pictures should be taken at different exposures to establish optimal exposure.62