Absolute fetopelvic disproportion is uncommon, but the performance of cesarean
delivery for that indication is common. It is assumed that the
physician factor is significant in the diagnosis of dystocia. Physicians
may be influenced by the patient’s attitude, the time of day, anesthesia
support, the medicolegal climate, and their own training
and experience. The patient’s level of anxiety and pain tolerance
also may influence the character and duration of labor. Medications given during labor may alter uterine contractility. β-Mimetics, calcium
channel blockers, magnesium sulfate, and antiprostaglandins
have been used to inhibit labor. Ethanol has a direct depressant
effect on smooth muscle and inhibits oxytocin release. Theophylline
and caffeine may lead to longer labors. The effects of barbiturates
are dose related. Pentobarbital and thiopental in anesthetic doses may
stop labor, whereas phenobarbital has little effect. Atropine and scopolamine
relax the lower uterine segment and decrease the frequency of
contractions. Tranquilizers have variable effects; usually, large doses
delay labor. Cocaine has been “marketed” as a drug that
reduces the duration of labor. Although there is accumulating evidence
that cocaine abuse is associated with an increased incidence of prematurity, it
does not significantly shorten the total duration of labor.12 Excessive use of analgesics can prolong the latent phase of labor; however, this
is not predictive of subsequent dystocia. Epidural anesthesia
may cause a transient decrease in contractility for 10 to 30 minutes. This
effect is much more pronounced if anesthesia is given in the latent
phase or with fetopelvic disproportion or malposition. The action
of voluntary muscles in correction of malposition may be impaired. Because
epidural anesthesia is associated with a prolongation of the second
stage of labor, intervention is not recommended unless the duration
of the second stage exceeds 3 hours with a regional anesthetic in a
nulliparous or 2 hours in a parous parturient.13 An increase in malpresentations and instrumental deliveries has been reported
with epidural anesthesia. In 1989, Thorp and colleagues14 reported a significantly increased frequency of oxytocin augmentation
and incidence of cesarean section for dystocia among patients receiving
epidural anesthesia compared with controls. The increased rate of cesarean
delivery for dystocia was not explained by other potentially confounding
variables, including gestational age, cervical dilation, use
of oxytocin, and birth weight. Other randomized trials investigating
early and late administration of epidural anesthesia have not shown an
increase in the rate of cesarean delivery.15,16,17 The latent phase of labor is particularly sensitive to narcotics. Although
active labor is considerably less sensitive than in the latent phase, heavy
sedation may slow dilation and increase the chance of protraction
and arrest of dilation. Inhalation agents also have a wide range
of effects that are dose dependent. Nitrous oxide has no effect on contractions, but
halothane and other halogenated agents are strongly inhibitory. Intravenous
nitroglycerin (in increments of 50 μg) results
in rapid and profound uterine relaxation. Halothane and nitroglycerin
can be used to relax the uterus to facilitate internal podalic version
of a second twin, delivery of entrapped breech head, or manual
removal of the placenta. The use of these agents can be associated with
postpartum hemorrhage, however. There is a close relationship between the cervical score and onset of spontaneous
labor. The state of the cervix may reflect myometrial sensitivity
to oxytocin. It is possible that the promotion of cervical ripening
may increase myometrial sensitivity and soften the cervix. Many agents
have been investigated and found to induce cervical ripening with
varying success, including estradiol and relaxin gels, Foley catheters, Laminaria tents, and prostaglandin E2 and F2α by various routes. Other newer induction agents, such as misoprostol, a
prostaglandin E1 analogue, may cause cervical ripening and labor induction and stimulation. Oxytocin Oxytocin is an octapeptide that is synthesized in the hypothalamus and
transported to axonal terminals in the posterior pituitary gland. Wide
variability exists regarding reported maternal plasma concentrations
of oxytocin during pregnancy and spontaneous labor. In addition to a tonic
baseline release, oxytocin is released in pulses occurring at 3- to 5-minute
intervals during normal labor, and fetal transfer of oxytocin
to the maternal side may be an important physiologic source.18 Most investigators report a gradual rise in plasma oxytocin levels throughout
gestation with a peak during the second stage of labor. Human
myometrial oxytocin receptors, which peak in early spontaneous labor, seem
to contribute to the initiation of uterine contractions. Receptor
concentration is probably the major determinant controlling uterine response
to either endogenous or exogenous oxytocin. Oxytocin is a potent uterotonic agent that can be safe and efficacious
when used appropriately. Friedman19 advocated the use of oxytocin with a secondary arrest of labor or failure
of descent when there is no suspicion of cephalopelvic disproportion. Many
physicians also use oxytocin for prolonged latent phase or protracted
active phase disorders. There is some evidence that preventing
prolonged labor by the use of oxytocin results in a better fetal and
maternal outcome. This improved outcome seems to apply even if cephalopelvic
disproportion is clinically suspected because the diagnosis may
be arrived at sooner. In general, when dysfunctional labor is present
and vaginal delivery is deemed possible by the enhancement of uterine
contractions, augmentation of labor may be indicated.20 In vivo studies determined that the interval to reach a steady-state concentration
of oxytocin in plasma (and maximal response) is 40 to 60 minutes
after initiating or altering the infusion. The in vivo half-life of oxytocin is approximately 10 to 15 minutes. Studies of plasma
oxytocin levels during continuous intravenous infusion show first-order
saturation kinetics, with a progressive, linear, stepwise increase
with each increase in the infusion rate. Pharmacologic data suggest
that the infusion rate of oxytocin should start low (0.5 to 2 mU/min) with
an increase arithmetically by 1 to 2 mU/min every 40 to 60 minutes. Because
of varied response ranges in individuals secondary to differences
in clearance rates and sensitivities, however, this rate of increase
is inefficient in a significant portion of patients. Clinically, this
variation resulted in an unacceptable delay in reaching an effective
maintenance dose of oxytocin in 31% of patients who required
greater than 4 mU/min. Seitchik and Castillo21,22,23 proposed, as a compromise, an increase in the infusion rate every 30 minutes
with cautious observation for hyperstimulation. This proposal was
tested in term nulliparas and multiparas with dysfunctional labor. When
the oxytocin infusion rate was increased at less than 30-minute intervals, there
was a twofold increase in the frequency of discontinuing
or decreasing the oxytocin infusion because of hyperstimulation or
fetal distress. A short (less than 30 minutes) interval of rate increase
also was shown to be the most important factor resulting in a higher
maximum dose of oxytocin and an actual delay in delivery by an average
of 3 hours. The preterm uterus is usually less sensitive to oxytocin than the term
uterus and may require larger doses. Theobald and coworkers24 reported that a 2 mU/min infusion of oxytocin at 32 weeks’ gestation
induced small contractions, but just before, during, or after spontaneous
labor, regardless of gestational age, the uterus responded to 0.5 mU/min. At
term, an oxytocin infusion rate of 2 to 8 mU/min is usually
sufficient for the successful induction of labor.25 Doses of 5 mU/min or less have been found to improve vaginal delivery
rates, improve 1-minute Apgar scores, and cause no hyperstimulation. A
dose greater than 20 mU/min at term is rarely necessary. The higher the
dose required to produce effective contractions, the less likely is
success. Seitchik and Castillo21,22,23 found in oxytocin augmentation of labor that with an initial dose of 1 mU/min
and an increase of 1 mU/min every 30 minutes until a dilation
rate of 1 cm/hr was reached, more than 90% of multiparas and 85% of
nulliparas required less than 4 mU/min. Oxytocic effects on the myometrium include increased strength, velocity, and
frequency of contractions and increased intrauterine resting pressure. After
maximum efficiency is reached, further increases in oxytocin
may result in excessive contraction frequency and increased baseline
tone, resulting in decreased effectiveness of the contractions and
the development of fetal distress. If the contraction pattern seems adequate (one
every 3 to 4 minutes), but cervical dilation is inadequate, clinical
experience indicates that occasionally a decrease in the oxytocin
dose may improve efficacy. The uterine contractile pattern may
provide an initial evaluation of response, but the quantitation of uterine
activity is far from perfect in identifying hypocontractile labor, identifying
excessive stimulation, or guiding oxytocin therapy. Because
normal labor has wide variability in uterine activity within and between
individuals, other criteria for titrating oxytocin also are recommended. Melmed
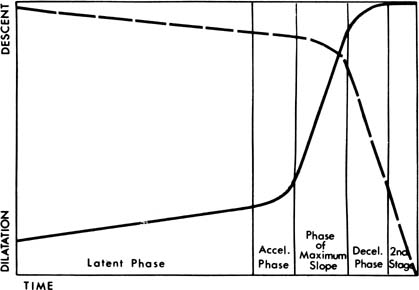
and Evans26 reported that if the initial cervical dilation rate was at least 1 cm/hr
among spontaneously laboring nulliparas, 93% had a spontaneous
vaginal delivery. In contrast, 67% required forceps or vacuum
delivery or cesarean section if the cervical dilation rate was less
than 1 cm/hr. Proponents of “active management of labor” use a cervical
dilation rate of 1 cm/hr among nulliparas to guide their use of oxytocin. As
described by O’Drisoll and associates,27 active management of labor included early routine amniotomy and oxytocin
infusions starting at 6 mU/min with increases every 15 minutes up to 40 mU/min, for
dilation rates less than 1cm/hr, provided that there
was no fetal distress. Among 3106 nulliparas, the cesarean section rate
for the diagnosis of dystocia was only 1.4%. Although some claim
that this policy for oxytocin use might result in too many patients
receiving oxytocin, evidence from clinical studies suggests that a 1cm/hr
rate of dilation may serve as a useful guide to determine the most
effective dose of oxytocin in induction or augmentation in nulliparous
women. In the United States, this active management approach resulted
in a shorter duration of labor but did not result in a decreased cesarean
section rate. Especially in the nulliparous patient, fetal heart rate response provides
a further, and perhaps more appropriate, basis for titration of oxytocin
than does uterine contractility. Placental venous outflow has been
shown to stop during contractions, but adequate exchange follows with
good relaxation. Arterial inflow continues until pressures of 30 to 70 mm
Hg, then may stop completely. The fetus continues to extract oxygen
from the intervillous blood but eventually may resort to anaerobic
metabolism until oxygen is restored after the contraction. Uterine blood
flow varies indirectly with resting pressure, frequency, intensity, and
duration of contractions. There are wide variations in fetal reserve, but
excessive uterine tone and frequency of contractions may lead
to acidosis, distress, and fetal death, even in the normal fetus. If
the intensity and frequency of contractions must be kept low to protect
a fetus with “decreased reserve,” more contractions
are needed, but it is possible that this patient still may deliver vaginally
with careful titration of the oxytocin dose. If, during oxytocin induction or augmentation, excess uterine activity
or fetal distress occurs, it may not be a pathologic state requiring immediate
cesarean delivery. When a patient moves to the supine position, contractions
may become more frequent but less intense. This position
also may result in increased risk of fetal hypoxemia and less progress
in cervical dilation. Placing the patient in a left lateral position
may be the only corrective measure necessary for hyperstimulation and
fetal distress. Additional measures include starting oxygen and decreasing
or stopping the infusion of oxytocin. Decreasing the oxytocin
dose, rather than stopping it, may correct the abnormal pattern and prevent
an unwarranted delay in delivery. When restarting the oxytocin, we
suggest decreasing the previous rate by at least half. It may be possible to decrease the dose of oxytocin when labor has been
established. Continuation of oxytocin throughout delivery may decrease
the risk for postpartum uterine atony. Monitoring of the fetal heart
rate and uterine activity should begin before and continue throughout
oxytocin induction or augmentation. This monitoring is vital in the current
medicolegal climate and may assist in determining the most efficient
dose of oxytocin. Accurate documentation of fetal heart tones, frequency
and duration of contractions, and oxytocin rate also is recommended. |