Vaginal reconstruction is critical for maintenance of sexual functioning, psychosocial
health, restoration of body image, and for pelvic support
to prevent bladder, rectal, and pelvic prolapse. Among the conditions
leading to impairment of vaginal function are the following: - Surgical removal of the upper vagina or the entire vagina at the time
of conservative, radical, or ultraradical surgery
- Contraction, constriction, erosion, or ulceration of the vagina after
irradiation for cervical, vaginal, or endometrial cancer
- Fistula formation
The technique of vaginal reconstruction depends on the length of the remaining
vagina, the viability of the tissue, and the accompanying deficit
as a result of the treatment method. Procedures that have been described
include split-thickness and full-thickness skin grafts, peritoneal
grafts, omental grafts, vulvovaginal grafts, large- and small-bowel
grafts, bladder segment grafts, and myocutaneous flaps. Split-Thickness Skin Grafting In the absence of irradiation, vaginal reconstruction can be achieved by
the placement of a split-thickness skin graft in the vaginal canal. Skin
can be taken from the lower abdomen, the lateral hairless inguinal
area, the posterior medial thigh, or the posterior medial buttocks. The
graft usually varies from approximately 0.015 to 0.017 inches in thickness. Initially, split-thickness grafts contract approximately 20% compared
with full-thickness grafts, which may contract up to 50%. However, the
secondary contracture, which occurs as the wound heals, is
much less for the thicker grafts. Therefore, thicker grafts are more pliable
and contract less. The donor site usually heals without difficulty
in 14 to 21 days; however, a residual scar will remain at the donor
site. Graft-take ranges from 75% to 90%. This method of creating a neovagina
can be used in patients who have had a partial or complete vaginectomy
for intraepithelial or invasive carcinoma of the vagina or in
patients who have had either an anterior or a total pelvic exenteration. When
the latter is performed, creation of a vascular bed may be required; this
can be created surgically with the use of an omental pedicle
flap or muscle pedicle flap. When using a split-thickness graft, a vaginal stent is necessary to keep
the vagina patent. The stents are tailored to the size of the vagina. They
should be easy to remove and should not remain in place for prolonged
amounts of time. Excessive pressure should not be exerted on the
graft as it can lead to vascular compromise and draft necrosis. After
stent removal, topical estrogen can be used and the vaginal canal is
kept open, either naturally or by the use of a mold. The mold is used
for approximately 3 to 4 months if the patient is not sexually active; less
time is required if the patient is sexually active. Complications of vaginal reconstruction include loss of viability of the
graft, stenosis of the vagina, and rectovaginal and vesicovaginal fistula. Long-term
complications of vaginal grafts include vaginal dryness, vaginal
prolapse, and the rare development of squamous cell carcinoma
of the graft.14 Thus, split-thickness skin grafts have emerged as one of the two more
commonly used techniques for reconstruction of the female genital tract
after treatment for gynecologic malignancies. Use of Pelvic Peritoneum or Omentum When the length of the vagina would be compromised at the time of radical
or ultraradical surgery, intraoperative procedures can be used to lengthen
the vagina. A simple procedure involves the use of pelvic peritoneum.15 Peritoneum from the vesicouterine pouch or from the cul-de-sac (extension
of the peritoneum from the bladder and rectum) is preserved at the
time of radical surgery and attached to anterior and posterior vaginal
edges. The most superior or cephalad peritoneal edges are sutured in
the midline. Thus, a peritoneal pouch that is an extension of the existing
vaginal canal is created. Using this method, the vaginal depth can
be extended by at least 2 to 3 cm (Fig. 4). In addition, omental pedicle16 or pelvic peritoneum15,17 can be used to cover the pelvic floor and the dome of the denuded vaginal
canal. Approximately 3 to 6 weeks after surgery, after vaginal patency
is maintained, a split-thickness skin graft can be applied to the
vaginal tube. 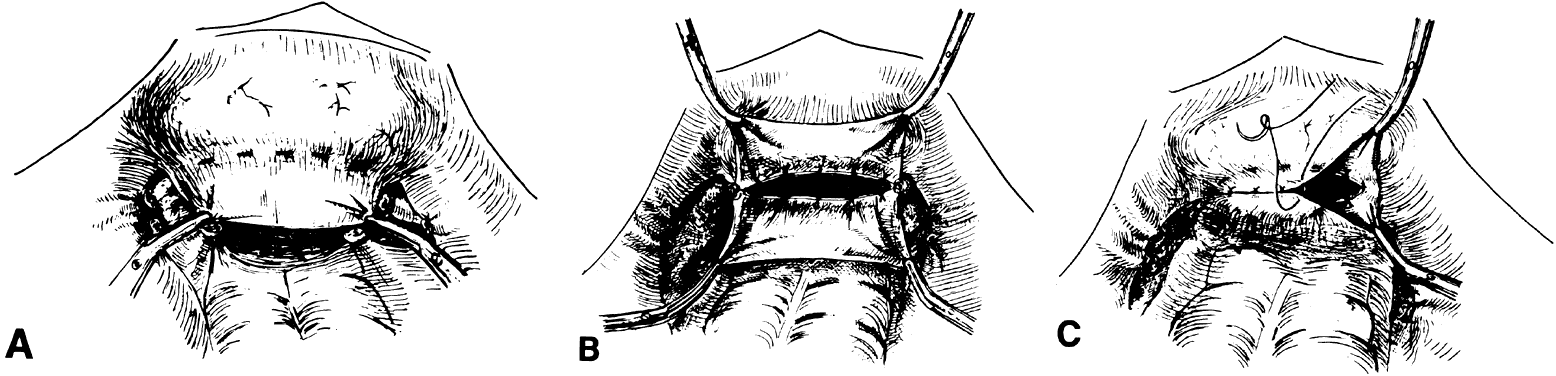 Fig. 4. Use of the pelvic peritoneum. A. A 2- to 3-cm segment of bladder flap peritoneum is left unattached. B. A corresponding rectal peritoneal flap is left unattached. C. The anterior and posterior flap edges are sutured to create a pouch at
the apex of the vaginal canal.(Saito M, Kumasaka T, Kato K et al: Vaginal repair in the radical operation
for cervical carcinoma. Acta Obstet Gynecol Scand 55:151, 1976.) Fig. 4. Use of the pelvic peritoneum. A. A 2- to 3-cm segment of bladder flap peritoneum is left unattached. B. A corresponding rectal peritoneal flap is left unattached. C. The anterior and posterior flap edges are sutured to create a pouch at
the apex of the vaginal canal.(Saito M, Kumasaka T, Kato K et al: Vaginal repair in the radical operation
for cervical carcinoma. Acta Obstet Gynecol Scand 55:151, 1976.)
|
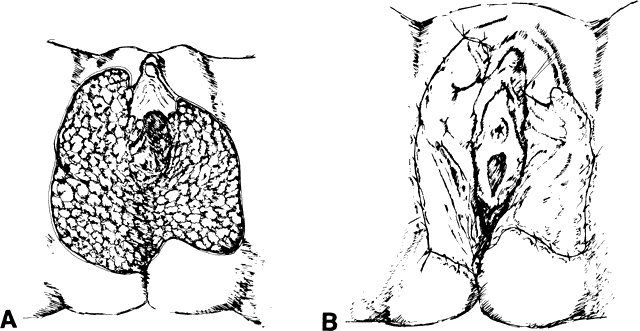
Variation of Standard Incisions VULVOVAGINOPLASTY. The current-day procedures use either the basic technique or minor modifications
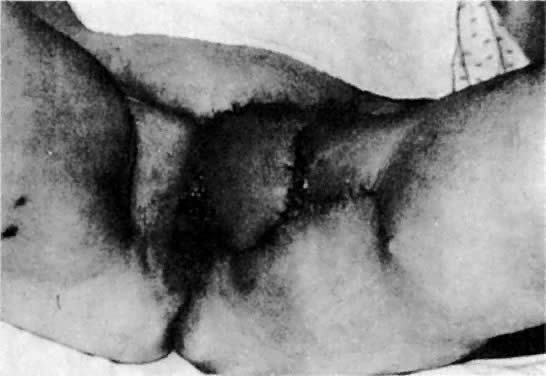
of the original Abbe-McIndoe-Williams procedure.18 This procedure begins with an incision of the labia majora as outlined
in Figure 5. The inner lateral edges of the incised skin are then approximated to
create a tubular vagina (Fig. 6). This tube is interior to the reapproximated outside edges of the incision. 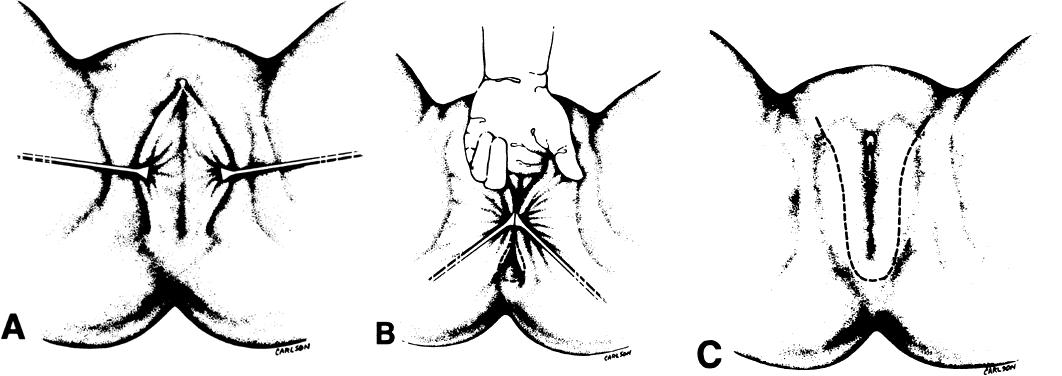 Fig. 5. Vulvovaginoplasty. A. Allis clamps outline the lateral margins of the neovagina. B. Approximation of the clamps allows an estimate of length and caliber of
the neovagina. C. The incision is outlined.(Day TG Jr: Vulvovaginoplasty in gynecologic oncology. Obstet Gynecol 50:362, 1977.) Fig. 5. Vulvovaginoplasty. A. Allis clamps outline the lateral margins of the neovagina. B. Approximation of the clamps allows an estimate of length and caliber of
the neovagina. C. The incision is outlined.(Day TG Jr: Vulvovaginoplasty in gynecologic oncology. Obstet Gynecol 50:362, 1977.)
|
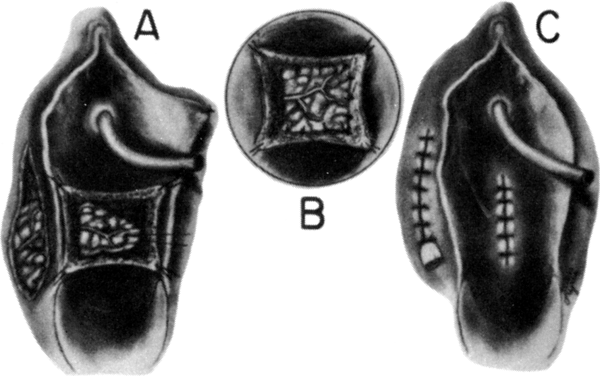
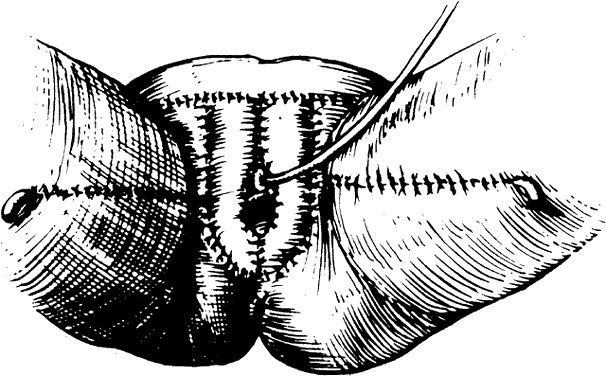
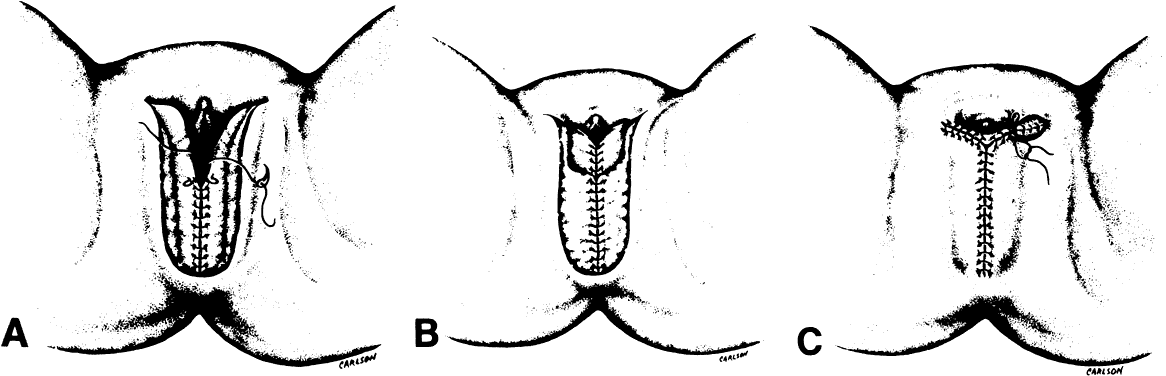 Fig. 6. A. Interrupted sutures approximate the inner margins of the incision. B. Subcutaneous tissue is next approximated. C. The outer margins are approximated and the superior aspect of the incision
is closed.(Day TG Jr: Vulvovaginoplasty in gynecologic oncology. Obstet Gynecol 50:362, 1977.) Fig. 6. A. Interrupted sutures approximate the inner margins of the incision. B. Subcutaneous tissue is next approximated. C. The outer margins are approximated and the superior aspect of the incision
is closed.(Day TG Jr: Vulvovaginoplasty in gynecologic oncology. Obstet Gynecol 50:362, 1977.)
|
This technique allows the creation of a functional vagina in approximately 6 to 8 weeks. Advantages of this procedure are that dissection of
the perineum is not necessary, the vagina retains its sensory function, routine
dilation is not required, hospitalization is decreased, and
the patient is able to ambulate early. This procedure is exceedingly useful after vaginectomy or when shortening
of the vaginal canal has occurred, such as after radical hysterectomy
or vaginal irradiation, when the length of the vagina is compromised. Among the complications of this operation are stenosis of the canal, prolapse
of the vagina, and the rare occurrence of either rectovaginal or
ileovaginal fistulas. A change in the direction of the vaginal canal
occurs after surgery. In addition, the vaginal skin usually is dry, and
lubrication is required before coitus. Occasionally, there is hair
growth in the neovagina. PERINEAL INCISION. Another technique reported by Simmons and Millard19 and West and coworkers20 for creation of a vagina involves the dissection and creation of a new
vagina between the compromised posterior vagina and the anterior rectum. This
rare surgical procedure is used when there is severe vaginal
stenosis secondary to irradiation. A vaginal pouch as large as 10 cm in
length and 5 cm in width can be created and lined with a split-thickness
skin graft. A vaginal mold and continued vaginal dilation are required
to prevent scarring. Organ Substitution SEGMENT OF BOWEL. In the past, the intestines have been used to fashion a vaginal tube. At
the time of exploratory celiotomy, a vaginal tube can be created by
using either a loop of ileum or a segment of the sigmoid colon.21 Preservation of the blood supply to the bowel segment is critical to the
success of this operation. The segment is used either to fashion a
neovagina entirely or to attach it to a vaginal remnant to augment vaginal
length. This method was popular with European surgeons but is rarely used today. It
served as an impetus for the development of current-day techniques, because
routine canal dilation and the use of stents were circumvented. Mucous
secretion from either the ileum or the sigmoid, primarily
the sigmoid, was bothersome to many patients. USE OF THE BLADDER DOME. A less-popular method for creation of a neovagina involves the use of
the bladder dome.22 A segment of bladder is resected, the epithelium is removed, and this
portion of the bladder is used to fashion an upper vagina. Usually, this
procedure is followed by application of a split-thickness skin graft
to fashion the lower vagina. This procedure is rarely used today. USE OF A MYOCUTANEOUS FLAP. The presence of gaping defects in the pelvic-vaginal cavity after ultraradical
surgery such as a total pelvic exenteration has necessitated
the use of larger flaps. Myocutaneous flaps have enjoyed increasing popularity
in these instances. One favorite technique involves using the
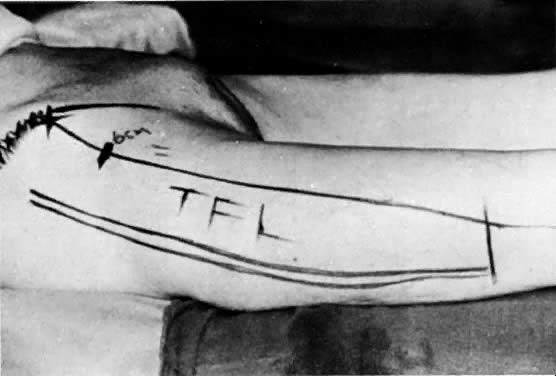
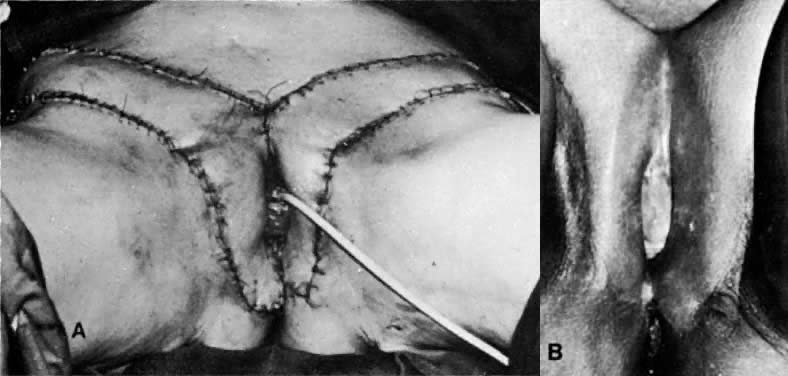
gracilis muscle to close the pelvic defect and create an adequate vagina (Figs. 7–11). To reduce the bulk associated with this flap, a shortening of the flap
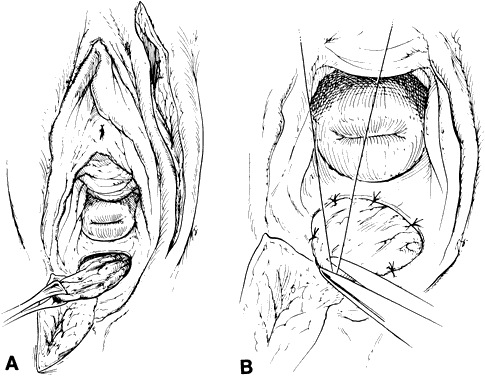
with equally efficacious results has been recommended.23 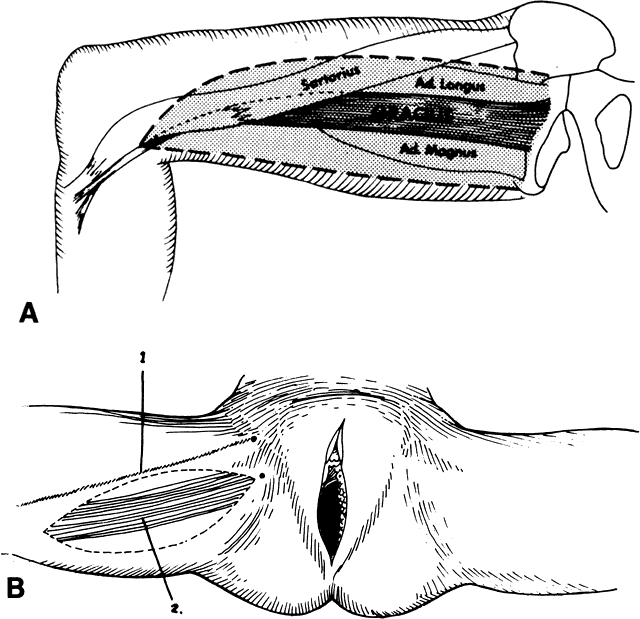 Fig. 7. A. The gracilis and adjacent muscles. The presence of a major vessel within
the proximal 8 cm of the muscle allows the muscle to be detached from
the distal insertion. The muscle belly and overlying tissue are reflected. (Franklin
EW, Bostwick J, Burrell MO et al: Reconstructive techniques
in radical pelvic surgery. Am J Obstet Gynecol 129:285, 1977.) B. The dotted line delineates skin and underlying gracilis muscle ( line 2 ). Origin of the gracilis from the lower pubic symphysis and pubic arch
and insertion on the upper tibia ( line 1) ( A ). (Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.) Fig. 7. A. The gracilis and adjacent muscles. The presence of a major vessel within
the proximal 8 cm of the muscle allows the muscle to be detached from
the distal insertion. The muscle belly and overlying tissue are reflected. (Franklin
EW, Bostwick J, Burrell MO et al: Reconstructive techniques
in radical pelvic surgery. Am J Obstet Gynecol 129:285, 1977.) B. The dotted line delineates skin and underlying gracilis muscle ( line 2 ). Origin of the gracilis from the lower pubic symphysis and pubic arch
and insertion on the upper tibia ( line 1) ( A ). (Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.)
|
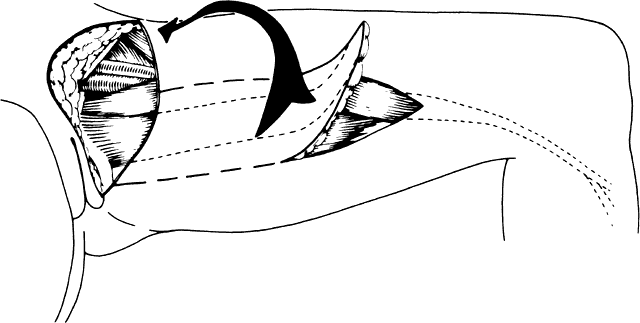
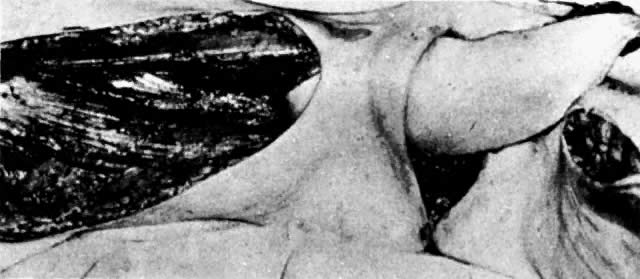 Fig. 8. The right gracilis muscle, skin, and fat are elevated and rotated under
the skin bridge.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.) Fig. 8. The right gracilis muscle, skin, and fat are elevated and rotated under
the skin bridge.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.)
|
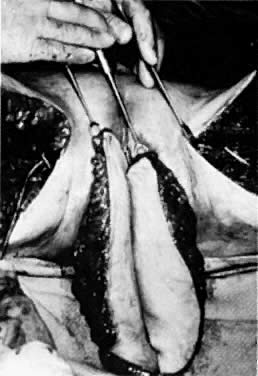 Fig. 9. Bilateral gracilis flaps are shown with skin, subcutaneous tissue, and
muscle.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.) Fig. 9. Bilateral gracilis flaps are shown with skin, subcutaneous tissue, and
muscle.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.)
|
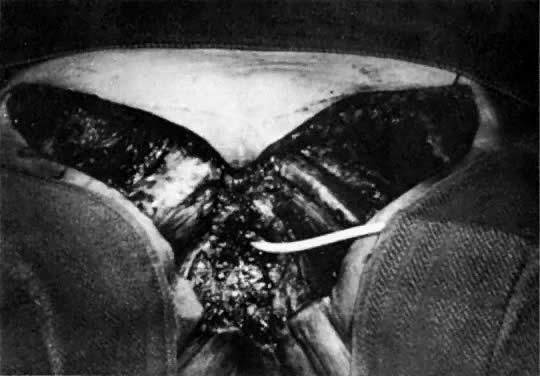
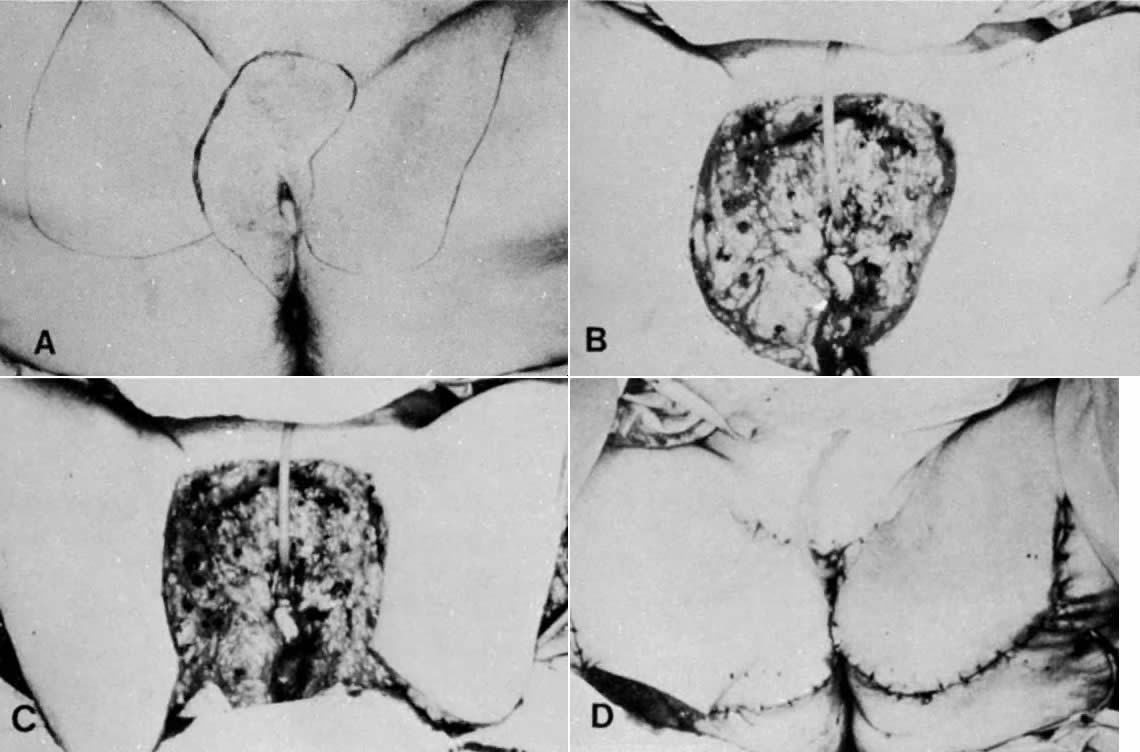
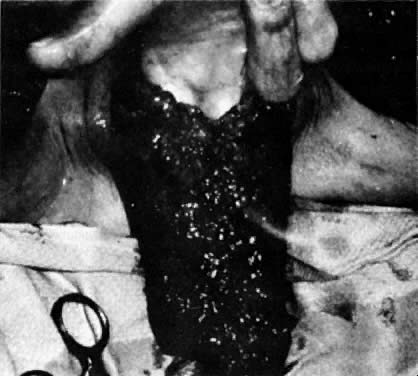 Fig. 10. Suturing of three sides of two flaps creates a new vaginal pouch. The fingers
are in the pouch. Rotation of the pouch posteriorly into the pelvic
defect will occur. The portion of the pouch closest to the viewer
becomes the posterior wall of the vagina.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.) Fig. 10. Suturing of three sides of two flaps creates a new vaginal pouch. The fingers
are in the pouch. Rotation of the pouch posteriorly into the pelvic
defect will occur. The portion of the pouch closest to the viewer
becomes the posterior wall of the vagina.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.)
|
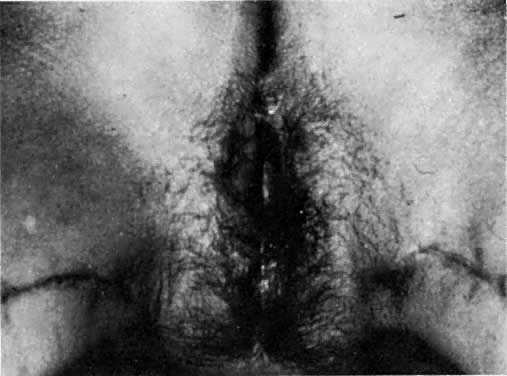 Fig. 11. A functional vagina 3 months after surgery. The thigh skin incisions are
healed.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.) Fig. 11. A functional vagina 3 months after surgery. The thigh skin incisions are
healed.(Becker DW Jr, Massey FM, McCraw JB: Musculocutaneous flaps in reconstructive
pelvic surgery. Obstet Gynecol 54:178, 1979.)
|
Rectus abdominal muscle flaps have also been used for vaginal reconstruction. One
advantage is that only a single flap is necessary, compared
with two gracilis flaps, and, second, the donor site incision can be
closed with the laparotomy incision in cases of exenterative procedures. Disadvantages
of the rectus muscle flap include difficulty of stoma
placement and that a two-team approach may no longer be possible.24,25 URINARY CONDUIT. With the trend toward better cosmesis oncologists have attempted to enhance
patient comfort and acceptance after radical surgery. One such advancement
has been the creation of a continent urinary conduit. The large
and small intestines are used to create a urinary reservoir intra-abdominally, and
although an abdominal wall stoma still is present, there
are several advantages for the patient. The stoma is smaller and
the patient does not have to constantly wear a urinary receptacle. In
addition, a low rectal anastomosis or anastomosis of the colon, rather
than an end sigmoid colostomy, allows the patient to be free of a conduit
for the stool. These two refinements significantly enhance the body
image of the patient undergoing radical pelvic surgery. |