In general, the bioeffects literature can be divided into two classes: one
based on the epidemiology of the large number of ultrasound examinations
performed both clinically and as laboratory experiments, and another
in which a causal relation between bioeffects and the applied acoustic
energy is developed. Both classes are discussed below. Effects of Ultrasound on Mammalian Tissues In Vivo Simple in vitro models, such as cells, are often used in the search for
biologic effects to gain an understanding of the possible mechanisms
of interaction between ultrasound and biologic tissue. The results of
the research on biologic effects in nonmammalian species, such as insects, amphibians, and
avians, indicate that studies on these relatively
simple organisms are helpful in understanding the mechanisms of interaction
between ultrasound and biologic systems.15 However, from the point of view of this work, studies of mammalian species
are of more relevance. The examples chosen for the present discussion
pertain mainly to in vivo insonation of mammalian tissue and include
exposure conditions at clinically relevant frequencies. Most of the
bioeffects studies were performed on small rodents, such as mice or
rats. Although these animals constitute a fairly inexpensive model for
in vivo bioeffects studies, the extrapolation of experimental results
to humans is not immediately obvious. A very comprehensive review of
the effects of ultrasound on mammalian development was prepared by Sikov.16 He evaluated bioeffects depending on gestational age and thus attempted
to extract information on the relation between exposure parameters and
stage of development at exposure. Because this approach seems useful for drawing some conclusions about the
adverse effects of diagnostic ultrasound in prenatal practice, it was
adapted in the following discussion of bioeffects findings. EARLY STAGE. Several researchers studied the influence of ultrasound exposure in the
period before implantation. In the CW studies, Takeuchi and co-workers40 used pregnant rats exposed to a 2.5-MHz ultrasound field on the second
and third day of gestation, at spatial average intensities of 150 mW/cm2. No increase in prenatal mortality was found. Similarly, no increase in
the rate of postnatal malformation was found after 20-minute exposures. These
results are not surprising in view of the fact that absorption
in the embryonic tissue is lower than that of the surrounding tissue. Therefore, it
is likely that more heat was generated in the vicinity
of the embryo than in the embryo tissue itself. Stolzenberg and associates41 exposed pregnant mice to 2-MHz of CW ultrasound in the first 3 days of
gestation. A 1-inch (about 25 mm) planar source transducer was used, and
the spatial average intensity was determined to be 1 W/cm2. A decreased uninterrupted pregnancy rate was noted after exposure for 5 minutes
on the third day and after exposure for 200 seconds on day
zero. Also, a reduction in fetal weight after delivery was observed at
thresholds corresponding to exposure for 100 and 200 seconds on day zero
and 200 and 400 seconds on the first day. In another series of studies,42 ultrasound exposure led to damage of maternal tissue, which was reflected
in increased mortality, decreased weight gain, and paralysis of the
pups. Thus, it appears that in vivo exposure to CW ultrasound at spatial average
intensities below 1 W/cm2 does not affect embryos at the early stage of gestation. However, limited
data suggest that levels of ultrasound of 1 W/cm2 may lead to undesirable changes in maternal tissue. The application of ultrasound in guided oocyte aspiration for in vitro
fertilization and embryo transfer is rapidly growing. Therefore, the recent
studies aimed at determining the interaction between ultrasound
exposure and successful fertilization are included in this discussion.43,44 In general, the clinically available data on ultrasound exposure of oocytes
during meiosis are confusing. Although some researchers reported
a deleterious effect on the fertility of patients undergoing artificial
insemination (they claimed a reduction in the cumulative rate of pregnancy),45 others claimed an increase in the success rate, allowing ultrasound monitoring
of follicular growth.46 An attempt to clarify this ambiguity was described by Mahadevan and colleagues.47 Their study sought to determine how oocytes obtained under ultrasound
guidance affected the pregnancy rate. The results obtained at 3.5 MHz
suggest that exposure of human oocytes to ultrasonic waves during the
different phases of meiosis does not significantly influence the developmental
potential of the in vitro fertilized embryos. Unfortunately, except
for ultrasound frequency, these researchers did not give any of
the relevant exposure parameters discussed earlier. ORGANOGENESIS. McClain and associates48 exposed rats to 10 mW/cm2 CW Doppler ultrasound for up to 2 hours at frequencies of 2.25 and 2.5 MHz. The
fetuses were examined on day 20, and no consistent increase
in mortality was observed, nor did the authors detect any other abnormalities. Stolzenberg and co-workers41 reported a decrease in the uninterrupted pregnancy rate of mice exposed
between days 6 and 8 to a spatial average intensity of 1 W/cm2. They reported statistically significant fetal weight reductions for exposures
longer than 140 seconds. More evidence of the possibility of
ultrasonically producing embryolethal effects during organogenesis has
been described.49,50,51,52 Sikov and colleagues49,50,51,52 exposed an exteriorized rat uterus to three frequencies (0.8, 2, and 3.2 MHz) at
day 9 and evaluated the offspring at day 20. The exposure was
performed at different intensity levels, with exposure times at 5 or 15 minutes. No
effect on fetal weight was observed, even at spatial
average intensities as high as 30 W/cm2, but prenatal mortality at 15 to 20 W/cm2 (spatial average) clearly increased with increasing exposure time. The
cause of this was ascribed to a thermal mechanism. FETAL BONE HEATING. Several researchers have investigated exposure conditions in which the
ultrasound energy was impinging on a bone embedded in tissue with relatively
little absorption or attenuation. This situation is particularly
relevant in obstetrics, because the developing fetus is exposed to
diagnostic ultrasound. Carstensen and associates53 insonified exposed mouse skulls that had thermocouples implanted. Both
adult and young mice were used. The results were reproducible, with the
older mice yielding a steady temperature rise of 5.6 ± 0.3°C
with a focal intensity of 1.5 W/cm2. In the first 15 seconds of ultrasound application, the temperature rose
to about 75% of the final value. Comparison of these results with the
theoretic predictions obtained from an appropriate tissue model18 indicated an agreement to within 20%; however, the theoretic transient
response indicated the possibility of a considerably faster rate of temperature
increase-the 75% was predicted to be achieved within 5 seconds
of exposure. These results indicate that there may be a reason for
concern, especially with the rate of temperature increase. Also, at this
rate of heating, the exposure time should be limited to about 1 minute.18 If the temperature increase had been, for example, 7°C, the exposure
time necessary for a bioeffect to occur would have been 15 seconds. It
cannot be ruled out that during an actual examination, the acoustic
beam might be held stationary for such a period. POSTNATAL DEVELOPMENT. Equally important in determining the safety of diagnostic ultrasound is
knowing the influence of prenatal ultrasound exposure on postnatal development. In
a carefully devised study,54 pregnant rodents were exposed to 500 mW/cm2 spatial average intensity at 2-MHz ultrasound for 1 to 3 minutes, or to 1 W/cm2 spatial average intensity for 40 to 60 seconds. No fetal mortality was
observed; however, a weight decrease in the pups delivered was observed
after exposure to 0.5 W/cm2 for 180 seconds. In another study, the effects of prenatal ultrasound exposure on adult
offspring behavior in the Wistar rat were investigated.55 The animals were exposed on days 15 through 17 and on 19 of gestation
to a 5-MHz, 1-kHz pulse repetition rate and intensities (ISpTp) of 500 and 1500 W/cm2. The total exposure time was 35 minutes. Two hundred seventy-eight offspring
were subjected on postnatal day 60 to two of four well-established
behavioral tests. The animals were tested in random order within sexes. The
results showed no consistent dose-related alterations in adult
behavior due to prenatal fetal exposure. Vorhees and co-workers56 used a unique approach to study the possible teratogenic effects of ultrasound
exposure. They eliminated the need for anesthesia (and therefore
the possible complications caused by forced restraint) by training
the rats to remain immobile during the ultrasound exposure. The animals
were exposed to 0.1, 2, or 30.0 W/cm2 (ISpTa), 3 MHz CW, for about 15 min/day on days 4 through 19 of gestation. No
dose-dependent changes in various maternal parameters, the incidence
of malformation, or fetal weight were observed. In a follow-up study, Vorhees
and colleagues57 exposed rats to these levels of ultrasound for 10 minutes on gestational
days 4 through 20. Although they did not observe exposure-related alterations
in maternal parameters, offspring survival and growth, or neonatal
psychophysiologic parameters, changes did occur in offspring adult
behavior, in locomotor activity, and in two measures of the multiple-T
water maze test performance at the highest dosage level. In the studies aimed at determining the effects of ultrasound exposure
on uteroplacental function, ter Haar and co-workers58 found that a spatial peak intensity of 2 W/cm2 at 3 MHz caused an exteriorized uterus to increase in frequency of contraction, which
lasted for about 4 minutes after the ultrasound was turned
off. They reported no increase in temperature and thus ascribed the
increased rate of contractions to an unspecified nonthermal mechanism. Placental function during ultrasound exposure was investigated by Kelman
and associates.59,60,61 In their experiments they insonified the placenta of a guinea pig with 2.5 MHz
of ultrasound at different intensity levels and in both pulsed
and CW modes. Functional changes were observed after 10 minutes of exposure
at SpTp intensities of 30 W/cm2 in pulsed mode (pulse length, 10 μs; PRR, 1 kHz) and SpTa intensities
of 28 to 46 W/cm2 in the CW mode. Again, it appears that at sufficiently high intensity levels, ultrasound
can affect vitelline blood flow and lead to increased uterine contractility
and changes in placental function. However, the exposure levels
far exceed those present in the clinical environment. Murrils and associates62 found that fetal Doppler monitoring did not influence fetal activity, which
would be an indication of ultrasound effects on the perinatal nervous
system. Bioeffects Related to Hyperthermia The evidence above indicates that there is a teratogenic effect of heat
on the prenatal development of mammalian tissues. Experiments with cultured
rat embryos stressed the importance of ambient temperature; no
bioeffects were observed when embryos were exposed to pulsed ultrasound
for 15 minutes at ISpTa levels of 1.2 W/cm2.63 However, the same experiment repeated with the temperature elevated 1.5°C
above normal (40°C) resulted in retardation of head growth. Although
the outcome of this experiment cannot immediately be extrapolated
to humans, it indicates that ultrasound exposure of a febrile pregnant
patient under certain conditions might constitute an increased
risk for potential bioeffects. There are relatively few papers containing diagnostically relevant information
on this subject; therefore, it is appropriate to draw attention
here to a recent survey.64 This survey established that no thermal bioeffects were observed at temperature
elevations of 39°C, regardless of how long the ultrasound
exposure lasts. However, for each increasing degree of temperature elevation, to
stay within safety limits, the duration of ultrasound examination
must be reduced by a factor of four. More specifically, the review
indicated that the maximum safe duration for a temperature of 43°C
is 1 minute, and for 42°C it is 4 minutes. Similarly, at 41°C
the exposure time may be increased to 16 minutes, and at 40°C
the duration of examination may be as long as 64 minutes. Based on
the data available, the survey concluded that if the maximum temperature
rise during the ultrasound exposure is kept less than 2°C, any
biologic effect (in a febrile patient) is highly unlikely. It is possible
to express the relation between the exposure time required for a bioeffect
to occur and the associated TI, namely, t = 4 < (6 - TI). Thus, for
a TI of 6, the exposure time should be limited to 1 minute. Similarly, a
TI of 4 means that the exposure time should be less than 16 minutes (see
Appendix). Recent findings indicating that the ultrasound imaging transducer may act
as a substantial heat source (Duck FA, unpublished observations) are
also of interest. The temperature at a clinically operated Doppler transducer
was reported to increase by 10°C when the Doppler was applied
to skin with a standard coupling gel. Although tissue heating from
the transducer is most likely limited to the tissue volume in the immediate
vicinity of the transducer, this effect should be kept in mind
for ultrasound examinations in which an endocavity (e.g., endovaginal) transducer is used, or when performing contact scanning
of the neonatal brain or in ophthalmologic applications. Bioeffects Related to Cavitation A few relevant papers reporting lung hemorrhage in mice and in monkeys
caused by cavitation-like phenomena have already been discussed under
Cavitational Mechanism.28,29 These papers describe significant biologic effects associated with gas
body activation. The effects were produced in vivo in mammalian lung
tissue in small rodents (mice) and in primates (monkeys). The bioeffects
were observed at pressure amplitudes and pulse durations representative
of those currently encountered at the output of diagnostic ultrasound
equipment, particularly when used in pulsed Doppler mode. As previously
noted, the results described in those papers are important for
the present discussion because they led to a modification of the AIUM
statement on “In Vivo Mammalian Bioeffects.” The statement, which
was renamed “Non-Human Mammalian In Vivo Biological Effects,” emphasizes
the importance of the MI.17 Recent data presented in the article by Dalecki and colleagues65 indicate that, in general, threshold pressures increase with increasing
frequency. The thresholds for hemorrhage in the intestine of adult mice
ranged from about 1 MPa at 1 MHz to 3 MPa at 3 MHz (10-μs pulse
duration, 100-Hz repetition rate). To date there is no evidence that flowing cardiovascular blood will permit
cavitation in vivo. Although there is some indication that microscopic
gas bodies may exist in biologic tissue, there is no evidence that
exposure of these bodies to the ultrasound levels similar to those used
in clinical diagnostic practice will cause any significant bioeffects. However, it
is conceivable that vibration of those bodies may introduce
cell membrane stresses that can lead to extravasation of blood
in lung tissue.5 This may indicate a need for appropriate modification during cardiovascular
examinations and transesophageal echocardiography. The above findings indicate that the body regions containing gas volumes
are very sensitive to the potential side effects of ultrasound exposure
and that biologic effects can be introduced by using a commercially
available diagnostic imaging system in tissues containing gas volumes. Carefully
designed studies are needed to assess any potential hazards
with conditions that are typical for diagnostic ultrasound. Such hazards
may also be associated with ultrasonic contrast agents injected
into the vascular system. To summarize, the thresholds for confirmed cavitation-induced biologic
effects in mammalian tissues in the diagnostic frequency range of 2 to 8 MHz
are above 1 MPa.17 All of the biologic effects that have been confirmed under diagnostically
relevant exposure conditions involve tissues that are known to contain
microscopic gas bodies. For tissues not containing gas (such as the
kidney), no damage was observed at peak positive pressures on the order
of 10 MPa.30 Lung hemorrhage has been observed in neonatal and adult mice, rabbits, monkeys, and
neonatal swine with diagnostically relevant ultrasound pressures
ranging from 1 to 3 MPa. No damage was observed in the lungs
of adult swine at pressures on the order of 5 MPa (see Appendix).28,29 Epidemiologic Studies The evidence of ultrasonically induced bioeffects in humans is perhaps
the most important information from the clinician's point of view. As
pointed out by Ziskin and Petitti,2 “No matter how many laboratory experiments show a lack of effect
from diagnostic ultrasound, it will always be necessary to study directly
its effect in human populations before any definitive statement regarding
risk can be made.” There are extensive reviews of the literature on the epidemiology of human
exposure to ultrasound.1,3,6 As would be expected, the major thrust of these reviews is the assessment
of the possible side effects of ultrasound exposure in utero. This
is mainly because most pregnant women are exposed to ultrasound examination
in the early stages of pregnancy and because any harmful effects
to the fetus may last for years to come. In general, few data pertaining to human ultrasound exposure are available. Some
of the published data are reviewed briefly here. The experiments
of O'Brien,66 who observed that in utero exposure to ultrasound resulted in reduced
birthweight in mice, prompted similar studies in humans. Bakketeig and
associates,67 in their randomized, controlled trial, reported that they were unable
to find any effect of ultrasound on birthweight in humans. A similar conclusion
was drawn from the earlier studies on the relation between ultrasound
exposure and birthweight.68 Although no fetal structural anomalies were observed in the recent studies
performed by Stark and co-workers68 and Bakketeig and colleagues,67 Stark and associates reported somewhat disturbing findings in their retrospective
follow-up examinations of 425 children exposed to ultrasound
in utero and 381 unexposed children. They reported an increased risk
of dyslexia; however, their study suffers from the fact that the subjects
were not selected at random. No evidence has been found that ultrasound
exposure in utero may lead to an increased rate of childhood cancer.69,70 Likewise, no relation between in utero exposure and children with hearing
deficiencies has been determined.68 Deficiencies of the Bioeffects Studies The data presented earlier indicate that the exposure of pregnant rodents
to CW ultrasound at sufficiently high intensities during organogenesis
can lead to undesirable biologic changes in the developing fetus. Also, recent
evidence has emerged indicating that the body regions containing
gas volumes may be particularly prone to potential side effects
when exposed to sufficiently high pressure amplitudes.28,29 Although the data were carefully selected to include only those studies
that can be readily related to diagnostically relevant exposure conditions, it
should be stressed that in most cases the bioeffects were observed
at exposure conditions that were different from those typical
of clinical diagnostic practice. Before 1990, most of the bioeffects observed
were ascribed to the thermal mechanism of interaction between
the ultrasound and biologic tissue, but in the past half decade it has
been demonstrated that exposure to ultrasound produced by commercially
available diagnostic imaging equipment can produce nonthermal, cavitation-like
bioeffects in the lung tissue of primates.29 Many bioeffects studies describe the effects of whole-body heating, which
most likely acts through a mechanism that is not immediately comparable
to ultrasound-induced hyperthermia. Few studies have been specifically
designed to determine threshold values for abnormal embryonic or
fetal development.64 On the basis of the thermal criterion, a diagnostic exposure that produces
a maximum temperature rise of 1.5°C above normal physiologic
levels can be used without reservation in clinical examinations (see Appendix). However, a
diagnostic exposure that produces an in situ temperature
elevation to or above 41°C for 15 minutes should be considered
hazardous to embryonic or fetal development. Any increase in the
tissue temperature will act as a potentiating factor. Therefore, ultrasound
examination in a febrile patient may constitute an additional embryonic
and fetal risk.5 The reviews on human exposure clearly indicate that epidemiologic studies
and surveys yield no evidence of adverse effects from diagnostic ultrasound, but
they also point out several serious deficiencies of all
the data available. These deficiencies are carefully discussed by Ziskin
and Petitti.2 Ziskin and Petitti point out that the available results of epidemiologic
studies are based on about 500,000 patient examinations that were collected
from 179 users of clinical ultrasound. However, the results allow
only a fairly cautious statement to be made, namely, that the users
overwhelmingly believe that their experience with diagnostic ultrasound
had been safe. Ziskin and Petitti also note that all clinical studies on the possible
harmful effects of ultrasound exposure to the fetus produced negative
results. However, the acoustic outputs of the diagnostic instruments used
were generally not known. In addition, the sample sizes (the number
of patients included in the studies) were limited to a few hundred. In
their careful assessment of the limitations of the epidemiologic studies, Ziskin
and Petitti focus on the lack of information or simply inadequate
ultrasound dosimetry. Also, the reasons for the examinations
were not clearly stated, and sparse information is available on the number
of examinations and the gestational age at the time of exposure. In
addition, most studies were performed with pulsed ultrasound. As shown
in Table 1, Doppler ultrasound results, in general, in higher values of ISpTa. Ziskin and Petitti suggest that the existing data on exposure of the
fetus in utero are inadequate to conclude that diagnostic ultrasound is
safe at all combinations of dose and period of exposure for both imaging
and Doppler devices. Another point addressed by these researchers is the importance of statistical
considerations, including sample size and statistical level of
significance. Because a more thorough treatment of statistics is beyond
the scope of this chapter, it can be simply stated that the larger the
sample, the higher the statistical level of significance that can be
achieved. The usefulness of small samples is extremely limited; studies
that use small samples usually have inconclusive results. In summary, the human studies that have been performed do not preclude
the possibility that adverse effects may be found under certain conditions. The
limited data available indicate that no relation has been found
between prenatal exposure to ultrasound and subsequent postnatal changes
in children, but statistical considerations show that minor chemical
and behavioral changes, long-term delayed effects, and certain genetic
effects could easily escape detection.2 There is still a need for a well-designed, randomized clinical study addressing
the risk for the fetus exposed in utero. | 
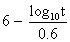
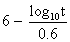
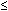 2°C.
2°C.