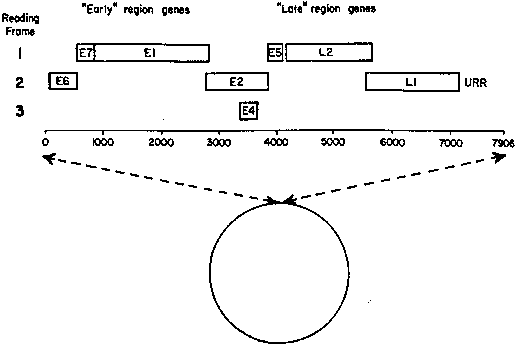
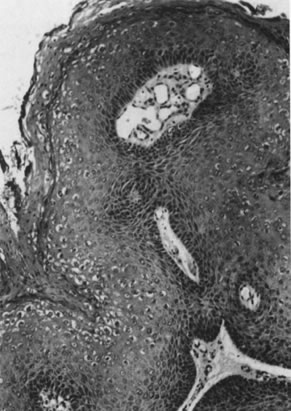
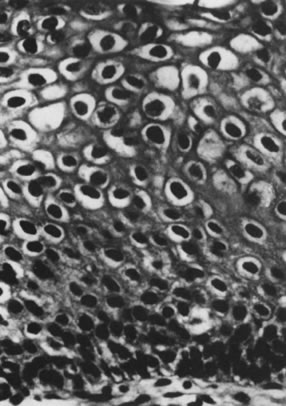
Infection by most sexually transmitted HPV types occurs throughout the
lower female genital tract, and multiple sites are commonly involved at
the same time. Initially, the total area of infected epithelium greatly
exceeds the area displaying lesions. The manifestation of the HPV
infection is influenced by cofactors, as previously discussed, and by
the viral subtype, involved organ, immune system, and local tissue factors. Viral Subtype According to their ability to induce cancers, HPV types often are grouped
as low-risk types (HPV-6 and -11), medium- or intermediate-risk types (HPV33, -35, -39, -40, -43, -45, -51, -46, and -58), and high-risk
types (HPV-16, -18, and -31). Intermediate- and high-risk viruses also
are referred to as potentially oncogenic types. High-risk viruses commonly
are isolated from high-grade dysplasias or condylomatous lesions
with atypical mitoses. Up to 35% of bland-appearing condylomas, however, may
contain potentially oncogenic viruses.69 Involved Organ The HPV-6 and HPV-11 types cause exophytic condylomas (genital warts, condylomata
acuminata) mostly on the vulva, vestibule, or perineum, and
rarely on the cervix. The same viral types also may be associated with
clinically inapparent or subclinical disease, which requires special
methods for diagnosis, such as colposcopy. The cervical transformation
zone appears particularly vulnerable to oncogenic viruses. Therefore, the
most severe effects of high-risk infections generally occur on the
cervix. Immune System An intact immune system prevents most HPV infections from becoming clinically
evident. In immunosuppressed patients, by contrast, HPV virtually
always produces recognizable epithelial proliferations, cellular atypia, or
neoplasia. Pregnancy is a state of relative immunosuppression, permitting
a higher replication rate of viral DNA with enhanced lesion
formation and growth. Local Tissue Factors Epithelial abnormalities induced by HPV tend to be localized, although
surrounding epithelium also is infected and may exhibit inconspicuous
epithelial changes. Localized disease, therefore, may represent a focal
breakdown of host control within a field of diffuse HPV infection.57 CERVIX. On the cervix, the classic condylomata acuminata associated with HPV-6 and
HPV-11 are uncommon and, when present, often are small and best assessed
using the colposcope after application of 5% acetic acid. Most
HPV-associated lesions are subclinical and present a large variety of
colposcopic findings. In some patients, islands of acetowhite epithelium (satellite
lesions) are found in the periphery of the cervix outside
the transformation zone (Fig. 8). 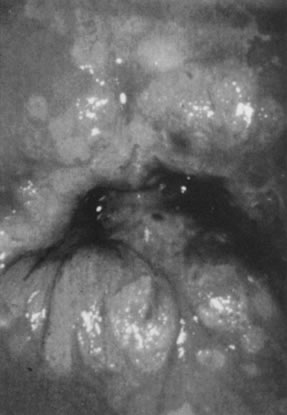 Fig. 8. Colpophotograph of flat condyloma of the cervix. Acetowhite epithelium
covers much of the ectocervix. Satellite lesions are seen in the periphery
of the lesion and outside the transformation zone. Fig. 8. Colpophotograph of flat condyloma of the cervix. Acetowhite epithelium
covers much of the ectocervix. Satellite lesions are seen in the periphery
of the lesion and outside the transformation zone.
|
The surface may appear micropapillary (spiked), flat, or sometimes microconvoluted
to a brainlike epithelial arrangement (Fig. 9). Many of the flat lesions have a pure, shiny-white color reminiscent
of pearls, in contrast to the full, oyster-white color of high-grade cervical
intraepithelial neoplasia (Figs. 10 and 11). Some HPV infections produce coarse capillary loops with a horizontal
or vertical orientation, giving the appearance of a mosaic or punctation. The
regular spacing of the vessels helps to differentiate these arrangements
from invasive carcinomas.73,74 Histologically, the lesions contain koilocytotic and parakeratotic changes
in the upper layers of the infected epithelium (Fig. 12). 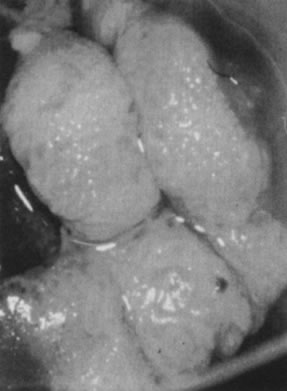 Fig. 9. Colpophotograph of a spiked condyloma on the ectocervix. Myriad tiny excrescences
cause the surface of the lesion to be irregular. Fig. 9. Colpophotograph of a spiked condyloma on the ectocervix. Myriad tiny excrescences
cause the surface of the lesion to be irregular.
|
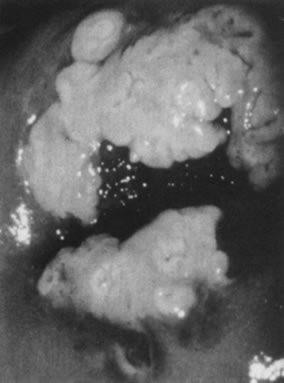 Fig. 10. Colpophotograph of a flat condyloma on the ectocervix. The surface is smooth
and has a shiny white color reminiscent of pears. Fig. 10. Colpophotograph of a flat condyloma on the ectocervix. The surface is smooth
and has a shiny white color reminiscent of pears.
|
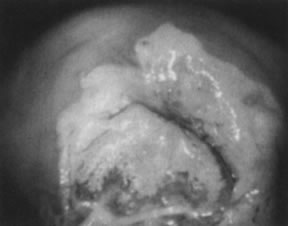 Fig. 11. Colpophotograph of high-grade cervical intraepithelial neoplasia on the
ectocervix. The acetowhite epithelium has a dull, oyster-white color. Fig. 11. Colpophotograph of high-grade cervical intraepithelial neoplasia on the
ectocervix. The acetowhite epithelium has a dull, oyster-white color.
|
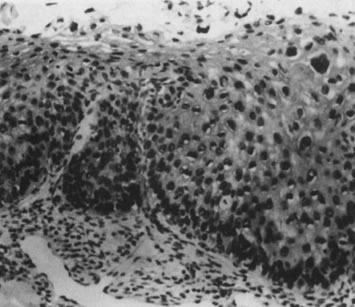 Fig. 12. Cervical intraepithelial neoplasia grade 1. Nuclear atypia, increased mitoses, nuclear
crowding, and occasional binucleated forms are seen on
the parabasal cell layers. Koilocytotic changes are prominent in the
upper cell layers. (Hematoxylin-eosin, ×500.) Fig. 12. Cervical intraepithelial neoplasia grade 1. Nuclear atypia, increased mitoses, nuclear
crowding, and occasional binucleated forms are seen on
the parabasal cell layers. Koilocytotic changes are prominent in the
upper cell layers. (Hematoxylin-eosin, ×500.)
|
VAGINA. Vaginal condylomata acuminata can be detected in one third of women with
vulvar condylomata by careful examination. The distribution usually
is patchy, with the proximal and distal thirds of the vagina being affected
most commonly. Subclinical vaginal HPV-associated lesions include
minute papillary epithelial projections (asperities), each containing
a central capillary (Fig. 13). The projections may be isolated, in clusters, or diffuse, covering large
areas of the vagina (micropapillomatosis vaginalis). These lesions
most commonly are associated with HPV-6 and HPV-11. Acetowhite epithelium, by
contrast, is more commonly found in infections due to potentially
oncogenic HPV types, most notably HPV-16. Sometimes, minute capillaries
are evident, giving rise to patterns reminiscent of punctation
and a mosaic pattern on the cervix. Histologically, vaginal intraepithelial
neoplasia often is present.75 A common subclinical finding of HPV infection is a pattern called reverse
punctation. It consists of a myriad of slightly raised, tiny acetowhite
dots, which are pronounced during pregnancy and may involve large
areas of the vagina (Fig. 14). Acetic acid may accentuate the changes, but there usually is no sharp
demarcation to normal epithelium. It is questionable whether this pattern
is due to HPV infection or simply represents a nonspecific response
to infectious, hormonal, or other stimuli. If HPV positive, HPV-6 and
HPV-11 are isolated from these lesions. Histologic findings are characterized
by mild and often focal koilocytosis, variable dyskeratosis, and
prominent intraepithelial capillaries. 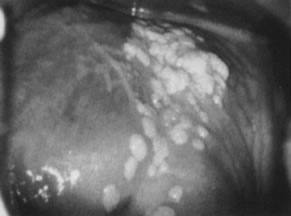 Fig. 13. Colpophotograph of vaginal condylomata. Multiple acetowhite papular lesions
crowd the vaginal fornices. Minute papillary epithelial projections (asperities) are
evident. Fig. 13. Colpophotograph of vaginal condylomata. Multiple acetowhite papular lesions
crowd the vaginal fornices. Minute papillary epithelial projections (asperities) are
evident.
|
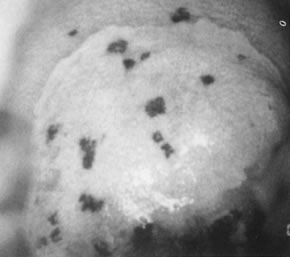 Fig. 14. Colpophotograph of reverse punctation on cervix and vagina in a pregnant
woman. Note the acetowhitening of the cervical portion of the epithelium. Fig. 14. Colpophotograph of reverse punctation on cervix and vagina in a pregnant
woman. Note the acetowhitening of the cervical portion of the epithelium.
|
VULVA. Condylomata acuminata in the vulvar area most commonly involve the perineum, the
posterior portion of the vestibule, and the labia minora; less
commonly involved are the labia majora, the clitoris, and the mons
pubis. Lesions on moist, mucosal surfaces tend to be pink, vascular tumors
with finger-like projections (Fig. 15). On keratinized skin, the condylomas are often white or dark because
of keratin and pigment formation (Fig. 16). The lesions have a typical histologic appearance of a papillary growth
with marked acanthosis, koilocytosis and hyperkeratosis, or parakeratosis. Condylomata
acuminata most commonly are associated with HPV-6 and
HPV-11. 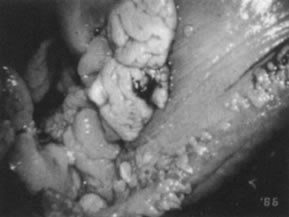 Fig. 15. Colpophotograph of condylomata acuminata of the vulva. The condylomata
near the hymenal ring ( top left )are microconvoluted to a brainlike epithelial arrangement. Condylomata
near the outside of the vestibule have an irregular papillary appearance. Fig. 15. Colpophotograph of condylomata acuminata of the vulva. The condylomata
near the hymenal ring ( top left )are microconvoluted to a brainlike epithelial arrangement. Condylomata
near the outside of the vestibule have an irregular papillary appearance.
|
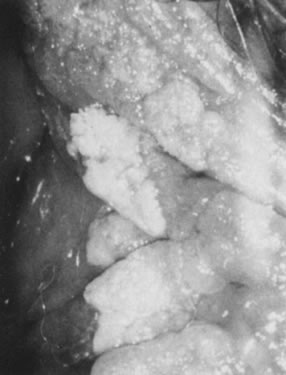 Fig. 16. Colpophotograph of condylomata on the inner aspect of the right labium
majus. Some lesions are white because of keratin formation. Fig. 16. Colpophotograph of condylomata on the inner aspect of the right labium
majus. Some lesions are white because of keratin formation.
|
Potentially oncogenic HPV types, especially HPV-16, also may give rise
to grossly visible lesions presenting as multiple, darkly pigmented, sometimes
white, pale, or fleshy papules, formerly often referred to as
bowenoid papulosis (Fig. 17). Histologic examination of the papules usually reveals vulvar intraepithelial
neoplasia (Fig. 18). 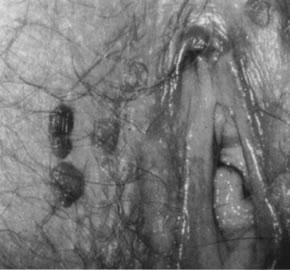 Fig. 17. Colpophotograph of several darkly pigmented papular vulvar lesions. Biopsy
showed vulvar intraepithelial neoplasia, grade 3 (see Fig. 18). Fig. 17. Colpophotograph of several darkly pigmented papular vulvar lesions. Biopsy
showed vulvar intraepithelial neoplasia, grade 3 (see Fig. 18).
|
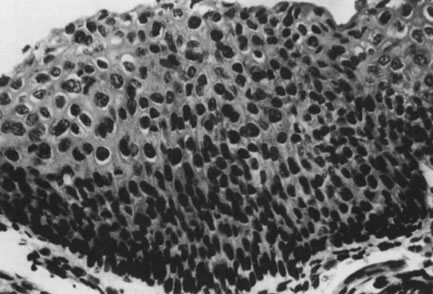 Fig. 18. intraepithelial neoplasia, grade 3. The entire thick- ness of the epithelium
is replaced by atypical cells. The nuclei are enlarged but maintain
some polarity. Increased cellularity and multiple mitoses are evident. (Hematoxylin-eosin, ×500.) Fig. 18. intraepithelial neoplasia, grade 3. The entire thick- ness of the epithelium
is replaced by atypical cells. The nuclei are enlarged but maintain
some polarity. Increased cellularity and multiple mitoses are evident. (Hematoxylin-eosin, ×500.)
|
Other HPV-associated lesions of the vulva consist of papillary changes
commonly visible with the naked eye but best appreciated colposcopically. The
papillae are multiple, small villous projections from mucous membranes
that may involve the entire vestibule and inner surface of the
labia minora. When extensive, the papillary changes are referred to
as micropapillomatosis vestibularis or labialis (Fig. 19). Some women with vestibular papillae have intense vulvar pain (vulvodynia), burning, irritation, and pruritus. Although the papillary formations
have been associated with various HPV types, especially HPV-6, they
frequently represent anatomic variants of vestibular mucosa.76 It is unlikely that HPV infections are responsible for vulvodynia, but
overly aggressive therapy directed against vulvar or vaginal HPV-associated
lesions may result in damage to the vulvar epithelium and chronic
vulvar pain. 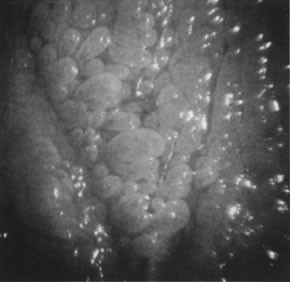 Fig. 19. Colpomicrograph of micropapillomatosis vestibularis. The entire vestibule
is covered with myriad small villous projections. The epithelium tested
negative for HPV. Fig. 19. Colpomicrograph of micropapillomatosis vestibularis. The entire vestibule
is covered with myriad small villous projections. The epithelium tested
negative for HPV.
|
Histologically, isolated papillary fronds are observed, with prominent
fibrovascular cores associated with chronic inflammation and dilated capillaries. Koilocytotic
transformation of superficial epithelial cells
is variable. Acetowhitening of the vulvar epithelium occurs most commonly within mucosal
surfaces but is not confined to it (Fig. 20). Although it often represents a nonspecific epithelial reaction to chemical, mechanical, and
infectious irritants, acetowhite epithelium frequently
is associated with HPV-16 and HPV-18, particularly when it is
multifocal and when significant epithelial atypia (vulvar intraepithelial
neoplasia) is present. 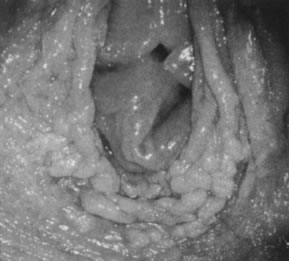 Fig. 20. Colpophotograph of acetowhitening of vulvar epithelium. Dots of acetowhite
epithelium surround the vaginal introitus in a horseshoe-shaped configuration. The
lesion tested positive for HPV-16. Histologically, vulvar
intraepithelial neoplasia, grade 1, was present. Fig. 20. Colpophotograph of acetowhitening of vulvar epithelium. Dots of acetowhite
epithelium surround the vaginal introitus in a horseshoe-shaped configuration. The
lesion tested positive for HPV-16. Histologically, vulvar
intraepithelial neoplasia, grade 1, was present.
|
|