Preeclampsia is a hypertensive disorder unique to human pregnancy. Several risk factors for the development of preeclampsia have been identified and are listed in Table 2. The risk to the fetus in patients with preeclampsia relates largely to the gestational age at delivery. Risk to the mother can be significant and includes the possible development of disseminated intravascular coagulation, intracranial hemorrhage, renal failure, retinal detachment, pulmonary edema, liver rupture, abruptio placentae, and death. Therefore, astute and experienced clinicians should be caring for women with preeclampsia.
Table 2. Risk Factors for Preeclampsia
| Nulliparity |
| Family history of preeclampsia |
| Obesity |
| Multifetal gestation |
| Preeclampsia/eclampsia in previous pregnancy |
| Poor outcome in previous pregnancy |
| Intrauterine growth retardation |
| Abruptio placentae |
| Fetal death |
| Preexisting medical conditions |
| Chronic hypertension |
| Renal disease |
| Diabetes mellitus (class B to F) |
| Thrombophilias |
| Antiphospholipid antibody syndrome |
| Protein C, S, or antithrombin deficiency |
| Factor V Leiden |
| Abnormal uterine artery Doppler studies |
| S/D ratio >2.6 |
| Resistance index >0.58 |
| Presence of a notch (uterine artery) |
| Increased angiotensin sensitivity at 28 weeks |
| Positive roll over test at 28–30 weeks |
Etiology and Pathogenesis
The etiology of preeclampsia remains unknown. During the past century, many theories regarding its etiology have been suggested, but most have not withstood the test of time. The syndrome is characterized by vasospasm, hemoconcentration, and ischemic changes in the placenta, kidney, liver, and brain.12 Reported abnormalities are summarized in Table 3.12,13,14 These abnormalities are usually seen in women with severe preeclampsia. Theories as to the causative mechanisms include placental origin, immunologic origin, and genetic predisposition among others. A great deal of research is dedicated to solving the etiologic enigma of preeclampsia. Without a definitive etiology, predicting patients at risk for the development of preeclampsia and effecting a treatment is difficult.
Table 3. Pathophysiologic Abnormalities in Preeclampsia
| Generalized vasospasm |
| Activation of coagulation system |
| Abnormal hemostasis |
| Altered thromboxane-to-prostacyclin ratio |
| Endothelial cell injury |
| Abnormal hemodynamics |
| Reduced uteroplacental blood flow |
Pathophysiology
CARDIOVASCULAR
The hypertensive changes seen in preeclampsia are attributed to intense vasospasm thought to be caused by increased vascular reactivity. The underlying mechanism responsible for the increased vascular reactivity is presumed to be alterations in the normal interactions of vasodilatory (prostacyclin, nitric oxide) and vasoconstrictive (thromboxane A2, endothelin) substances. Another vascular hallmark of preeclampsia is hemoconcentration. Patients with preeclampsia have lower intravascular volumes and have less tolerance for the blood loss associated with delivery.
HEMATOLOGIC
The most common hematologic abnormality in preeclampsia is thrombocytopenia (platelet count less than 100,000/mm3). The exact mechanism for thrombocytopenia is unknown. Another possible hematologic abnormality is microangiopathic hemolysis as seen in association with HELLP syndrome and can be diagnosed by schistocytes seen on peripheral smear and increased lactate dehydrogenase (LDH) levels. Interpretation of the baseline hematocrit level in a preeclamptic patient may be difficult. A low hematocrit level may signify hemolysis and a falsely high hematocrit may be caused by hemoconcentration.
RENAL
Vasospasm in preeclampsia leads to decreased renal perfusion and subsequent decreased “glomerular filtration rate” (GFR). In normal pregnancy the GFR is increased up to 50% above prepregnancy levels. Because of this, serum creatinine levels in preeclamptic patients rarely increase above normal pregnancy levels (.8 mg/dL). Close monitoring of urine output is necessary in patients with preeclampsia, because oliguria (defined as less than 500 cc/24 hours) may occur because of renal insufficiency. Rarely, profound renal insufficiency may lead to acute tubular necrosis. The pathognomonic renal lesion in preeclampsia is called glomerular capillary endotheliosis, which is swelling of the glomerular capillary endothelial and mesangial cells.
HEPATIC
Hepatic damage in association with preeclampsia can range from mildly elevated liver enzyme levels to subcapsular liver hematomas and hepatic ruptures. The latter are usually associated with HELLP syndrome. The pathologic liver lesions seen on autopsy are periportal hemorrhages, ischemic lesions, and fibrin deposition.
CENTRAL NERVOUS SYSTEM
Eclamptic convulsions are perhaps the most disturbing central nervous system (CNS) manifestation of preeclampsia and remain a major cause of maternal mortality in the third world. The exact etiology of eclampsia is unknown but is thought to be attributed to hypertensive encephalopathy and vasospasm with resultant ischemia or microhemorrhages and cerebral edema. Radiologic studies may show evidence of cerebral edema and hemorrhagic lesions, particularly in the posterior hemispheres, which may explain the visual disturbances seen in preeclampsia. Other CNS abnormalities include headaches and visual disturbances such as scotomata, photophobia, blurred vision, and, rarely, temporary blindness.
Fetus and Placenta
The hallmark placental lesion in preeclampsia is acute atherosclerosis of the decidual arteries. This is caused in part by the abnormal adaptation of the spiral artery/cytotrophoblast interface and results in poor perfusion. This may lead to poor placental perfusion, resulting in oligohydramnios, intrauterine growth restriction, placental abruption fetal distress, and, ultimately, fetal demise.
Prediction
Prevention of any disease process requires knowledge of its etiology and pathogenesis, as well as the availability of methods to predict or identify those at high risk for this disorder. Numerous clinical, biophysical, and biochemical tests have been proposed for the prediction or early detection of preeclampsia. Unfortunately, most of these tests have poor sensitivity and poor positive predictive values, and most of them are not suitable for routine use in clinical practice.15 The reported differences in the predictive values of these tests may be attributed to one or more of the following: populations studied, definitions and prevalence of the disorder, and techniques and methodology used in performing these tests. As a result, there is no single screening test that is considered reliable and cost-effective for predicting preeclampsia.15
Given that nulliparity has a 5% to 7% risk of preeclampsia and multiparity has only a 3% risk, an accurate and thorough maternal history with identification of risk factors is the most cost-effective screening method available.
Maternal and Perinatal Outcome
Maternal and perinatal outcomes in preeclampsia are usually dependent on one or more of the following: gestational age at onset of preeclampsia as well as at time of delivery, the severity of the disease process, the presence of a multifetal gestation, and the presence of preexisting medical conditions such as pregestational diabetes, renal disease, or thrombophilias. In women with mild preeclampsia, the perinatal death rate, rates of preterm delivery, small-for-gestational-age (SGA) infants, and abruptio placentae are similar to those of normotensive pregnancies6,8,9 (Table 4). The rate of eclampsia is less than 1%, but the rate of cesarean section is increased because of increased rates of induction of labor.6,8,9
Table 4. Pregnancy Outcome in Women With Mild and Severe Preeclampsia
| Hauth et al6 | Buchbinder et al8 | Hnat et al9 | ||||
| Mild n = 217 | Severe n = 109 | Mild n = 62 | Severe n = 45 | Mild n = 86 | Severe n = 70 | |
| Delivery <37 wk (%) | N/R | N/R | 25.8 | 66.7 | 14.0 | 33.0 |
| <35 wk (%) | 01.9* | 18.5* | 09.7 | 35.6 | 02.3 | 18.6 |
| SGA infant (%) | 10.20 | 18.50 | 04.8 | 11.4 | N/R | N/R |
| Abruptio placentae (%) | 0.5 | 3.7 | 03.2 | 06.7 | 0 | 01.4 |
| Perinatal death (%) | 1.0 | 1.8 | 0 | 08.9 | 0 | 01.4 |
SGA = Small for gestational age; N/R = not reported.
*These rates are delivery <34 weeks.
This study included women with previous preeclampsia. The other studies included only nulliparous women.(From Sibai BM: Obstet Gynecol 102:181–192, 2003.)
In contrast, perinatal mortality and morbidities rates and the rates of abruptio placentae are substantially increased in women with severe preeclampsia (see Table 4).16 The rate of neonatal complications is markedly increased in those with severe preeclampsia that develops in the second trimester, whereas it is minimal in those with severe preeclampsia beyond 35 weeks' gestation.
Severe preeclampsia is also associated with increased risk of maternal mortality (.2%) and increased rates of maternal morbidity (5%) such as convulsions, pulmonary edema, acute renal or liver failure, liver hemorrhage, disseminated intravascular coagulopathy (DIC), and stroke. These complications are usually seen in women with preeclampsia that develops before 32 weeks' gestation and in those with preexisting medical conditions.16
Mild Preeclampsia
Patients with preeclampsia should ideally be hospitalized at the time of diagnosis for evaluation of maternal and fetal conditions. A small percentage of these cases are associated with reduced uteroplacental blood flow. In addition, the mother is at slightly increased risk for the development of abruptio placentae or convulsions, particularly in cases remote from term. Thus, women with mild disease who have a favorable cervix at or near term should undergo induction of labor for delivery. The pregnancy should not continue past term (beyond 40 weeks' gestation), even if conditions for induction of labor are unfavorable, because the uteroplacental blood flow becomes suboptimal.
The optimal management of mild preeclampsia remote from term (less than 37 weeks' gestation) is controversial. In general, there is considerable disagreement regarding the need for hospitalization versus ambulatory management, the use of antihypertensive drugs, and the use of sedatives. A few studies have reported on the pregnancy outcome in women with mild pregnancy-induced hypertension (PIH) (without proteinuria) remote from term who were randomized to either bedrest at hospital or normal activity at home.17,18 These studies questioned the value of hospitalization and came to the conclusion that management at home is safe and cost-effective. By contrast, others reported that early and prolonged hospitalization for patients with mild hypertension remote from term had improved perinatal survival, reduced maternal morbidity, and was cost effective.19
There are numerous clinical reports (controlled and uncontrolled) describing the use of various drugs in an attempt to prolong gestation and improve perinatal outcome in women with mild preeclampsia remote from term. However, it is almost impossible to evaluate and to compare the results of these studies, because of the heterogeneous populations studied (all forms of hypertension), various parities, and absence of a control group in most of these studies. It is important to note that these drugs are used overseas and are rarely used in this country for this purpose. In addition, none of these studies has reported a better perinatal outcome than the respective outcome in studies using hospitalization alone.
There are few controlled studies comparing the use of beta blockers versus either placebo or no treatment in the management of mild preeclampsia remote from term.20,21,22,23,24 Perinatal outcome derived from these studies is summarized in Table 5. Two of the six studies reported lower neonatal morbidity rates in the treatment group, and the other four reported no differences in perinatal outcome between treatment and control patients. However, all studies reported a lower incidence of progression to severe hypertension in the treatment group. Of note, the sample was not adequate in any study to evaluate perinatal mortality. In addition, none of these studies showed a better perinatal outcome compared with studies that included hospitalization only for management of mild preeclampsia.
Table 5. Randomized Trial of Beta Blockers Versus Placebo
or No Treatment in Mild Preeclampsia
| No. of Patients | GA at Entry | GA at Delivery | Birth Weight (g) | No. SGA (%) | No. Neonatal Deaths (%) | ||
| Wichman21 | Metoprolol | 26 | 33.0 | 38.0 | 2700 | 0N/A---- | 0---00 |
| Placebo | 26 | 33.0 | 38.0 | 2962 | 0N/A---- | 1---00 | |
| Plouin23 | Oxprenolol | 78 | 28.0 | 38.4 | 3079 | 05 (7)0.1 | 2 (2.6) |
| No treatment | 77 | 28.2 | 38.1 | 3023 | 08 (11).0 | 3 (3.9) | |
| Rubin22 | Atenlol | 46 | 33.8 | 39.0 | 2961 | 07 (15).0 | 1 (2.2) |
| Placebo | 39 | 33.8 | 38.0 | 3017 | 07 (18).0 | 2 (5.1) | |
| Sibai20 | Labetalol | 92 | 32.6 | 35.4 | 2204 | 18 (19.1) | 1 (1.1) |
| No treatment | 94 | 32.4 | 35.5 | 2258 | 09 (9.3)- | 0---00 | |
| Pickles24 | Labetalol | 70 | 34.0 | 37.8 | 2948 | 10 (14).1 | 0---00 |
| Placebo | 74 | 34.2 | 37.5 | 2913 | 05 (7)0.1 | 0---00 |
GA = gestational age, SGA = small for gestational age
Recently, Sibai and colleagues25 (in a prospective, randomized trial) compared no therapy to the use of nifedipine in the management of mild preeclampsia remote from term. They found that nifedipine was effective in reducing maternal systolic and diastolic blood pressure in women with mild preeclampsia. However, this reduction in maternal blood pressure was not associated with a reduced number of antepartum hospital days in the nifedipine group. In addition, the use of nifedipine led to a lower incidence of delivery for severe hypertension (9%) as compared with the no therapy group (18%). However, such reduction in incidence of severe hypertension in the nifedipine group was not associated with a concomitant improved pregnancy prolongation when compared with the no therapy group. This was because of the fact that a larger percentage of patients in the nifedipine group (16%) were delivered for fetal reasons (abnormal antepartum testing and fetal growth retardation) as compared with the no therapy group (9%). Consequently, the two groups had similar gestational ages at the time of delivery and similar birth weights. In addition, the incidence of preterm births, SGA infants, and infants admitted to the special care nursery were similar in the two groups.
Our management plan at the University of Cincinnati for patients with mild preeclampsia is summarized in Figure 1. All patients with mild preeclampsia require maternal and fetal evaluation at the time of diagnosis. Subsequent management will depend on maternal and fetal findings as well as gestational age. If the patient has mild hypertension in the absence of significant proteinuria (<1000 mg in 24 hours) or maternal symptoms (headaches, visual disturbances, or epigastric pain), outpatient observation may be considered on a selective basis. This form of management is appropriate in a reliable patient only during the early stages of the disease and in the absence of fetal jeopardy (abnormal testing or abnormal fetal growth). These patients are instructed to have rest at home, daily urine dipstick measurements of proteinuria, and blood pressure monitoring (by self or by nurse). The patient is also instructed to keep fetal movement counts and to report any symptoms of impending eclampsia. The patient is then evaluated in the antepartum testing area for maternal and fetal well-being at least two times per week. If there is any evidence of disease progression and if acute severe hypertension or proteinuria develops, then prompt evaluation is indicated. If there are any signs of worsening maternal or fetal conditions at any time during outpatient management, then hospitalization or delivery is indicated. In addition, after 37 weeks' gestation, labor should be induced as soon as the cervix is favorable (Bishop score greater than 6).
|
Severe Preeclampsia
The clinical course of severe preeclampsia may be characterized in some patients by progressive deterioration in both maternal and fetal conditions. Pregnancies complicated by severe preeclampsia are associated with increased rates of perinatal mortality and increased risks of maternal morbidity and mortality. As a result, there is universal agreement that all such patients should be delivered if the disease develops after 34 weeks' gestation or before that time if there is evidence of maternal or fetal distress. There is also agreement on delivery of such patients before 35 weeks' gestation in the presence of any of the following: premature rupture of membranes, labor, or severe fetal growth retardation (less than fifth percentile for age). In this situation, appropriate management consists of parenteral medications to prevent convulsions, control of maternal blood pressure within a safe range, and then induction of labor to achieve delivery. If delivery of a preterm infant (less than 36 weeks' gestation) is anticipated at a level 1 or level 2 hospital, the mother should be transferred to a tertiary care center with adequate neonatal intensive facilities.
There is considerable disagreement about management of patients with severe disease before 34 weeks' gestation. Some authors consider delivery as the definitive therapy for all cases, regardless of gestational age, whereas others recommend prolonging pregnancy in all severe preeclamptic gestations remote from term until either development of fetal lung maturity, fetal or maternal distress, or gestational age of 36 weeks is achieved. Some of the measures used in these cases have included one or more of the following: antihypertensive agents, diuretics, sedatives, chronic parenteral magnesium sulfate, plasma volume expanders, and antithrombotic agents. It is important to note that such measurement requires intensive monitoring of both maternal and fetal well-being on a daily basis. Unfortunately, these studies were retrospective and uncontrolled except for three recent studies described subsequently.
In the first recent study, the results of individualized management were reported in 58 women with severe preeclampsia at 28 to 34 weeks' gestation.26 These patients were treated initially with magnesium sulfate, hydrazine, and corticosteroids for fetal lung immaturity. All received intensive maternal and fetal evaluation in an obstetric ward for patients at high risk. Nonstress tests were performed at least three times daily and laboratory tests were evaluated at least twice weekly. Twenty of the 58 women were delivered because of maternal and/or fetal reasons within 48 hours after hospitalization. The remaining 38 were then randomized to either aggressive or expectant management (n = 20). Patients assigned to the aggressive management group received steroids and were delivered within 72 hours. Patients assigned to the expectant management group were treated with hydrazine to maintain blood pressure between 140/90 to 150/100 mmHg. In addition, they received frequent evaluation of maternal and fetal well-being. These patients were delivered at 34 weeks' gestation or before in the presence of maternal or fetal distress. The authors found fewer neonatal complications and fewer number of days spent in the neonatal intensive care unit in the expectant management group.
In a second study,27 the randomized clinical trial was conducted in which patients with severe preeclampsia between 26 weeks' and 36 weeks' gestation were assigned to be treated with either nifedipine (n = 24) or hydrazine (n = 25). Patients assigned to the nifedipine group received 10 mg to 30 mg sublingually initially, then 40 mg to 120 mg per day orally. Those assigned to the hydrazine group received 6.25 mg to 12.5 mg intravenous (IV) initially. Maternal evaluation included frequent measurements of blood pressure, heart rate, platelet indices, urine output, and laboratory tests. Fetal evaluation included daily fetal heart rate monitoring, biophysical profile three times per week, and weekly ultrasonographic assessment of fetal growth. The authors found better control of blood pressure and a lower incidence of fetal distress in the group managed with nifedipine. In addition, the group receiving nifedipine had a better perinatal outcome than those receiving hydrazine. They concluded that nifedipine is a safe and effective drug in the management of patients with severe preeclampsia remote from term.27
Sibai and coworkers28 studied 95 women with severe preeclampsia at 28 to 32 weeks' gestation who were randomly assigned to either aggressive management (AM) (n = 46) or expectant management (EM) (n = 49). The two groups were similar at the time of randomization with respect to several clinical and laboratory findings. The average pregnancy prolongation in the EM group was 15.4 ± 6.6 days (range: 4–36 days), which was significantly higher than the average of 2.6 days in the AM group (p < .0001; range: 2–3days). Indications for delivery in the EM group were maternal reasons (n = 16), fetal compromise (n = 13), attainment of 34 weeks' gestation (n = 10), preterm labor or rupture of membranes (n = 7), or vaginal bleeding (n = 3). The maternal indications for delivery were thrombocytopenia (n = 5), uncontrolled severe hypertension (n = 3), headache/blurred vision (n = 3), epigastric pain (n = 2), severe ascites (n = 1), and maternal demand (n = 2). Table 6 compares the pregnancy outcomes in the two groups. Gestational age at delivery, placental weight, and birth weight were significantly higher in the EM group. The two cases of abruptio placentae in the AM group were found at time of cesarean section, whereas the two cases in the EM group were suspected because of abnormal fetal heart rate testing and vaginal bleeding. There were no cases of eclampsia, pulmonary edema, renal failure, or disseminated coagulopathy in either group. No fetal or neonatal deaths occurred in either group. The number of infants admitted to neonatal care unit (37% vs 46%), average duration of stay in that unit (20% vs 37%), the frequency of respiratory distress syndrome (22% vs 50%), and necrotizing enterocolitis (0% vs 11%) were significantly lower in the EM group.
Table 6. Pregnancy Outcome in the Management of Severe Preeclampsia
| Aggressive Management (N = 46) | Expectant Management (N = 49) | Significance | |
| Gestational age at delivery (wk) | 30.8 ± 1.7 | 32.9 ± 1.5 | P <0.0001 |
| Placental weight (g) | 355 ± 88 | 435 ± 117 | P <0.01 |
| Birth weight (g) | 1233 ± 287 | 1622 ± 360 | P = 0.0004 |
| Cesarean section N (%) | 39 (85) | 36 (73) | NS |
| Abruptio placentae N (%) | 2 (4.3) | 2 (4.1) | NS |
| HELLP, N (%) | 1 (2.1) | 2 (4.1) | NS |
| Postpartum stay (days) | .3 ± 2.1 | 5.1 ± 1.9 | NS |
HELLP = hemolysis, elevated liver enzymes, and low platelets
(Sibai BM, Mercer MM, Schiff E, et al: Aggressive versus expectant management of severe preeclampsia at 28–32 weeks' gestation: A randomized controlled trial. Am J Obstet Gynecol 171:818, 1994)
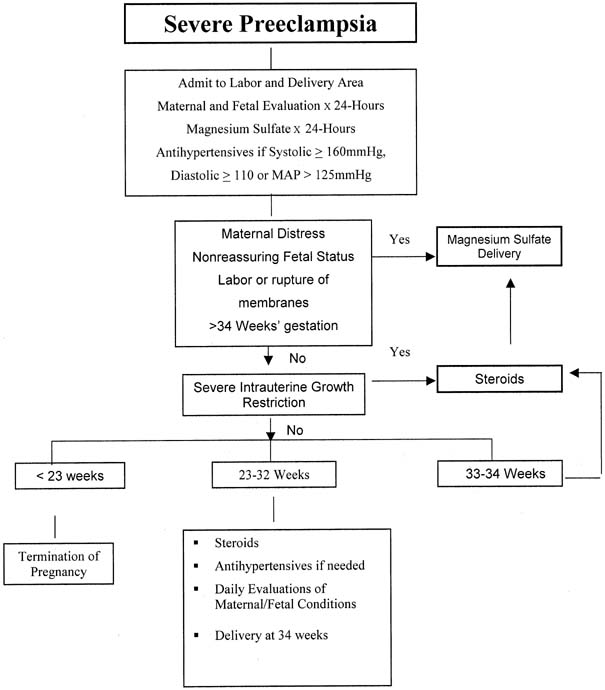
At the University of Cincinnati, patients with severe preeclampsia remote from term are admitted initially to the labor and delivery area for continuous evaluation of maternal and fetal conditions for at least 24 hours (Fig. 2). During observation, they receive continuous infusion of magnesium sulfate to prevent convulsions and IV doses of hydrazine (5 mg–10 mg) or oral nifedipine (10 mg) as needed to keep diastolic blood pressure below 110 mmHg. Maternal evaluation includes continuous monitoring of blood pressure, heart rate, urine output, cerebral status, and the presence of epigastric pain. Laboratory evaluation includes a platelet count and liver enzymes. Fetal evaluation includes continuous fetal heart monitoring, a biophysical profile, and ultrasonographic assessment of fetal growth. Patients with resistant severe hypertension or other signs of maternal or fetal deterioration are delivered within 48 hours, irrespective of gestational age or fetal lung maturity. In addition, patients in labor or those with fetuses with a gestational age older than 34 weeks and those with evidence of fetal lung maturity (by amniocentesis) at 33 to 34 weeks also are delivered within 24 hours. Patients at 33 to 34 weeks' gestation with immature lung studies receive steroids to accelerate fetal lung maturity and are delivered 24 hours after the last dose of steroids in the absence of any change in maternal or fetal condition. Patients at 28 to 32 weeks' gestation receive individualized management based on their clinical responses during the observation period. All of these patients receive steroids to accelerate fetal lung maturity. Some demonstrate marked diuresis and improvement in blood pressure during the observation period. If the blood pressure remains below 100 mmHg diastolic (without antihypertensive therapy) after the observation period, magnesium sulfate is discontinued, and the patients are followed-up closely in the ward for patients at high risk until fetal maturity is achieved. During hospitalization, they receive antihypertensive drugs (usually oral nifedipine 40–120 mg/day) to keep their diastolic blood pressure between 90 and 100 mmHg, with daily evaluation of maternal and fetal well-being. Steroids are used as indicated. In general, most of these patients will require delivery within 2 weeks. However, some patients may continue their pregnancies for several weeks. It is important to note that such pregnancies should be managed at tertiary care centers, because the course of pregnancy in such patients is very unpredictable.