Prolapse of the umbilical cord is a true obstetric emergency, and understanding of its predisposing factors can assist in speedy diagnosis and treatment. Although cord compression occurs routinely in normal labors and is commonly seen during fetal heart rate monitoring, severe cord occlusion can result in dangerous alterations in fetal and placental circulation. Historically, incidence of cord prolapse has been reported as approximately 1 in 300 deliveries. However, recently, the reported incidence has fallen to about 0.2%, or 1 in 500 of all deliveries, mainly as a result of changing obstetric management, including antenatal ultrasound and continuous electronic fetal monitoring.10 Morbidity and mortality associated with prolapse of the cord also have declined. Murphy and MacKenzie report that despite ominous fetal heart rate tracings, abnormal fetal blood gas readings, and low Apgar scores, most of the mortality is attributable to congenital anomalies and prematurity.10
Cord prolapse may be frank or occult. Occult cord prolapse frequently is encountered intrapartum, when fetal heart rate abnormalities lead to palpation of loops of cord around or ahead of the presenting part. Yla-Outinen and associates report that the cord was found free in the vagina in 45%, at the introitus in 11%, and presenting in 4% of the cases.11
Predisposing Factors
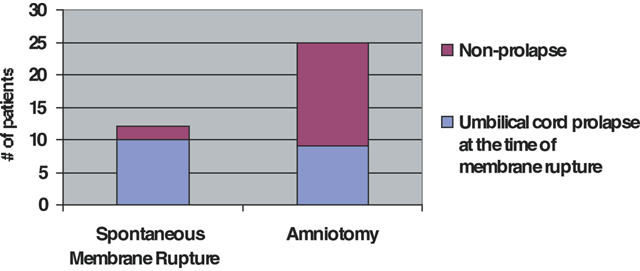
Umbilical cord prolapse mostly is seen in the clinical setting of a poorly engaged or unengaged presenting part, such as with malpresentation, prematurity, multifetal pregnancy, hydramnios, and uterine anomalies (Table 1). However, most cases of cord prolapse are reported with singleton cephalic presentation in 50% to 63% of the cases.12 Yet, proportionally, breech and transverse presentations have higher risk of prolapse per case. There has been concern over the role of obstetric maneuvers in promoting prolapse of the cord. Intrapartum interventions and procedures such as amniotomy, amnioinfusion, cervical ripening, and labor induction have been thought to increase risks of cord prolapse. Amniotomy especially has received blame for cord prolapse. In a study by Roberts and colleagues, no increased risk was identified between artificial and spontaneous rupture of the membranes in their series, nor a difference in cervical dilation at the time of diagnosis of prolapse.13 Figure 1 illustrates the proximity of ruptured membranes to cord prolapse, with 83% of diagnoses made after spontaneous rupture versus 36% of the artificially ruptured group. In the group with artificial rupture, two thirds of the patients with cord prolapse had abnormal fetal heart tracings, which prompted intervention for applying an internal fetal electrode or performing amnioinfusion to treat cord compression. In addition, those diagnosed with prolapse had a significantly higher station and little descent of the presenting part during labor. Labor induction, amnioinfusion, and cervical ripening also were not found to be associated with increased risk of cord prolapse in that series. Often, amniotomy was performed for evaluation of existing intrapartum fetal heart rate abnormalities. Other investigators have not been able to confirm risk associated with other maneuvers, including application of fetal scalp electrode, insertion of intrauterine pressure catheters, and external cephalic version.
TABLE 1. Factors Associated With Cord Prolapse in 79 Patients
Factor | No. | % |
Abnormal presentation | 40 | 50.6 |
Prematurity <2500 g or <37 wk | 30 | 38.0 |
Obstetric manipulation | 15 | 19.0 |
Multiparity (<5 pregnancies) | 10 | 12.7 |
Multiple gestation | 8 | 10.1 |
Placental problems (abruption, abnormal implantation, bleeding) | 6 | 7.6 |
Polyhydramnios or excessive amniotic fluid (index above 25, or vertical pocket of 8 cm) has been shown to increase the risk of umbilical cord prolapse. Many patients with excessive fluid have high station at labor onset. In a retrospective study of more than 60,000 singleton deliveries at term, Maymon and colleagues noted an almost 10-fold increase in the risk of cord prolapse (2.2% versus 0.3%) in patients with hydramnios.14 Multifetal pregnancies have the risk of cord prolapse, and twin pregnancy, even in vertex-vertex presentation, has a higher proportion of cord prolapse with the second twin, requiring cesarean delivery. In a study of 106 sets of vertex-vertex twins, Sullivan and associates noted a 35% incidence of cord prolapse in the second twin among those who required cesarean delivery of the second twin.15 Abnormal cord insertion, placenta previa, and low-lying placenta also are risk factors for prolapse of the cord.
Antepartum Diagnosis and Management
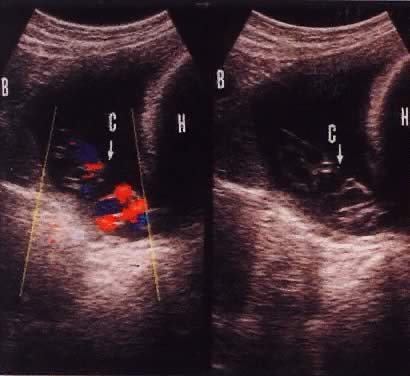
Funic presentation before rupture of membranes is a predisposing factor to umbilical code prolapse. This is visualized on ultrasound in approximately 1 in 167 (0.6%) live births.16 The increased use of antepartum obstetric ultrasound, especially with color flow Doppler, has assisted in making the diagnosis of a presenting cord and preparing for cesarean delivery before overt or occult prolapse. Figure 2 illustrates the usefulness of color flow Doppler in this diagnosis. Every patient with fetal malpresentation, multiple gestation, or known abnormal placentation should undergo ultrasound examination before labor to exclude the possibility of funic presentation. Ultrasound also can be helpful for the preterm fetus in assessing the contents of hour-glassing membranes.
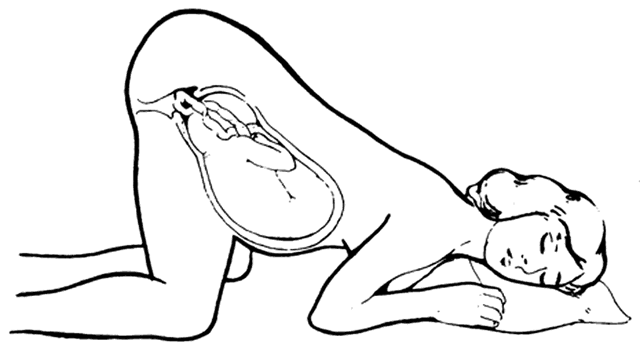
During labor, the diagnosis of cord prolapse must be in the differential diagnosis whenever fetal heart rate abnormalities are noted, especially recurrent variable decelerations. If risk factors for cord prolapse are present, gentle vaginal examination should be performed, possibly with the assistance of confirming bedside ultrasound. Once the diagnosis has been confirmed, the cord should be manipulated as little as possible because the vessels of the cord may spasm and further compromise blood flow. Cervical dilation, effacement, and station of the presenting part should be ascertained. Because of the possibility of spasm, controversy exists as to whether an attempt at replacement of the cord should be done. If the cervix is completely dilated and rapid vaginal delivery is feasible, then an effort at replacement is not harmful, during which simultaneous preparations for abdominal delivery are made. The patient should be immediately repositioned in knee-chest position (Fig. 3), which reduces the pressure of the presenting part on the cord. If the patient is unable to assume the knee-chest position because of epidural or body habitus, or for patient comfort during prolonged positioning, steep Trendelenburg position also may be used. Oxygen should be administered to the mother. In addition, the examiner's hand should remain in the vagina to manually elevate the presenting part, if possible. Another technique described by numerous authors is that of filling the mother's urinary bladder with 500 to 1000 mL of fluid to assist in elevating the presenting part while preparations for delivery are made. This may be combined with the maneuvers previously described. If the cord has prolapsed beyond the introitus, it should be wrapped in sponges or a sterile towel soaked with warm saline. Another useful therapy is to decrease force and frequency of contractions of labor using subcutaneous terbutaline. Immediate delivery is the treatment of choice, providing the fetus still is viable.
|
Depending on the setting and the availability of anesthesia and surgical facilities, vaginal delivery may be the most rapid route of delivery using forceps or vacuum, given the appropriate cervical dilation and station. Vaginal breech delivery also is an option, including breech extraction for a second twin. However, other presentations, such as transverse, shoulder, and other unstable presentations, should be delivered abdominally. In a large British series of cord prolapses, 71% of 132 patients were delivered by cesarean section, with a perinatal mortality of 91 of 1000.10 Low cord pH (less than 7.10) and low Apgar scores were not predictive of intact outcome. Only one baby did not survive as a direct result of cord prolapse. In this study, time to delivery was not a critical factor; however, Praboulous and Phillipson, in their study of 65 cases of cord prolapse of 26,000 deliveries, found that infants in the group having a shorter time to delivery (mean 11 minutes) had a worse outcome, including all cases of asphyxia, suggesting a pre-existing problem such as anomaly or prematurity.17 The presence of a limp, pulseless cord does not necessarily indicate fetal death. Auscultation and ultrasound assessment of fetal cardiac activity also should be performed. Driscoll and colleagues report patients having pulseless cord but also the presence of cardiac activity by ultrasound, with subsequent delivery of liveborn infants who did well.18 After the delivery, whether vaginal or abdominal, analysis of cord blood gases should be performed, as well as pathologic evaluation of the placenta.
Included in the differential diagnosis of fetal heart tracing abnormalities is abruptio placentae and umbilical cord prolapse. Often, emergent abdominal delivery is done without knowing which entity is present. According to Johnson and Richards, umbilical cord acid-base values at delivery can be highly predictive.19 The data suggest that careful analysis of umbilical arterial and venous blood gases may elucidate the cause of fetal acidosis. The study noted acidosis in arterial pH for both abruptio and cord prolapse of 6.87 and 7.05, respectively. There was, however, a marked difference in umbilical venous pH values, which also were acidotic, with a mean of 6.98 in the abruptio group and a near-normal value at 7.28 in the cord prolapse group.19 Acidosis in the newborn usually results from either of two different clinical situations: reduced maternal oxygen delivery to the placenta, or reduced fetal placental blood flow. In abruptio placentae—as well as maternal hypotension, hypoxemia, or increased arterial resistance, as in uterine hyperstimulation—there is poor oxygen delivery to the placenta and fetus, giving rise to low pH in both umbilical arterial and venous gases. In contrast, umbilical cord prolapse, as well as in other conditions of reduced fetal cardiac output, leads to reduced fetal perfusion of the placenta, large differences in arterial and venous oxygen saturation and pH, and a totally different mechanism for fetal acidosis.