Until a few years ago, one of the first prerequisites for laparoscopy would have been general anesthesia, but office laparoscopy currently is performed with conscious sedation.14,15 Generally, these procedures are diagnostic, and minimal operative procedures are performed; for more complex procedures, general anesthesia still is advisable. As the saying goes, preparation is everything, and laparoscopy is no exception. Before beginning the procedure, the bladder is emptied and, if necessary, the stomach is suctioned to prevent injury at the time of trocar placement. The patient is placed in the lithotomy position. In adults or adolescents, a cervical cannula is placed for uterine manipulation during the procedure. Several types of cannulas are available that allow for uterine manipulation and chromopertubation. Disposable catheters such as the HUMI are quite flexible and small in caliber, which are useful for younger or nulliparous patients. Reusable manipulators such as the Pelosi and Valtchev are heavier articulating tools that come with several adaptable head pieces and provide excellent laparoscopic exposure.
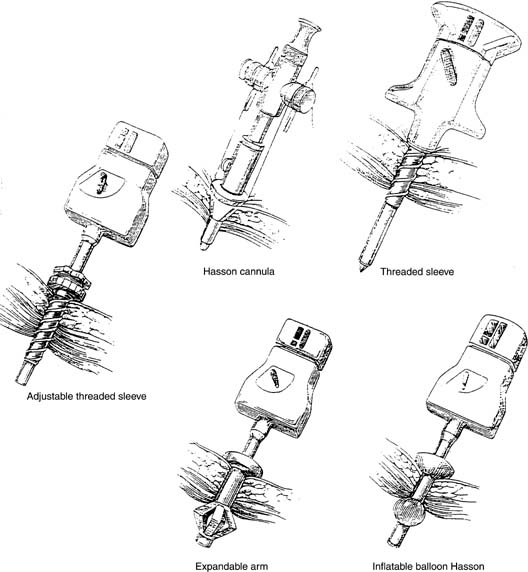
A small incision is made within the umbilicus and should be large enough to accommodate a 5- or 10- to 12-mm trocar. A Veress needle, directed at the inferior margin of the umbilicus (the aponeurosis), may be placed in the incision for insufflation. Obtaining a pneumoperitoneum with incorrect placement of the Veress needle has been associated with complications. Because most laparoscopic injuries occur on entry, aspirating with a 5-mL syringe, performing a water-drop test, and observing lower opening pressures (< 8 mmHg) are essential before insufflating with carbon dioxide gas. Several studies have shown that direct trocar insertion can be equally effective, obtaining a more rapid pneumoperitoneum.16,17 In a series of 1655 laparoscopic procedures, Hasaniya and colleagues18 report no complications from direct trocar insertion. In patients who have had previous abdominal surgery, in children, and in pregnant patients, an open technique may be preferred. Vascular and abnormally situated bowel injuries may be reduced with this technique because the fascia and peritoneum are incised under direct visualization;19 however, similar complication rates with both closed and open laparoscopy have been reported.20 Many surgeons use a Hasson cannula with a blunt trocar for open laparoscopy. With this technique, two sutures are placed through the fascia, lateral to the incision, and secured to a bar along the proximal shaft of the cannula to prevent loss of gas. Several new disposable cannulas are available with an inflatable balloon (Marlow, Willoughby, OH) or an expandable arm (Surgiport; US Surgical, Norwalk, CT), eliminating the need for stay sutures. Also, a cannula with a threaded sheath (Endopath, Somerville, NJ) is available that is literally screwed into the fascia to stabilize the sleeve (Fig. 1).21 The fascial defect must be reapproximated on completion of the procedure.
|
In 1999, the Food and Drug Administration (FDA) approved a new trocar system, InnerDyne. This system has a radially expanding access. It uses a 1.9-mm insufflation needle fitted into a radially expandable sleeve to penetrate the abdominal wall. The needle is withdrawn, leaving the expandable sleeve in place. The sleeve preserves the needle tract and becomes the access channel through which a blunt cannula or dilator is advanced, eliminating axial force during entry. The sleeve translates linear force to radial force.
Two other alternatives to open laparoscopy are the Visiport (US Surgical) and the Endopath Optiview (Ethicon), which permit visualization for cutting the subcuticular tissue and fascia (Fig. 2).
The Optiview allows entry into the abdominal cavity under direct vision. An incision can be made through the abdominal layers under direct vision. The Optiview allows identification of abdominal layers as they are dilated with the blunt conical tip. This method provides an easy way to avoid intestinal and vascular injury during initial trocar entry and permits access to the retroperitoneum for lymphadenectomy and incontinence procedures without entry into the peritoneal cavity.22
If the surgeon prefers to insufflate the abdomen before trocar placement, insufflation needles 3.6 mm in diameter are available that have a transparent conical tip. A small laparoscope then can be attached to this needle for visualization during insertion.23
Many different sizes of trocars are available. Most laparoscopists prefer to place a 10- to 12-mm trocar at the umbilicus. Left upper quadrant port placement may be used when peri-umbilical adhesions are suspected. Deflate the stomach with suction tubing and direct a 5-mm trocar just beneath the twelfth rib in the midaxillary line. Accessory ports may then be placed in the lower abdomen, typically one in the midline and two lower quadrant ports. Care must be taken to avoid vessels when placing ports in the lower abdomen. The inferior epigastric vessels are subfacial and cannot be seen during transillumination. First, identify the insertion of the round ligament into the inguinal canal; then, follow the obliterated umbilical artery cephalad along the anterior abdominal wall. The vessels can often be seen in a ridge lateral to this embryologic remnant. Direct the trocar perpendicular to the abdominal wall and avoid wandering on entry. Transillumination of the superficial branch of the epigastric arteries, after pneumoperitoneum has been achieved, is helpful. The mean distance between these two vessels has been reported to be as far as 1.4 cm.24
Cannulas are constructed of stainless steel, plastic, or fiberglass. Most metal cannulas are insulated; nevertheless, a mixture of metal and plastic or fiberglass can create a capacitance effect, leading to a burn injury.21
Trocars can have blunt, pyramidal, or conical tips. Pyramidal trocars require less force for insertion, but conical trocars may cause less tissue trauma during insertion.25 Several disposable trocars have spring-loaded tips that retract into a safety shield after the peritoneum is entered. If excessive force is used during insertion, the mechanism may not work properly; if the bowel is adherent to the fascia, injury will not be prevented. These should not be regarded as fool-proof safeguards.
Disposable instruments, in addition to their various designs, also have the advantage of always being sharp and readily available. Unfortunately, the cost to the hospital for disposable trocars and cannulas can run from $50 to $400. One study demonstrates that using disposable instruments only if reusable instruments were unavailable could markedly reduce the total cost of laparoscopy.26
If insufflation before trocar insertion is desired, several types of Veress needles are available, both reusable and disposable. A standard Veress needle is spring-loaded so that the dull inner stylet protrudes distal to the sharp outer needle on entering the peritoneal cavity. The Janicki needle has a vacuum sensor that can sense the negative pressure of the peritoneal cavity on placement.27 This sensor causes the light on the needle to change from red to green, signifying that the needle is in the peritoneal cavity.
For operative laparoscopy, carbon dioxide should be used for insufflation. The insufflator should be capable of pumping 3 to 4 L/min of gas to a maximum of 10 to 15 mmHg in adults, 8 to 10 mmHg in children, and 6 to 8 mmHg in infants. High-flow insufflators pump carbon dioxide gas up to a rate of 40 L/min. These maintain adequate visualization in the setting of small leaks encountered throughout the case. After insertion of the Veress needle, a syringe with irrigation solution should be attached to the needle for aspiration of any blood or fecal material. This information should be appropriately documented at the time of surgery. At 15 mmHg, 94% of the abdominal volume is obtained in adults, and there is no change in the pressure required for trocar insertion up to 30 mmHg. Therefore, insufflation beyond 15 mmHg may be dangerous.28 Nevertheless, some gynecologists prefer higher intraperitoneal pressures of up to 25 mmHg for entry, followed by a return to 15 mmHg for the remainder of the procedure. An abdominal wall lifter can be used rather than insufflation for abdominal wall elevation, but visualization generally is poor with this instrument.
Should extraperitoneal insufflation occur, Kabukoba and Skillern29 describe a technique in which the laparoscope is advanced until it is 4 cm above the symphysis in the subcuticular tissue. The Veress needle then is introduced through a small incision just superior to the symphysis and directed toward the pouch of Douglas. The tip of the needle can be observed entering the fascia by the laparoscope. The trocar valve then is opened to release gas from the subcuticular tissue as the intra-abdominal pressure increases with insufflation.
The laparoscope contains fiberoptic bundles for image and light transmission. Laparoscopes ranging up to 11 mm are available. Although much work is being performed to improve the optics of smaller laparoscopes, most surgeons prefer 10-mm laparoscopes for operative procedures.
The camera equipment is composed of a charge-coupled device camera (CCD) and an output monitor. The CCD converts optical images into electrical signals. The information is transferred to the camera control unit, which transforms the electrical signals into optical images on the video monitor. Picture resolution is important; this term refers to the number of horizontal lines on the video monitor. These horizontal lines are composed of pixels, and the greater the number of pixels, the better the resolution.30
One of the disadvantages of laparoscopy is that video images are two-dimensional, and depth perception is difficult to judge. Undoubtedly, the use of three-dimensional laparoscopy will become widespread. A recent study demonstrates that surgeons were able to perform complex tasks more quickly and efficiently with three-dimensional compared with two-dimensional laparoscopy.31,32 Also, the use of digital rather than electrical signals from the CCD will greatly improve images transmitted to the output monitor.
Complications related to port closure have been reported with an incidence of .17% to 6.3%.33,34,35 Incisional hernias occur infrequently; however, because of the increased incidence encountered with larger ports, we recommend closure of those beyond 5 mm. Although the purpose is to secure facial defects, Richter's hernias may occur when large or small bowel is herniated through the peritoneum. These are especially devastating because they are more subtle, without a palpable mass, and may occur beyond the expected time frame for a facial herniation (approximately 10 days).
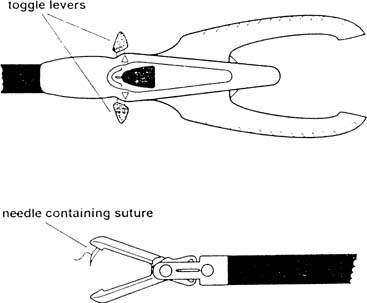
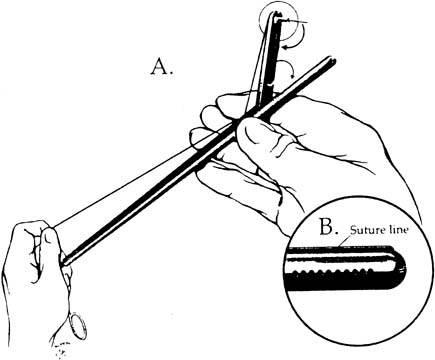
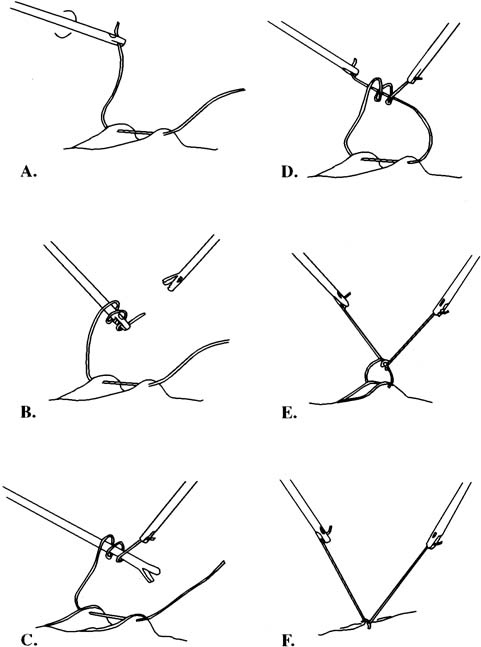
Approximating the fascia and peritoneum together is facilitated with closure devices such as the Carter-Thomason, which passes a free ligature through these layers under direct laparoscopic guidance. The diagnosis is primarily clinical and the presentation may be similar to that of an ileus, bowel obstruction, or wound hematoma. Computed tomography may also be useful in confirming the diagnosis. The small intestine, because of its caliber and mobility, herniates more frequently than the large bowel. Treatment is via laparoscopy or laparotomy and resection is seldom required.