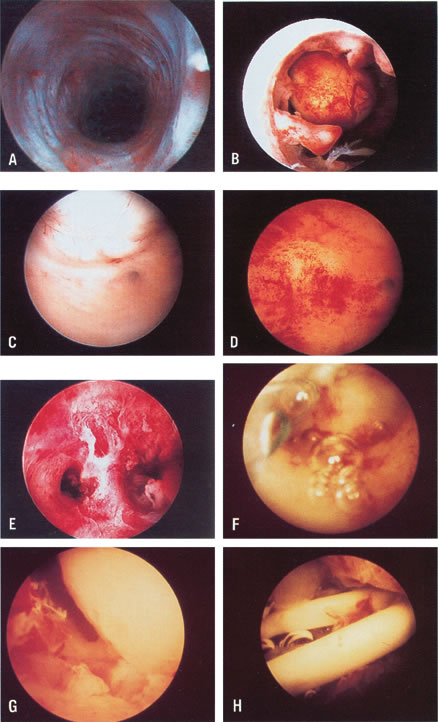
The hysteroscope offers not only the opportunity to visualize the intrauterine
structures and provide diagnosis, but also therapeutic capabilities
for conditions that in the past required a hysterotomy and, obviously, a
laparotomy. Instruments can be guided visually and targeted to
areas in the uterine cavity in need of treatment for uterine septa, intrauterine
adhesions, polyps and submucous myomas, and tubal cornual
occlusions.17,18 Treatment of Uterine Septa Uterine anomalies, specifically the uterine septum, can interfere with
reproduction in about 25% of patients afflicted with this condition.19 Traditionally, the symptomatic uterine septum producing pregnancy wastage
has been treated by Jones or Tompkins abdominal metroplasty.19–21 The ability to manipulate instruments inside the uterine cavity has provided
the opportunity to treat the uterine septum transcervically. This
method was tried blindly for many years, following Ruge's attempt
at transcervical treatment.22 Although abdominal metroplasty gave excellent results in reproduction, it
required a laparotomy and a hysterotomy with their inherent morbidity, particularly
related to pelvic adhesions. Patients were hospitalized, experienced
the usual postoperative convalescence, and waited 3 to 6 months
to attempt conception. These patients were subjected to routine
cesarean section when they achieved pregnancy and viability. Such
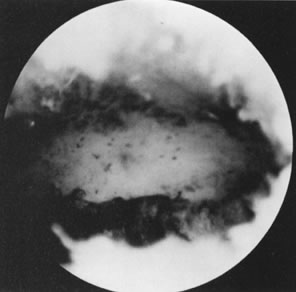
drawbacks were overcome by the ability to transect the septum hysteroscopically
on a day-surgery basis without invading the uterine walls and
the abdominal cavity (see Fig. 20; see Color Plate 1).23–25 There are several methods to treat the uterine septum hysteroscopically. In
the most widely employed method, mechanical semi-rigid scissors are
used to divide the septum systematically until reaching the junction
between the fibrotic septum and the myometrium. This is done by following
the symmetry of both uterine tubal openings, and observing by hysteroscopy
the rich myometrial vascularization of the area, and by laparoscopy
the uniform translucency of the hysteroscopic light. Patients
are given prophylactic antibiotics, using cephalosporins with 1 g of
cefazolin sodium (Kefzol) intravenously during the procedure followed
by cephalexin (Keflex) 500 mg by mouth four times daily for 3 to 4 additional
days. Estrogens are given in the form of conjugated estrogens (Premarin) 2.5 mg
twice daily for 30 days, and progesterone in the form
of medroxyprogesterone acetate (Provera) 10 mg in the last 10 days of
this artificial cycle to allow withdrawal bleeding. At the completion
of hormonal treatment, a hysterosalpingogram is performed to assess
the results of the hysteroscopic treatment; if satisfactory, the patient
is allowed to conceive. The results obtained have surpassed the previous metroplastic operations
used in the treatment of the anomaly.26,27 As an alternative to mechanical methods, fiberoptic lasers (neodymium:yttrium-aluminum-garnet [Nd:YAG], argon, and KTP-532 laser) with
sculptured or extruded fibers can be used to divide the uterine septum.24 Care should be taken not to destroy the peripheral endometrium and to
avoid uterine perforation by observing carefully the hysteroscopic light
through the laparoscope. Bleeding may not be apparent once the myometrium
is reached because of the coagulating power of the lasers.28 Alternatively, the resectoscope may be used with a modified loop electrode
placed forward to cut the septum, or a narrow knife electrode can
divide the septum by pushing forward. Nonetheless, care must be taken
to avoid perforation. The coagulating power of the electricity may interfere
with the observation of bleeding, which usually occurs at the
myometrium. Therefore, the laparoscopic assistant using dimmed laparoscopic
light should observe for uniform illumination provided by the hysteroscopic
light (Table 4).24–27 Table 4. Hysteroscopic Metroplasty
| | | | | | | Pregnancy |
Author | No. Patients | Medium | Technique | IUD* | E/P† | Antibiotics | Term | Premature | Abortion | In Progress |
Edstrom (1974) | 2 | Dextran 70, 32% | Rigid biopsy foreceps | + | + | — | — | 19 wks | — | — |
Chervenak and | 2 | Dextran 70, 32% | Scissors adjacent | + | — | + | 1 | — | — | — |
Neuwirth (1981) | | | to hysteroscope | | | | | | | |
Rosenberg et al | 1 | Dextran 70, 32% | Flexible scissors | NA‡ | NA‡ | NA‡ | NA‡ | — | — | — |
(1981) | | | | | | | | | | |
Daly et al (1983) | 25 | Dextran 70, 32% | Flexible scissors | — | + | — | 7 | — | 1 | — |
Perino et al (1985) | 11 | CO2 | Flexible semi-rigid | + | — | — | NA‡ | — | — | — |
| | | scissors | | | | | | | |
DeCherney et al (1986) | 72 | Dextran 70, 32% | Resectoscope | — | — | — | 58 | — | 4 | 4 |
Corson and Batzer | 18 | Dextran 70, 32%, | Resectoscope and | — | — | — | 10 | 1 | 2 | 2 |
(1986) | | CO2 | rigid scissors | | | | | | | |
Fayez (1986) | 19 | Dextran 70, 32% | Rigid scissors | Foley | — | + | 14 | — | — | — |
| | | | catheter | | | | | | |
March and Isreal | 91 | Dextran 70, 32% | Flexible scissors | + | + | — | 44 | 4 | 7 | 7 |
(1987) | | | | | | | | | | |
Valle (1987) | 59 | D5W/Dextran 70, 32% | Flexible, semi-rigid | — | + | + | 44 | 2 | 5 | — |
Choe and Baggish | 19 | Dextran 70, 32% | Nd:YAG with bare or | Foley | + | + | 10 | 1 | 1 | 3 |
(1992) | | | sculptured fibers | catheter | | | | | | |
Fedele et al (1993) | 102 | Dextran 40, 10% in | Semi-rigid scissors (80) | +(21) | +(39) | + | 45 | 10 | 11 | NA‡ |
| | normal saline | Argon laser (10) | | | | | | | |
| | | Resectoscope (12) | | | | | | | |
Valle (1996) | 124 | D5 in 1/2 normal | Semi-rigid scissors (98) | — | + | + | 84 | 7 | 12 | — |
| | saline Glycine 1.5% | Resectoscope (20) | | | | | | | |
| | | Nd:YAG laser (6) | | | | | | | |
Totals | 545 | | | | | | 317(78.3%) | 26(6.4%) | 43(10.6%) | 18(4.4%) |
*IUD, Intrauterine device
†E/P, Estrogen/progestrone
‡N/A, Nonapplicable
Modified from Siegler AM, Valle RF: Therapeutic hysterocopy procedures. Fertil
Steril 50:685, 1988.
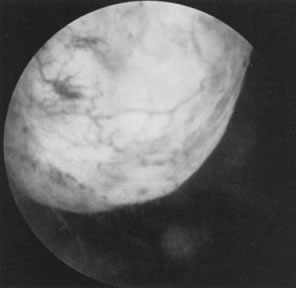
Treatment of Intrauterine Adhesions Intrauterine adhesions resulting from trauma to the postpartum or postabortal
endometrium are known as Asherman's syndrome. This condition
usually results in menstrual abnormalities, particularly amenorrhea, hypomenorrhea, or
both and may also result in infertility and pregnancy
wastage.29 The best screening method to rule out intrauterine adhesions is hysterosalpingography. Hysteroscopy will confirm the presence of adhesions, evaluate
the type of adhesions, and serve to divide these adhesions under
visual control (see Color Plate 1E). A 7- to 8-mm OD operative hysteroscope is used, with semi-rigid scissors
and biopsy forceps to systematically divide the adhesions. Concomitant
laparoscopy is used when extensive adhesions are present and when
tubal occlusion is demonstrated on the hysterosalpingogram, as a guide
to the hysteroscopic surgery. Perioperative and postoperative antibiotics are usually prescribed in the
form of cephalosporins with 1 g IV during the procedure, followed by
cephalexin 500 mg orally four times daily for 5 to 6 days, particularly
in women requiring an intrauterine splint after the surgery. Adjunctive
hormonal treatment to stimulate re-epithelialization is used with
conjugated estrogens, Premarin 2.5 mg orally twice daily for a 30- to 40-day
cycle, with additional terminal progesterone as medroxyprogesterone
acetate, 10 mg once a day in the last 6 to 10 days of this artificial
cycle. At the conclusion of the hormonal therapy, a hysterosalpingogram
is performed to evaluate the results of this therapy. To determine the prognosis of the treatment, a three-stage classification
of the adhesions (mild, moderate, and severe) is used based on the
extent of adhesions seen on the hysterosalpingogram and the type of adhesions
involved as determined by hysteroscopy. Mild adhesions are usually filmy adhesions composed of basalis endometrium; they partially
or totally occlude the uterine cavity. Moderate adhesions are composed of fibromuscular tissue covered by endometrium; they usually
partially or totally occlude the uterine cavity, and bleed on division. Severe adhesions are composed of connective tissue. They usually do not have endometrial
lining and may partially or totally occlude the uterine cavity.30 The restoration of normal menstruation is achieved in about 90% of the
patients treated. The reproductive outcome parallels the extent and type
of adhesions. The milder the adhesions, the better the prognosis. Older
adhesions that more extensively occlude the uterine cavity have
a poorer prognosis. Valle and Sciarra30 reported on 187 patients evaluated and treated by hysteroscopy with restoration
of normal menstruation in 88.2%. The reproductive outcome correlated
with the type of adhesions and extent of uterine occlusion, and
ranged from a term pregnancy rate of 81.3% in patients with mild disease
to 31.9% in patients with severe disease. Although early diagnosis and treatment of intrauterine adhesions seem to
improve prognosis, the therapeutic outcome after hysteroscopic division
of adhesions has surpassed any blind treatment, adding precision in
the re-establishment of normal symmetry to the uterine cavity, and avoiding
unnecessary trauma to the rest of the endometrium (Table 5). Table 5. Hysteroscopic Lysis of Intrauterine Adhesions
| | | | Reproductive Outcome |
Author | No. Patients | Medium | Technique | Menses NL No. (%) | Pregnancy No. (%) | Term No. (%) |
Levine and | 10 | Hyskon | Flexible scissors | 5(50) | 2(20) | — |
Neuwirth (1973) | | | | | | |
Edstrom (1974) | 9 | Hyskon | Biopsy forceps | 2(22) | 1(11) | 1(11) |
Siegler and | 25 | CO2 | Target abrasion/ | 13(52) | 11(44) | 12(44.4) |
Kontopoulos (1981) | | | scissors/curettage | | | |
March and Israel | 38 | Hyskon | Flexible scissors | 38(100) | 38(100) | 34(79.1) |
(1985) | | | | | | |
Neuwirth et al (1982) | 27 | Hyskon | Scissors alongside | 20(74) | 14(51.8) | 13(48.1) |
Sanfilippo | 26 | CO2 | Curettage | 26(100) | 6 (100) | 3 (50) |
et al (1982) | | | | | | |
Hamou et al (1983) | 69 | CO2 | Target abrasion | 59 (85.5) | 20 (51.3) | 15 (38.4) |
Sugimoto et al (1984) | 258 | Hyskon/normal | Target abrasion/Kelly | 180 (69.7) | 143 (76.4) | 114(79.7) |
| | saline | forceps | | | |
Wamsteker (1984) | 36 | Hyskon | Scissors/biopsy Forceps | 34 (94.4) | 17 (62.9) | 12 (44.4) |
Friedman et al (1986) | 30 | Hyskon | Resectoscope/scissors | 27 (90) | 24 (80) | 23 (76.6) |
Zuanchong and Yulian | 70 | Normal Saline | Biopsy forceps/flexible | 64 (84.3) | 30 (85.7) | 17 (48.5) |
(1986) | | | scissors | | | |
Valle and Sciarra (1988) | 187 | D5 W/Hyskon | Flexible/semirigid/rigid | 167 (89.3) | 143 (76.4) | 113 (79.7) |
| | | scissors | | | |
Lancet and Kessler (1988) | 98 | Hyskon | Flexible scissors/electrosurgery | 98 (100) | 86 (87.8) | 77 (89.5) |
Pabuccu et al (1999) | 40 | Glycine | Murphy probe scissors | 33 (82.5) | 27 (67.5) | 23 (57.5) |
Feng et al (1999) | 365 | Dextrose 5% | Biopsy forceps/scissors | 294 (83.7) | 156 (83.8)† | 145 (92.9) |
Totals | 1298 | | | 1060 (87.5) | 718 (72.3) | 603 (87.2) |
*NI, Normal
† Of 186 desiring pregnancy
Modified from Siegler AM, Valle RF, Lindemann HJ et al: Therapeutic Hysteroscopy: Indications
and Techniques, Ch 6, p 103. St Louis, CV Mosby, 1990
Polyps and Submucous Leiomyomas Although benign tumors of the endometrium or the muscle seldom cause infertility, those
located in the uterine cavity may interfere with nidation
or appropriate growth and development of an established pregnancy. Polyps
may be removed accidentally at curettage or exploration with
forceps. To establish a definite diagnosis and location of a polyp, hysteroscopy
is mandatory. Assurance that the polyp has been removed completely
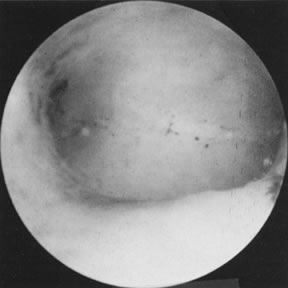
can only be offered by the hysteroscope, however (Fig. 19). Most submucous leiomyomas are symptomatic, generally causing excessive
uterine bleeding. Their removal is best accomplished by hysteroscopy, with
transection of the pedicle in those that are pedunculated allowing
subsequent removal of the myoma, or systematic shaving of sessile leiomyomas
that are partially intramural, using the resectoscope and a cutting
loop. When significant distortion of the uterine cavity is caused
by these leiomyomas on hysterosalpingography, a postoperative evaluation 2 to 3 months
after resection is useful by repeating the hysterosalpingogram
and assessing the re-establishment of uterine cavity symmetry (Fig. 20).31–34 Helpful adjuncts in the preoperative evaluation of patients with submucous
leiomyomas are vaginal sonography and the use of gonadotropin-releasing
hormone (GnRH) analogues. Vaginal sonography can help detect intramural
leiomyomas left behind, and assess the penetration of the submucous
leiomyoma in the uterine wall. The use of GnRH analogues is helpful
in controlling the excessive bleeding and preparing the uterine cavity
by atrophying the endometrium for best visualization during surgery. Additionally, the
leiomyoma may become less vascular and may also
decrease in size, making the hysteroscopic surgery easier (Table 6).35 Table 6. Hysteroscopic Myomectomy for Abnormal Bleeding
| | Type of Myomas | | | | | | |
Author | No. Patients | Pedunculated | Sessile | Method | IUDa | E/Pb | Antibiotics | Cure (%) | Recurrent (%) |
Haning et al (1980) | 1 | — | + | Resectoscope | — | + | + | 1 | — |
DeCherney and | 8 | + | + | Resectoscope | Foley | + | + | 8 | — |
Polan (1983) | | | | | | | | | |
Neuwirth (1983) | 28 | + | + | Resectoscope | Foley | + | + | 17 (60.7) | 8 (28.5) |
Lin et al (1986) | 13 | + | — | Resectoscope (9) | Foley | + | + | 9 (69.2) | 4 (30.7) |
| | | | Rigid scissors(4) | | | | | |
Hallez and Perino | 300 | + | + | Resectoscope | + | + | + | 299† | — |
(1988) | | | | | | | | | |
Baggish (1989) | 23 | + | + | Nd:YAG laser | Foley | — | + | NR* | NR* |
| | | | | (5 patients) | | | | |
Valle (1990) | 52 | + | — | Semi-rigid scissors | — | — | — | 52(100.0) | NR* |
Donnez, et al (1990) | 60 | 48 | 12 | Nd:YAG laser | — | — | — | 48(80.0)§ | 12 (20.0) |
Loffer (1990) | 53 (10 were | 18 | 25 (2 had 2 | Resectoscope | NR* | — | — | 40 (93.0) | 3(6.9) |
| polyps) | | procedures) | | | | | | |
Corson and Brooks | 92 | 92 | — | Resectoscope | NR* | — | + | 65 (81.2)‡ | 15 (18.7)‡ |
(1991) | | | | | | | | | |
Derman et al (1991) | 94 | 94 | — | Resectoscope | Rubber | + | + | 69 (75.0) | 23 (24.5) |
| | (2 intraoperative laparotomies) | | | balloon | | | | |
Wamsteker et al (1993) | 51 | 25 | 26 (several patients had | Resectoscope | — | — | + | 48 (94.1) | 3 (5.9) |
| | | 2–3 procedures) | | | | | | |
Emanuel et al (1999) | 285 | 73 | 266 | Resectoscope | — | — | + | 225 (78.8)§ | 41 (14.4) |
Totals | 1040 | | | | | | | 881 (81.2) | 109 (10.5) |
*IUD, intrauterine device; E/P, estrogen/progesterone; NR, not reported
†1 patient required laparotomy
† From 80 patients
§17 lost to follow-up
Modified from Siegler AM, Valle RF: Therapeutic hysterocopy procedures. Fertil
Steril, 50:68, 1988.
Tubal Cornual Occlusion About 30% of infertile women have fallopian tube obstructions causing infertility. Although 10% to 20% of women demonstrate cornual tubal occlusion
in their evaluation by hysterosalpingography, many of these occlusions
are due to physiologic tubal spasms and must be evaluated by laparoscopy
under general anesthesia.13,14,36–38 In a significant number of patients with cornual tubal occlusion demonstrating
occlusion at laparoscopy, no fibrosis is found on microsurgical
tubal reconstruction, only obstruction by debris or proteinaceous material
plugging the tubal lumen. In fact, Sulak and coworkers39 described an amorphous material present in the tubal lumen at histopathology
in 6 of 18 (33%) patients operated for proximal fallopian tubal
occlusion. Three patients (17%) had tubes with normal anatomy and no
occlusion. Seven of eighteen patients (39%) had tubal occlusion by fibrosis
or salpingitis isthmica nodosa on histopathological evaluation: Over 50% of
patients who had surgery and underwent histopathological evaluations
demonstrated pseudo-occlusions or obstructions produced by
debris or thick mucous tissue. For these reasons, tubal cannulation, begun
in the late 1960s and early 1970s, was revived in the early 1980s.40–44 Better, softer, and thinner catheters were introduced by manufacturers
based on experience with angiographic techniques using coaxial catheters, and
tubal cannulation was made easier, safer, and more reproducible.{39} The important elements in this procedure are a 3-French soft catheter
with a soft wire-guide of less than 0.5 mm diameter. A 5-French co-axial
catheter can be used to guide these two elements. The procedure should
be performed in the early follicular phase when the endometrium is
thin and no significant debris is present in the uterine cavity. Concomitant
laparoscopy is used to assess tubal patency and to aid in the
cannulation procedure. The wire guide is passed into the tubal lumen
and the 3-French catheter then is guided over the wire guide, bypassing
the intramural portion; the wire guide is removed and indigo carmine
is injected directly through the 3-French catheter. An assistant using
the laparoscope assesses tubal patency. Patients who fail tubal cannulation
are candidates for microsurgical tubal reconstructions on the
assumption that the occlusion is fiberoptic. Patency after hysteroscopic
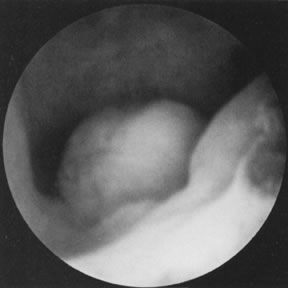
tubal cannulation is 72% to 92% and intrauterine pregnancy approaches 50%.43 Most tubal cannulations can be performed using the rigid hysteroscope. The
new flexible operating hysteroscopes, which are steerable and can
be directed toward the uterotubal cones, are particularly helpful in
small uteri with deeply recessed uterotubal cones (Fig. 21; see Color Plate 1C through G) (Table 7). 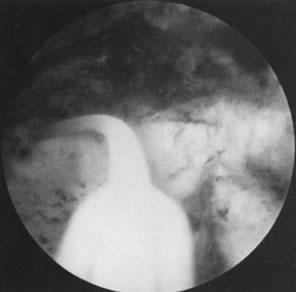 Fig. 21. Hysteroscopic tubal cannulation of right fallopian tube. Fig. 21. Hysteroscopic tubal cannulation of right fallopian tube.
|
Table 7. Hysteroscopic Cannulation for Proximal Tubal Obstruction
| | | | Complications | |
Author | No. Patients/Failed | No. Tubes/Failed | Catheter | (Perforation) | Pregnancies |
Confino et al (1986) | 1/0 | 1/0 | Balloon | 1 | — |
Daniell and Miller (1987) | 1/0 | 1/0 | Urological | 1 | 1 |
Sulak et al (1987) | 2/0 | 4/0 | Epidural | 0 | 1 |
Huang Yu-Lian et al (1988) | 78*/0 | — | Epidural | 0 | 46 |
Confino et al (1988) | 12/5 | 23/14 | Balloon | 3 | 2 |
Novy et al (1989) | 10/1 | 12†/1 | Cornual coaxial set | 1 | 2 |
Deaton et al (1990) | 11/4 | 18/5 | Urological | 2 | 6 (3 ectopics) |
Lin et al (1990) | 10/0 | 18/2 | Urological | — | 5(1 ectopic) |
Flood and Grow (1993) | 27/3 | 46/10 | Cornual coaxial set | 4 | 15 |
Valle (1994) | 63/11 | 120/19 | Cornual coaxial set | 2 | 27 (1 ectopic) |
Total | 215/24 (11/1%) | 244/61 (25.0%) | | 14 (6.5%) | 105 (49.7%) |
*15 patients refused additional procedures.
† Some performed under fluoroscopy
Vaucallie T, Schmidt EH: The uterotubal junction. A proposal for classifying
its morphology as assessed with hysteroscopy. J Reprod Med 33:624, 1988
Impacted and Misplaced Intrauterine Foreign Bodies An intrauterine foreign body such as a forgotten intrauterine device (IUD) will
interfere with fertility. This type of foreign body usually is
detected by sonography, but fragments of a broken IUD may remain unnoticed. Additionally, bony
fragments from previous late first-trimester
or second-trimester abortions can remain in the uterine cavity or form
osseous metaplasia acting as osteoblastic grafts. These formations
can impair fertility by acting as a foreign body. Because blind removal
of this type of foreign body is difficult, hysteroscopy is used to aid
in proper complete removal. Patients will assume fertility following
these procedures. It is important, nonetheless, to add sonography in
the evaluation of these patients, should fragmented IUDs or bony fragments
have penetrated the myometrium (see Color Plate 1J and K).45,46 Hysteroscopy and New Reproductive Technologies Hysteroscopy can be used as a platform to invade the fallopian tubes; it
can be used for intratubal insemination and for delivery of gametes
and zygotes, procedures that with refinements in technology may become
simpler, less invasive alternatives to laparoscopy.47 The endometrial changes occurring throughout the menstrual cycle can be
explored visually, with added magnification, and may eventually help
to determine appropriate endometrial maturation and receptivity for embryo
transfers.48 Similarly, the cumulative pregnancy rates in patients who had normal hysteroscopic
examinations before embryo transfers seem to be significantly
better than those in women in whom some abnormalities are detected. This
suggests the need for better assessment of the uterine cavity
before embryo transfers are performed.47,48 Hysteroscopy and Ectopic Gestations Evaluation of the patient with a possible ectopic pregnancy has been greatly
facilitated by the use of quantitative human chorionic gonadotropin (hCG) titration
and abdominal and vaginal ultrasound. Interest has
arisen in using less invasive methods to treat ectopic pregnancies, particularly
with methotrexate. This method requires systematic administration
of the drug, and produces frequent side effects. Alternative methods
of delivering the methotrexate locally used laparoscopic guidance, sonography, and
blind tubal cannulations. The hysteroscope remains
another alternative, particularly for pregnancies located at the proximal
tubal openings and for confirming the pregnancy location determined
by ultrasound. This approach may be expanded to include selected patients
with early ectopic pregnancies, and women desirous of further fertility
who have β-hCG levels of less than 1400 mLU/mL.49 Hysteroscopy can be useful in patients who have flattened or not decreasing
levels of β-hCG; in patients in whom ultrasound does not provide information of the
location of the pregnancy to rule out early threatening or incomplete
abortions; and in determining the possibility of an ectopic gestation
when the uterine cavity is completely normal.50 |