As noted in the historical review at the beginning of this chapter, the
cesarean operation has undergone a number of technical changes as the
procedure has evolved. Many different practitioners extol the benefits
of various techniques of skin incision, uterine incision, uterine closure, and
many other technical aspects of the operation. However, there
are relatively few randomized trials to support many of the commonly
used techniques in performing a cesarean section.28 Preoperative Evaluation The preoperative assessment should include a full history and physical
examination, past medical and surgical history, current medications, drug
allergies, and indication for cesarean section. In the uncomplicated
patient, no preoperative laboratory investigation is needed except
for the routine labor and delivery admission laboratory values. Rarely
will chest x-ray films and electrocardiograms be indicated unless
there is a history of significant maternal medical disease. In instances
in which these studies are indicated, preoperative consultation
with an anesthesiologist, cardiologist, or both should be considered. Abdominal Preparation Although abdominal preparation and shaving the maternal abdomen the night
before the procedure have been the norm in the past, there are little
data to support the use of night-before preparations. There
is evidence that any abdominal shave performed should be performed in
the operating room just before applying the antibacterial preparations
and not the night before. Shaving the patient the night before surgery
actually increases the bacterial count on the maternal abdomen.29 Shaving should be performed only to remove the hair that will physically
interfere with the operation itself. There is no reason to shave most
patients. Placing the patient in the left lateral tilt position using
either a hip wedge or an operative table with lateral tilt capability
will help avoid uterine compression of the inferior vena cava, which
can cause fetal bradycardia during preparation for and performance of
the cesarean section. Before the abdominal preparation and draping of
the patient, a Foley catheter should be placed to allow the bladder
to drain during the operation so that urinary output can be evaluated
intraoperatively and the presence of the bladder in the operative field
can be minimized. Skin Incision A number of skin incisions have been used in abdominal deliveries. The
most frequently used type of skin incision in the United States is the
Pfannenstiel incision; the midline vertical incision is the next most
common (Fig. 3). Other skin incisions used include the Maylard, Cherney, right paramedian, and
the low transverse. In general, the skin incision should
be determined by the physician based on maternal body habitus, clinical
situation, time available to deliver the infant, and skill of the
surgeon. Midline vertical incisions are generally more hemostatic and
require less dissection; therefore, less time from incision to birth than
transverse incisions. Transverse incisions fall along the lines of
expression of the anterior abdominal wall and therefore should create
less pronounced scarring and risk of dehiscence. Transverse incisions
have also been associated with less postoperative pain. 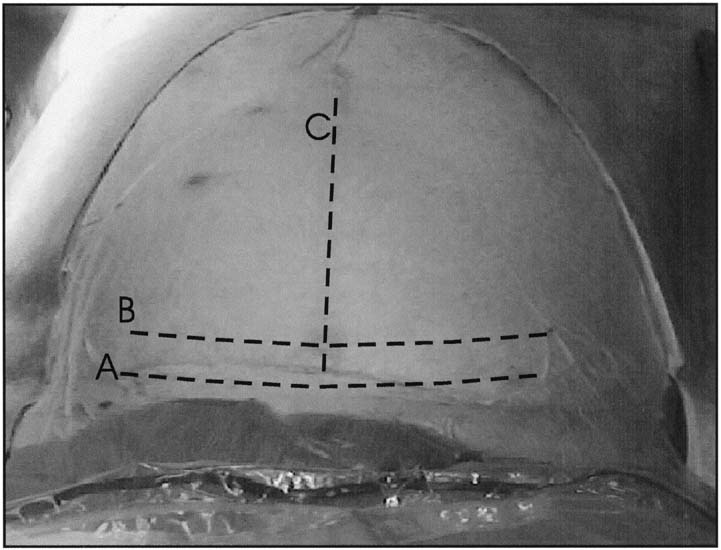 Fig. 3. Abdominal incisions. A. Pfannenstiel
incision should be made in a curvilinear fashion approximately 2
cm above the pubic symphysis. B. Joel-Cohen incision should
be made in a linear fashion approximately 2 to 3 cm above the traditional
placement of the Pfannenstiel incision. C. Midline vertical
incision should be made in the midline and extend from just below
the umbilicus to just above the symphysis pubis and may be continued
around the umbilicus if more exposure is necessary. D. Wiser
(right paramedian) incision should be made several cm to the right
side of the umbilicus and somewhat higher than the normal midline
vertical incision.
Fig. 3. Abdominal incisions. A. Pfannenstiel
incision should be made in a curvilinear fashion approximately 2
cm above the pubic symphysis. B. Joel-Cohen incision should
be made in a linear fashion approximately 2 to 3 cm above the traditional
placement of the Pfannenstiel incision. C. Midline vertical
incision should be made in the midline and extend from just below
the umbilicus to just above the symphysis pubis and may be continued
around the umbilicus if more exposure is necessary. D. Wiser
(right paramedian) incision should be made several cm to the right
side of the umbilicus and somewhat higher than the normal midline
vertical incision.
|
TRANSVERSE INCISIONS Although transverse incisions are commonly performed because of the widely
held belief that there is a decreased incidence of wound dehiscence
and incisional hernia and greater cosmetic appeal, more recent studies
have not supported this belief and point to infection as the greatest
risk for dehiscence regardless of incision type.30,31,32 The Pfannenstiel incision is made transversely in the maternal abdomen
approximately 3 cm above the symphysis pubis and is curvilinear, with
the lateral apices of the incision smiling up toward the anterior superior iliac spines (see Fig. 3). This incision is performed sharply to the level of the anterior
rectus fascia. The anterior rectus fascia is then sharply incised with
the scalpel in a transverse manner in the midline to expose the belly
of the rectus muscle on either side of the midline. At this time, the
incision in the anterior rectus fascia may be extended laterally using
either the scalpel or the Mayo scissors. Care must be taken not to cut the underlying rectus muscles. This may be
accomplished by placing the Mayo scissors, with the tip up, underneath
the fascia, and then sliding the scissors laterally along the length
of the proposed fascial incision, opening the blades of the scissors
at the proposed apex of the incision and withdrawing the scissors before
closing the blades. At this point, the Mayo scissors can be used to
extend the fascial incision laterally by merely pushing the blades of
the scissors against the fascia. Care should be taken to avoid the transverse
oblique muscle when incising the fascia. After the fascia is
incised, the anterior rectus fascia can then be dissected from the underlying
rectus muscles in both the cephalad and caudad directions. This
is accomplished by grasping the cut edges of the fascia with a pair
of strait Kocher clamps and using a combination of blunt and sharp dissection
to free the muscle from the overlying fascia. This dissection
allows the rectus muscles to be retracted laterally without being cut. During
this dissection, care must be taken to identify and ligate or
electrocoagulate the perforating vessels between the rectus muscles and
the anterior fascia; this can be performed at entry, or in the event
of an emergency cesarean delivery, at the time of closure. The posterior
sheath consists of the fascia of the transversalis muscle and is
closely opposed to the peritoneum. These tissues may be incised in either
a longitudinal and transverse manner. Regardless of which manner is
chosen, the entry point should be high in the operative field to avoid
injury to the maternal bladder. Sharpening of the peritoneum may be
performed by elevating the peritoneal membrane between two hemostats, palpating
the opposing pieces of membrane for evidence of entrapped bowel, and
making circumcision with a scalpel or by bluntly introducing
a finger through the peritoneum at the level of the umbilicus. Once the
peritoneal cavity is entered, the peritoneal incision is extended using
Metzenbaum scissors to maximize surgical exposure, with care being
taken to avoid inadvertent damage to the bladder or to any bowel or
omentum that may be adherent to the anterior abdominal wall. The Maylard and Cherney incisions differ from the Pfannenstiel incision
in the manner in which the anterior rectus sheath and the rectus muscles
are approached. With the Maylard incision, once the anterior rectus
sheath is incised in a transverse fashion, the fascia is not dissected
free from the underlying rectus muscles; instead, the inferior epigastric
arteries are identified and ligated, and the rectus muscles are
incised, usually with electrocautery to minimize bleeding. The posterior
rectus sheath and the peritoneum are then incised in a transverse
fashion. The Cherney incision is performed in the same manner as the Pfannenstiel
and the Maylard incisions except that the rectus fascia is not entered; instead, the
rectus muscles are cut free from the symphysis pubis
at their tendinous insertion and reflected superiorly. There are few if
any indications for the use of this type of incision for an abdominal
delivery. The Joel-Cohen incision is performed in a transverse manner several
cm above the location of a Pfannenstiel incision and is linear, not
curvilinear. The fascia is not dissected off of the rectus muscles, and
the peritoneum is entered transversely, as in the Maylard incision. An
advantages of this type of incision include decreased operating time, however
there are no maternal or fetal advantages other than speed.33,34 In the moderately obese patient, a variation of the Pfannenstiel incision
is performed several cm higher than the true Pfannenstiel to avoid
placing the incision in the fold created by the abdominal pannus and thereby
decreasing the rate of wound complications. VERTICAL INCISIONS Historically, the midline vertical skin incision has been the preferred
incision for cesarean section because of the speed and ease of entry
into the peritoneal cavity. The decreased dissection that is required
reduces intraoperative blood loss. Vertical incisions remain useful in
situations such as cesarean section for fetal bradycardia and in the
morbidly obese patient in whom a transverse incision may not allow for
adequate exposure of the operative field. The incision is performed vertically
from just below the umbilicus and extended to just above the
symphysis pubis and can easily be extended around the umbilicus if exposure
of the upper abdomen is required. When making a midline vertical
incision, it is important to remember that the linea nigra may not represent
the true midline. The incision is carried sharply down to the
level of the rectus sheath, which is then incised sharply with the scalpel
in a vertical direction. This incision may be completed with the
scalpel or by using the Mayo scissors. The fascial edge closest to the
midline is then grasped with a pair of Kocher clamps, and sharp and
blunt dissections are used to separate the rectus muscles from the overlying
fascia. The rectus muscles are then separated in the midline, and
the peritoneum is entered vertically as described previously. A right paramedian incision is useful in the morbidly obese patient in
whom the abdominal pannus is grossly displaced when the patient is placed
in the left lateral tilt position. Advantages of this incision are
that once the skin has been incised, an incision that is continued perpendicular
toward the floor of the operating room will incise the fascia
approximately in the midline of the patient, resulting in better exposure
for the delivery of the infant.
Repeat cesarean delivery account for the majority of cesareans. In patients
undergoing repeat cesarean delivery, the abdominal scar may be revised
at the time of repeat operation. In the case of an emergency cesarean
section, any scar revision should be performed at the time of abdominal
closure and not at entry. It is also important to remember that
the choice of skin incision should be that which the primary surgeon
believes will be most beneficial for the present operation and should
not be dictated by the location of a previous scar. Uterine Incisions
There are three standard uterine incisions that can be performed for
delivery of the fetus: low transverse, low vertical, and classical (Fig.
4). The specific type of uterine incision should be determined by
the primary surgeon at the time of the operation based on gestational
age and lie of the fetus and any uterine anomalies.
|
 Fig. 4. Uterine incisions. A. Low-transverse
uterine incision should be made through the thin, noncontractile
portion of the lower uterine segment in a curvilinear fashion. Also
pictured is a low-vertical incision, which is made through the noncontractile
lower uterine segment in a vertical fashion. B. J-extension
of the low-transverse incision. When additional exposure to the
uterine cavity is required to deliver the fetus, the low-transverse
incision can be extended laterally and cephalad to increase the
length of the incision without endangering the uterine arteries.
C. Another option in this situation is to use a T-extension
in the midline. D. The classical uterine incision is made
through the contractile portion of the myometrium above the bladder
reflection.
Fig. 4. Uterine incisions. A. Low-transverse
uterine incision should be made through the thin, noncontractile
portion of the lower uterine segment in a curvilinear fashion. Also
pictured is a low-vertical incision, which is made through the noncontractile
lower uterine segment in a vertical fashion. B. J-extension
of the low-transverse incision. When additional exposure to the
uterine cavity is required to deliver the fetus, the low-transverse
incision can be extended laterally and cephalad to increase the
length of the incision without endangering the uterine arteries.
C. Another option in this situation is to use a T-extension
in the midline. D. The classical uterine incision is made
through the contractile portion of the myometrium above the bladder
reflection.
|
Historically, the creation of a bladder flap was advocated before making
any uterine incisions. More recently, randomized controlled trials have
noted that the omission of the bladder flap provides short term advantages
such as reduction of operating time and incision-delivery
interval, reduced blood loss and need for analgesics. Long-term
effects remain to be evaluated.35 When developing a bladder flap, a segment of loose areolar peritoneum
can be visualized at the area where the bladder is adjacent to the lower
uterine segment. The peritoneum is grasped with a pair of forceps, elevated, and
then incised transversely with Metzenbaum scissors, with
care being taken not to extend the incision laterally into the vascular
broad ligament. Next, the inferior portion of the incision is elevated, and
careful blunt and sharp dissection is used to separate the posterior
wall of the bladder from the lower uterine segment. This dissection
serves two purposes: it allows better access to the lower uterine
segment and it allows the bladder to be retracted out of the operative
field. Before making the uterine incision, the surgeon should also
identify the round ligaments to properly orient the degree of dextrorotation
of the uterus and to evaluate for the presence of any myomas or
other malformations that might affect the choice and/or placement
of the incision.
The standard low-segment transverse incision accounts for 90% of
all uterine incisions.28 It is initiated sharply in the lower uterine segment, perpendicular to
the long axis of the uterus. This incision is made sharply with the scalpel
in the midline and performed down to the level of the fetal membranes, with
care being made not to incise the membranes. This incision
is then extended laterally using either blunt dissection with the fingers
or bandage scissors (Fig. 5). There was thought to be no difference between the two methods in
amount of blood lost or in the rate of extension of the incision into
the lateral uterine vessels when they were compared and correlated by
the stage of labor.36 However, a recent investigation revealed a greater risk of subsequent
blood transfusion in woman whose incision was extended sharply compared
to those extended bluntly.37 When blunt dissection is used, an upward curve of the incision may be
created by the surgeons placing their thumbs on the patient's anterior
superior iliac spines and index fingers in the uterine incision. By
keeping the hand in this position, the incision is pulled open in
an arc. 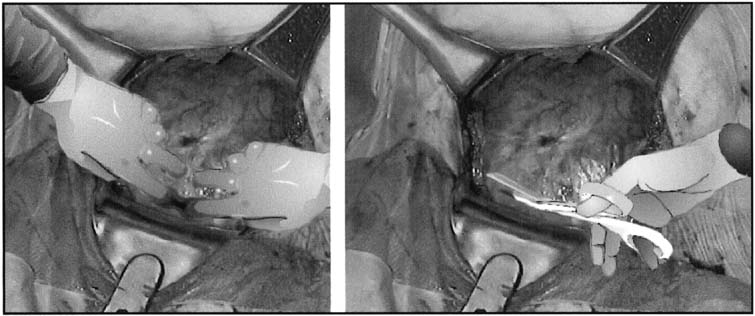 Fig. 5. Extension of the lower uterine incision
may be accomplished either by inserting fingers into the uterine
cavity and bluntly stretching the myometrial incision in a curvilinear
fashion or by sharply cutting the lower uterine segment with bandage
scissors. When the uterus has a poorly developed lower uterine segment,
using bandage scissors is often preferable.
Fig. 5. Extension of the lower uterine incision
may be accomplished either by inserting fingers into the uterine
cavity and bluntly stretching the myometrial incision in a curvilinear
fashion or by sharply cutting the lower uterine segment with bandage
scissors. When the uterus has a poorly developed lower uterine segment,
using bandage scissors is often preferable.
|
Intentional extension of the low-transverse incision is necessary
in 1% to 2% of cases.38 Typically, the extension of the low transverse incision is performed by
creating a low vertical incision in the midline, T-ing the uterine incision, or creating a vertical incision at the lateral aspect of the uterine
incision, a J-extension. These extensions are commonly performed for malpresentations, poorly
developed lower uterine segment, or deep transverse arrest.38 When performed, extensions of the low-transverse incision are associated
with increased incidence of maternal blood loss, broad ligament
hematoma, and uterine artery laceration compared with low-segment
transverse incisions that do not require extension. The low-vertical uterine incision is made parallel to the longitudinal
axis of the uterus in the midline, with care being taken to remain
below the contractile portion of the uterus and within the thin lower
uterine segment. Other than the direction of the incision, technical
aspects are carried out as described for the low-transverse
uterine incision. Studies have shown that there is no increased risk of
uterine rupture in patients with this type of incision compared with
the low-segment transverse incision as long as the incision remains
primarily in the thin lower uterine segment.39 A classical uterine incision is made by incising the uterus parallel to
the longitudinal axis of the uterus through the contractile portion of
the myometrium. Indications for classical uterine incision include situations
in which the lower uterine segment is not adequately developed
to accommodate a low-transverse or a low vertical incision; cases
of abnormal fetal lie such as back-down transverse lie, in
which the low-transverse or low-vertical incision will
not allow the operator adequate access to the fetus for manipulation
and delivery, or when myomas or uterine abnormalities distort the uterus
in such a way as to make a low transverse incision inadvisable.
Delivery of the Fetus
After the uterine incision has been made, the fetal membranes, if still
intact, are ruptured with an Allis clamp. If the fetus is in a noncephalic
presentation, leaving the membranes intact until the fetal feet or head
can be moved into the uterine incision will increase the ease of delivery.
When the fetus is in a cephalic presentation, delivery is performed by
the surgeons placing their dominant hand into the uterine cavity and elevating
the fetal head into the uterine incision (Fig.
6). If the fetus is not in an occiput anterior position, rotating
the head into this position will allow the fetal neck to extend around
the upper portion of the incised myometrium and more closely mimic the
cardinal movements of vaginal delivery. When the fetal head is impacted
in the maternal pelvis, such as in deep transverse arrest, there are a
number of options to assist with delivery of the fetal head. The surgeon
can place a hand in the lower uterine segment in the standard fashion
to cup and then disengage the fetal head. Care must be taken by the surgeon
not to flex the wrist, because this often causes extension of the uterine
incision caudally toward the bladder and vagina. If this does not work,
an assistant can place a sterile, gloved hand into the vagina from the
introitus and disengage the fetal head from below (Fig.
7). Another option is for the surgeon to the dominant hand between
the lower uterine segment and the reflected bladder and attempt to disengage
the head by cupping it through the lower uterine segment. It is our experience
that care must be taken with this maneuver to ensure that the bladder
is not damaged by inadvertent blunt cystotomy.
|
 Fig. 6. Extraction of the fetal head. The surgeon's
dominant hand is placed into the uterine incision so that the back
of the hand is against the inside of the lower uterine segment and
the fingers cup the fetal head. Firm, gentle traction is used to
elevate the fetal head toward the incision. The fetal head may then
be rotated to an occiput anterior position and delivered through
the uterine incision with the assistance of fundal pressure.
Fig. 6. Extraction of the fetal head. The surgeon's
dominant hand is placed into the uterine incision so that the back
of the hand is against the inside of the lower uterine segment and
the fingers cup the fetal head. Firm, gentle traction is used to
elevate the fetal head toward the incision. The fetal head may then
be rotated to an occiput anterior position and delivered through
the uterine incision with the assistance of fundal pressure.
|
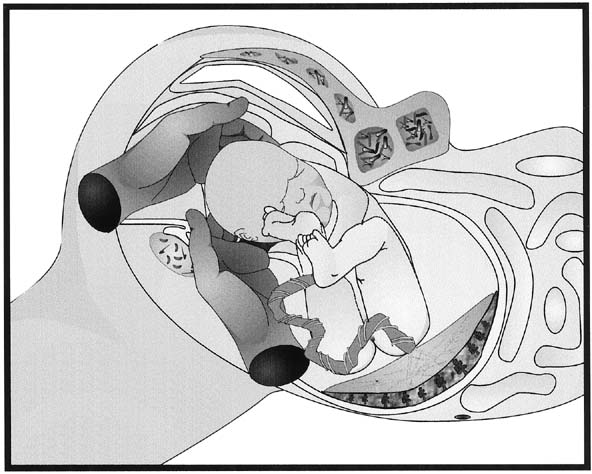 Fig. 7. Disimpaction of the fetal head. When
the fetal head has descended so far into the vagina that extraction
of the fetal head is difficult, having an assistant place a gloved
hand into the vagina and elevate the fetal head from below can increase
the ease of delivery and decrease the trauma to the lower uterine
segment and vagina.
Fig. 7. Disimpaction of the fetal head. When
the fetal head has descended so far into the vagina that extraction
of the fetal head is difficult, having an assistant place a gloved
hand into the vagina and elevate the fetal head from below can increase
the ease of delivery and decrease the trauma to the lower uterine
segment and vagina.
|
Once the fetal head is at the uterine incision, mild fundal pressure by
the first assistant will encourage the expulsion of the fetal head from
the uterus. At this point, the nares and mouth of the fetus should
be suctioned and, after checking for and reducing any nuchal cord, the
fetal body is delivered by standard maneuvers as in a vaginal delivery. After
the infant is delivered, the cord is doubly clamped and cut, and
the infant is handed to the personnel who have been assigned to care
for the newborn. It is important to remember that if the newborn requires
resuscitation, the obstetrician is responsible for its care as
well as the care of the mother. Qualified personnel should be available
to assume care of the newborn. Attention is now turned to the delivery of the placenta. The delivery of
the placenta may be accomplished either by manual extraction or by awaiting
spontaneous delivery. Spontaneous delivery of the placenta, when
assisted with uterine massage and gentle traction on the umbilical
cord, is associated with a lower rate of postpartum endomyometritis and
maternal blood loss compared with manual extraction.40,41,42 Once the placenta has been delivered, the uterus may be either exteriorized
or left in situ to be repaired. Blood loss is not significantly
different with either method.42 Exteriorization of the uterus does allow for better visualization of the
adnexal structures and increases the ease with which tubal ligation
can be performed. 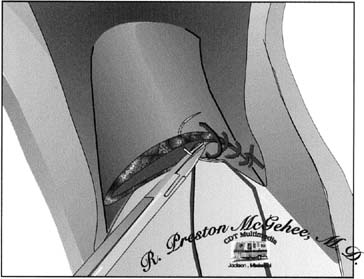 Fig. 8. Single-layer repair of the low-transverse
uterine incision. To obtain optimal hemostasis of the incision in
a single layer, the surgeon should be careful to include all layers
of incised myometrium while taking care to avoid including excess
decidua and serosa.
Fig. 8. Single-layer repair of the low-transverse
uterine incision. To obtain optimal hemostasis of the incision in
a single layer, the surgeon should be careful to include all layers
of incised myometrium while taking care to avoid including excess
decidua and serosa.
|
Uterine closure may be performed with either a single- or double-layer
closure technique. Single-layer closure using a running
locking stitch (Fig. 8) has been shown to be associated with decreased operative time and
fewer additional hemostatic sutures. A large Canadian study found a
four-fold increase in the risk of uterine rupture in woman who
had a single layer closure in their previous pregnancy.43,44,45,46,47 Chromic catgut has been the suture of choice for closure of the uterine
incision by many obstetricians for a number of years. However, the use
of a synthetic absorbable suture, such as polyglycolic acid or polyglactin, has
several advantages over the use of catgut. The method of absorption
of catgut suture is by phagocytosis, and this results in significantly
more inflammation than the absorption of synthetic sutures
by hydrolysis.48 The decreased inflammation associated with synthetic absorbable sutures, as
well as the increased time interval to the loss of suture strength, are
both advantageous in this situation. After the uterus is closed and has been returned to the peritoneal cavity, irrigation
can be employed. Routine irrigation in low-risk
populations does not reduce intrapartum or postpartum maternal morbidity.49 Next, attention should be turned to ensuring that the operative field
is hemostatic, with special attention given to the uterine incision and
bladder flap, if these have been previously placed on tension because
of exteriorization of the uterus, and to the rectus muscles. Hemostasis
may be achieved by either suture ligation or electrocoagulation of
bleeding points. There is no advantage to closure of the visceral or
parietal peritoneum. When repaired with suture, the peritoneum undergoes
more inflammation and scarring in animal models.50 Operating time and postoperative analgesia requirements are reduced in
patients who do not undergo closure of the visceral and parietal peritoneum. There
is also a decrease in adhesions found at repeat operation
when the visceral and parietal peritoneum is not closed.51 Fascial closure in a Pfannenstiel incision is performed in a single layer
with a synthetic absorbable suture. In patients who have undergone
more than one laparotomy through the same scar, or in patients who are
at increased risk for fascial separation or dehiscence such as diabetic
patients or patients who are on chronic corticosteroids, the use of
a synthetic delayed absorbable suture such as polydioxanone may be preferable
because of its ability to maintain suture strength for a longer
period of time.52 For the closure of a vertical fascial incision, a continuous running delayed
absorbable suture has been shown to be as effective as the Smead-Jones
closure and to reduce operating time without increasing
morbidity. Whenever sutures are placed within the fascia, it is important
to remember that a 10-mm zone of collagenolysis occurs surrounding
the incision; therefore, sutures should be placed more than 1 cm
from the fascial edge to achieve maximal wound strength.53 The subcutaneous tissue may be closed with an absorbable suture or simply
reapproximated by closure of the skin. Closing this layer has not been
associated with decreased rates of superficial wound disruption in
several studies.54 Skin closure may be accomplished by either a subcuticular stitch or staples. Subcuticular
stitch has been associated with less immediate postoperative
pain and more cosmetically appealing at 6 weeks when compared
to the stapling device.55 Postoperative Care There is little literature to support any specific postoperative regimen
in postcesarean patients; however, common sense and extrapolation of
data from other postlaparotomy patients allow for the development of
a rational plan of care. Most cesarean sections are relatively uncomplicated, and
in these patients, care should be similar to that given after
a vaginal delivery. In the first hour after cesarean section, the patient should be monitored
closely in a recovery area where urine output, pulse, blood pressure, respirations, and
any evidence of bleeding can be closely observed; if
the patient remains stable and without complication, she may then
be transferred to the postpartum ward. Once any nausea and vomiting has abated, the patient should be encouraged
to take fluids orally. This may be followed by oral intake of solid
food as soon as the patient feels that she is ready; this should occur
no later than the first postoperative day. Early institution of feeding
in the postsurgical patient with minimal intraoperative bowel manipulation
does not increase the incidence of postoperative ileus.56 Early ambulation should also be encouraged. Getting the patient out of
bed as soon as regional anesthesia has worn off or as soon as she has
recovered from general anesthesia will decrease the incidence of pulmonary
complications such as atelectasis and pneumonia and the incidence
of thrombotic complications. This will also facilitate the removal of
bladder catheters, therefore decreasing the incidence of catheter-associated
urinary tract infections. In the uncomplicated patient
with adequate urine output, the catheter should be removed no later than
the first postoperative day. Encouragement of deep breathing and coughing
with the use of incentive spirometry will also help prevent collapse
of alveoli in the lung and resulting infection. Routine laboratory studies are probably unnecessary in most postcesarean
patients who have no unexpected symptoms. However, a single hemoglobin
determination on postoperative day one or two is probably reasonable
to screen for significant anemia. Most postpartum patients with asymptomatic
anemia respond well to oral iron therapy. The wound should be cared for in the standard manner, with occlusive dressings
removed on the first postoperative day and the wound examined
daily during the hospitalization for evidence of infection, seroma, or
hematoma. Skin staples can be removed on the second or third postoperative
day with Pfannenstiel incisions and at the fifth to seventh postoperative
day with vertical incisions. The placement of SteriStrips after
staple removal may help maintain skin edge approximation with earlier
removal. The patient may be discharged when she is able to care for herself and
her newborn. Many patients are ready to leave the hospital by postoperative
day two or three. Discharge instructions should include patient
education concerning expectations on activity level, lochia, breastfeeding
or milk suppression, contraception, and newborn care. |