Squamous cell carcinoma is the most common malignant tumor of the vulva, constituting
more than 90% of all vulvar malignancies and accounting
for 4% of all genital malignancies in women. The frequency of this disease
increases with age, and it is most common in women older than 75 years
in whom its incidence is 19.9 per 100,000 women.14 Epidemiologic studies support the hypothesis that vulvar carcinoma has
at least two origins: one HPV-related and one not. In younger patients, these
lesions are frequently HPV-related with VIN as an apparent precursor. The
VIN lesions are usually of the warty or basaloid type and
are associated with basaloid or warty types of squamous cell carcinoma. These
women are often heavy cigarette smokers as a group. Cigarette
smoking alone appears to increase the odds ratio. The other group of women
is older and does not appear to have HPV-related vulvar lesions, nor
does there appear to be an association with cigarette smoking in this
group. This older group of women may have associated vulvar dermatoses, usually
lichen sclerosus. Other risk factors for vulvar carcinoma
in all groups include carcinogen exposure, immunosuppression, chronic
granulomatous disease, and prior cervical or vaginal carcinoma. The
association between squamous cell carcinoma of the cervix or vagina and
squamous carcinoma of the vulva is well established. As in VIN lesions, HPV
infection is a common etiologic factor leading to multifocal genital
neoplasia.23 Obesity and poor perineal hygiene also are implicated in many cases. Pregnancy
and high parity do not appear to be associated. Clinical Manifestations Squamous carcinoma of the vulva usually appears as an ulcerated, exophytic, or
indurated mass that may be associated with pruritus, local infection, and
bleeding. Squamous cell carcinoma of the vulva is typically
divided into two categories: (1) superficially invasive squamous cell
carcinoma and (2) frankly invasive squamous cell carcinoma. VIN may
be found adjacent to invasive carcinoma in approximately 60% of cases
and is found more commonly among younger women and in those with more
superficially invasive tumors. Conversely, superficially invasive vulvar
carcinoma may be encountered in the treatment of VIN. Vulvar lichen
sclerosus coexists with carcinoma in approximately 15% to 40% of cases.14 Although squamous cell hyperplasia has been found at the edges of invading
tumor, it is most probable that the hyperplastic lesions seen immediately
adjacent to squamous cell carcinomas are induced by factors associated
with the carcinoma, or by the carcinoma itself, and may not
necessarily be a precursor lesion. The tissue adjacent to the tumor may
harbor VIN, lichen sclerosus, or nonspecific hyperplasia or may appear
normal.24 In most cases of vulvar carcinoma, the tumor occurs within a single focus, usually
within the clitoris, perineal body, or medial aspects of
the labia majora or minora. Squamous carcinoma of the vulva is generally slow growing and spreads by
extension to contiguous skin, vagina, and rectum. The lesion may also
invade deeper tissues and become fixed to the pelvic bones. Metastases
to lymph nodes involve the femoral and inguinal nodes before they progress
to the deep obturator, internal lilac, or periaortic group. When
nodal metastases occur, the tumor typically involves the ipsilateral
nodes and, in rare cases, the contralateral nodes. When the tumor is
midline, bilateral nodal involvement may occur.25 The current clinical staging system for vulvar carcinoma was defined in 1995 by
FIGO. The International TNM staging is also commonly used26 (Table 6). The system no longer relies on the clinical assessment of groin nodes, a
procedure that can be quite inaccurate, based on a comparison of
clinical findings with surgical and pathologic findings.27 Table 6. Vulvar Carcinoma Staging: Comparison of the AJCC and FIGO Nomenclatures, TNM
Stage Groupings, and Correlation With FIGO Staging
TNM | | | | FIGO |
Stage 0Tis | N0 | M0 | | |
Stage IT1 | N0 | M0 | | Stage I |
Stage IA | T1a | N0 | M0 | |
Stage IB | T1b | N0 | M0 | |
Stage II | T2 | N0 | M0 | Stage II |
Stage III | T1 | N1 | M0 | Stage III |
| T2 | N1 | M0 | |
| T3 | N0,N1 | M0 | |
Stage IVA | T1 | N2 | M0 | Stage IVA |
| T2 | N2 | M0 | |
| T3 | N2 | M0 | |
| T4 | Any N | M0 | |
Stage IVB | Any T | Any N | M1 | Stage IVB |
AJCC, American Joint Comission on Cancer.
FIGO, International Federation of Gynecology and Obstetrics.
The prognosis for vulvar carcinoma is primarily related to the size of
the original lesion and the presence or absence of nodal metastases. Patients
with tumors confined to the vulva with tumor-free groin nodes
have a corrected 5-year survival rate of 90% after adequate excision of
the tumor and usual ipsilateral inguinal-femoral lymphadenectomy. If
the tumor has spread to the groin nodes, the corrected 5-year survival
rate decreases to 65%. If the tumor has spread to involve the pelvic
nodes as well, the survival rate is less than 25%.14,25,28 Large squamous carcinomas of the vulva have been reported in association
with hypercalcemia, without bone metastasis.29 This is primarily mediated through the production of parathyroid hormone
from the neoplastic keratinocytes.2,29 In addition, attempts at medical correction of the hypercalcemia have
been unsuccessful. After excision of the tumor, the serum calcium level
returns to normal but rises again with recurrent disease. Superficially Invasive Carcinoma of the Vulva The concept of superficially invasive carcinoma of the vulva implies that
lesions with limited dermal invasion may constitute a separate prognostic
category.28,30 The term microinvasive carcinoma has not been accepted for reference to vulvar carcinoma. Solitary tumors
with a depth of invasion of 1 mm or less have essentially no risk of
regional node metastasis, and node sampling or resection is not contributory
in these. HISTOPATHOLOGY The FIGO staging of vulvar carcinoma has defined a stage IA subset of stage
I carcinoma as originally set forth by the ISSVD, as a tumor with
a diameter of 2 cm (20 mm) or less with a depth of invasion of 1 mm or
less (Fig. 8). Measurement of tumor diameter alone is insufficient to define the risk
of nodal metastasis. Most vulvar carcinomas with a depth of invasion
of 1 mm or less are less than 2 cm in diameter. Clinically, VIN surrounding
the tumor may be included erroneously in the measurement of the
tumor diameter. In superficially invasive vulvar carcinoma, it is essential
that the entire tumor be available for study before an attempt
is made to determine the depth of invasion. Cases in which there is more
than one tumor are not included in the stage IA group. The depth of
invasion is defined as the measurement from the epithelial-stromal junction
of the adjacent most superficial dermal papilla to the deepest
point of invasion, as described by Wilkinson and colleagues.1,30,31 The ISGP and the WHO have accepted this definition of the “depth
of invasion” in the vulva.1,2 This measurement should be distinguished from the “thickness of
the tumor,” which is defined as the measurement from the surface, or
bottom of the granular layer of keratin, if present, to the deepest
point of invasion (Fig. 9A, B). The ISGP and WHO recommend that the pathologist report both measurements
whenever possible. The stage IA group includes patients whose tumors
involve capillary-like spaces, provided skin measurements are available. A
protocol for the evaluation of vulvar specimens drafted by the
senior author (Wilkinson) has been published by the College of American
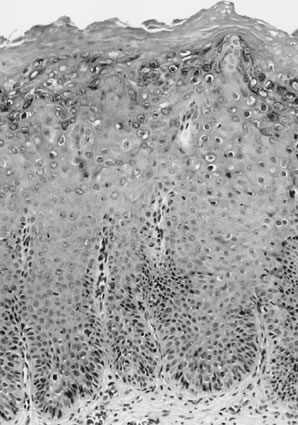
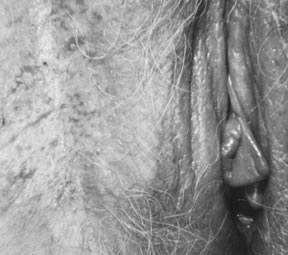
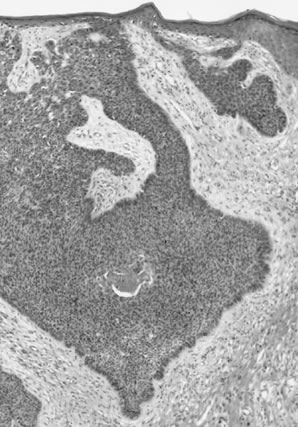
Pathologists.26 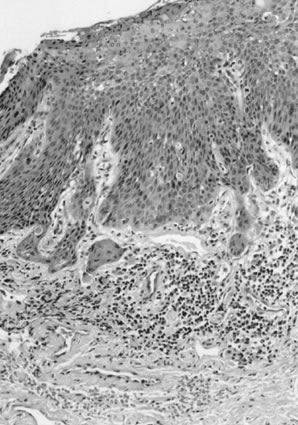 Fig. 8. Superficially invasive squamous cell carcinoma with vulvar intraepithelial
neoplasia (VIN) 3. There is a marked inflammatory response. Fig. 8. Superficially invasive squamous cell carcinoma with vulvar intraepithelial
neoplasia (VIN) 3. There is a marked inflammatory response.
|
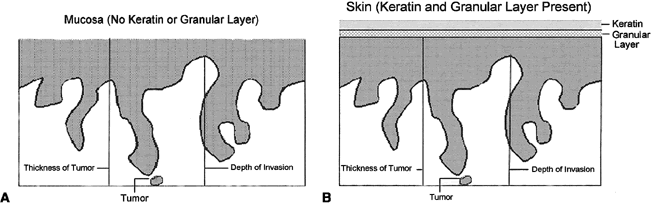 Fig. 9. Measurement methods of squamous cell carcinoma of the vulva. Depth of invasion
is defined as the distance from the epithelial-stromal junction
of the adjacent most-superficial dermal papilla to the deepest point
of invasion. Tumor thickness is defined as the distance from the surface (mucosa) or
bottom of the granular layer of the keratin (skin) to
the deepest point of invasion. Fig. 9. Measurement methods of squamous cell carcinoma of the vulva. Depth of invasion
is defined as the distance from the epithelial-stromal junction
of the adjacent most-superficial dermal papilla to the deepest point
of invasion. Tumor thickness is defined as the distance from the surface (mucosa) or
bottom of the granular layer of the keratin (skin) to
the deepest point of invasion.
|
TREATMENT Stage IA carcinomas, as defined in Table 7, can be treated by wide and deep local excision without lymphadenectomy. In
excising a squamous carcinoma, the entire tumor should be included
in the excision so that accurate measurements of the diameter, depth
of invasion, and thickness of tumor can be assessed. An excision with
margins of approximately 2 cm, extending to the fascia (partial deep vulvectomy), is recommended.25 If the tumor is then found to be deeper than 1 mm (no longer a stage IA) on
pathologic examination, ipsilateral lymphadenectomy is indicated
unless the tumor is midline, in which case bilateral inguinal-femoral
lymphadenectomy is necessary. Total or more complete partial deep vulvectomy
may be necessary if the tumor extends to a margin of resection
or is more than 3-mm deep. Table 7. Suggestions for Sampling Tissue Removed for Diagnosis or Treatment
of Vulvar Cancer
CLINICAL FOLLOW-UP Superficially invasive vulvar squamous cell carcinomas that meet the definition
of stage IA have essentially no risk of nodal metastases. In
contrast, with tumors 3-mm deep, the risk of nodal metastasis is approximately 12%. With
a 5-mm depth of invasion, or thickness, there is a
reported risk of nodal metastases of approximately 15%.1,25 Follow-up of women with vulvar superficially invasive carcinoma is essential
because it is recognized that these women, although they have a
relatively low risk of local recurrence, are at risk of having a “reoccurrence” of
a new primary tumor or the vulva, independent
of the original tumor site. Although this risk is low, awareness and
observation, with biopsy when indicated, can reduce the risk of metastases
or death from a recurrent or new “reoccurrent” vulvar
carcinoma.25,31 Frankly Invasive Squamous Cell Carcinoma of the Vulva Frankly invasive squamous cell carcinomas include all of those that invade
to a depth beyond the limit used to define superficially invasive
carcinoma (1 mm). HISTOPATHOLOGY Histologic subtypes of squamous cell carcinomas are listed in Table 8. Squamous cell carcinomas with keratin pearls and obvious squamous cellular
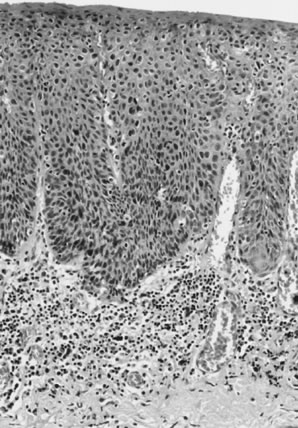
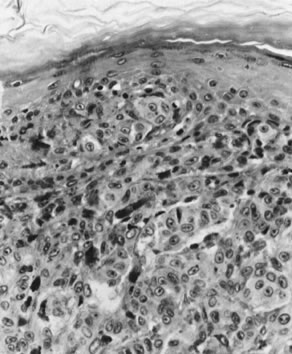
characteristics are considered well differentiated (Fig. 10). This pattern is usually the most frequent histologic type encountered. Other
terms that have been suggested for the keratin pearl-forming
carcinomas include large cell keratinizing, keratinizing, and epidermoid carcinoma. These tumors are more common in older women and are usually not associated
with HPV.1,10 The less well-differentiated squamous cell carcinomas are less obviously
squamous in origin without prominent intercellular bridges or keratinization (Fig. 11). Table 8. Histologic Subtypes of Vulvar Squamous Cell Carcinoma Including
Basal Cell Carcinomas
Squamous cell carcinoma, well-differentiated (not otherwise specified)
Basalold carcinoma
Warty (condylomatous) carcinoma
Verrucous carcinoma
Giant cell squamous carcinoma
Spindle cell squamous carcinoma
Acantholytic squamous cell carcinoma (adenoid squamous carcinoma)
Lymphoepithelioma-like carcinoma
Basal cell carcinoma Metatypical basal cell carcinoma (basosquamous carcinoma)
Adenoid basal cell carcinoma
Sebaceous cell carcinoma
From Wilkinson EJ: Premalignant and malignant tumors of the vulva. In Kurman
RJ: Blaustein's Pathology of the Female Genital Tract, 5th
ed. New York, Springer-Verlag, 2001.
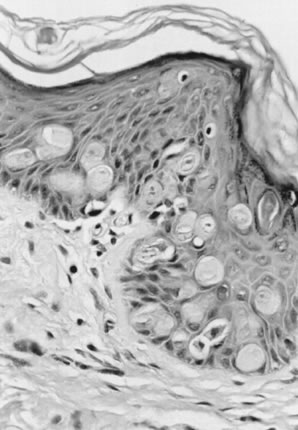
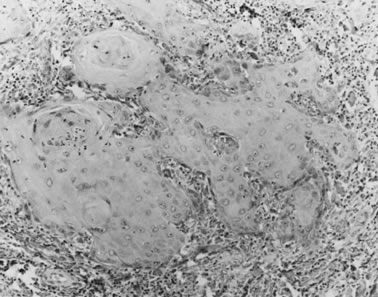 Fig. 10. Well-differentiated squamous carcinoma. Large cells with abundant cytoplasm
form keratin pearls (magnification, ×80). Fig. 10. Well-differentiated squamous carcinoma. Large cells with abundant cytoplasm
form keratin pearls (magnification, ×80).
|
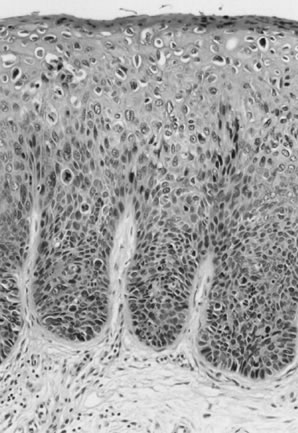
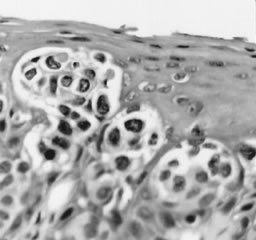
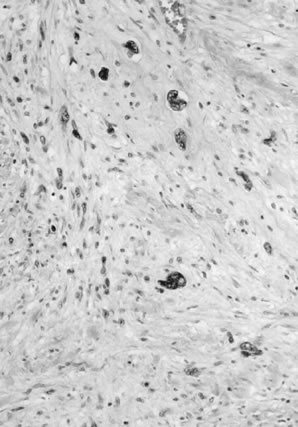
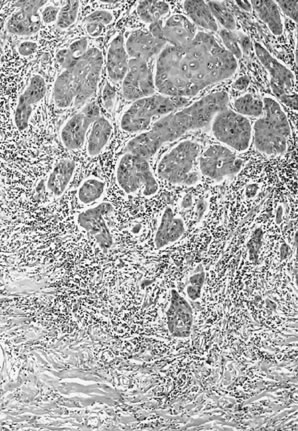 Fig. 11. Poorly differentiated squamous cell carcinoma of the vulva. The tumor cells
are nonkeratinized, without prominent intercellular bridges. The
tumor has a “finger-like” pattern of invasion. Fig. 11. Poorly differentiated squamous cell carcinoma of the vulva. The tumor cells
are nonkeratinized, without prominent intercellular bridges. The
tumor has a “finger-like” pattern of invasion.
|
Basaloid carcinomas are described as squamous tumors that do not form keratin
and are composed of smaller cells with an increased nuclear-to-cytoplasmic
ratio. These are less common than the well-differentiated
tumors and are associated with HPV, especially type 16.10 Warty (condylomatous) carcinomas have superficial features of condyloma
acuminatum. Their prognosis appears to be intermediate between well-differentiated
squamous cell carcinomas and verrucous carcinomas. They
also are associated with HPV, especially type 16.10 The giant cell forms of vulvar carcinoma are characterized by multinucleated
tumor giant cells and may have a poorer prognosis than the well-differentiated
carcinoma. These tumors must be distinguished from malignant
melanoma, which also may have tumor giant cells. An analysis of
cases classified as giant cell carcinoma of the vulva showed that criteria
described for giant cell carcinoma may be observed in vulvar malignant
melanoma and that the use of immunohistochemical studies is essential
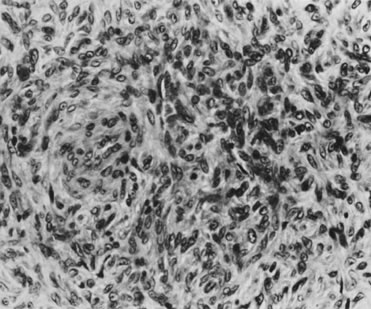
to distinguish between the two (see section on melanoma, below).32 In a study of 50 squamous cell carcinomas, Lasser and coworkers33 described two patients in whom the tumor showed primarily an adenoid-squamous
cell pattern. In 15 other patients, loci of adenoid-squamous change
were evident in isolated areas. The adenoid-squamous pattern of
growth is characterized by the formation of pseudoglandular spaces lined
with a single layer of squamous cells (Fig. 12). Within the spaces, dyskeratotic and acantholytic cells may be present. Mucin
stains do not show evidence of secretion. These adenosquamous
areas are usually found in tumors that would otherwise be considered
well-differentiated carcinomas with keratin pearl formation, and this
pattern of growth has not been observed in metastases or in recurrent
tumors. However, neoplasms with a predominantly adenoid pattern are poorly
differentiated and have a poor prognosis. One study noted a 6% 5-year
survival for such patients compared with a 77% survival for patients
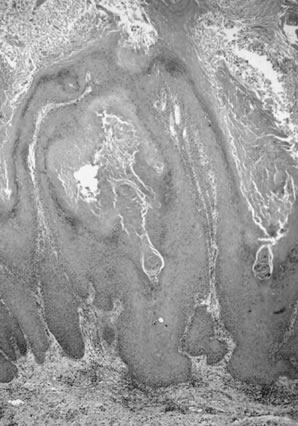
with conventional squamous cell carcinoma.34 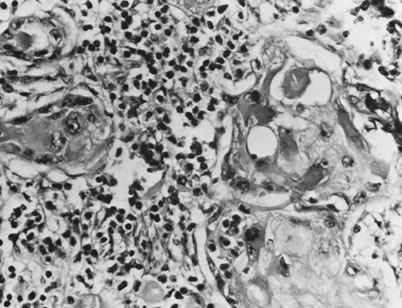 Fig. 12. Adenoid-squamous pattern with pseudoglandular spaces lined by squamous
cells (magnification, ×500). Fig. 12. Adenoid-squamous pattern with pseudoglandular spaces lined by squamous
cells (magnification, ×500).
|
Other Types of Vulvar Carcinoma Other primary carcinomas that have been reported arising within the vulva
include verrucous carcinoma, basal cell carcinoma, small cell,35 Merkel cell carcinoma,36–38 and adenocarcinoma.39 The ISGP has recommended that pathologists report the following information
for partial or total vulvectomy specimens1: - Depth of tumor invasion (in millimeters)
- Thickness of tumor (in millimeters)
- Whether there is vascular space involvement by tumor
- Diameter of the tumor (in millimeters) measured in the fresh or fixed state
- Clinical measurement of the diameter of the tumor
TREATMENT The treatment for vulva squamous cell carcinoma is directed toward sparing
as much of the vulva as possible with wide local excision (partial
deep vulvectomy) and associated ipsilateral regional lymph node dissection (inguinal-femoral
lymphadenectomy). A recent study has shown that
limited lymphadenectomy may be adequate in assessing the lymph node
status in patients with vulvar squamous carcinoma.40 If the tumor is midline or extends near the midline, bilateral inguinal-femoral
lymphadenectomy is usually performed.41 If the superficial lymph nodes are involved with tumor, radiation of the
deep pelvic nodes is usually recommended.41 |