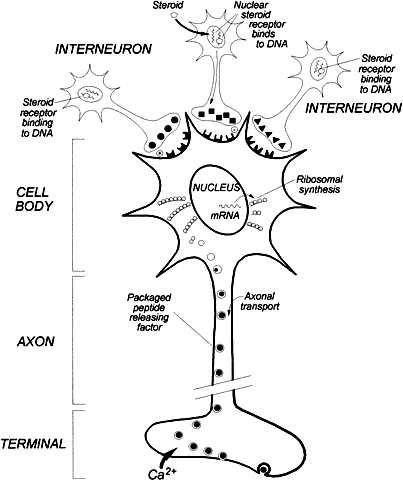
Thyrotropin-Releasing Hormone The first hypothalamic-releasing factor isolated was the tripeptide thyrotropin-releasing
hormone (TRH), which was discovered independently by
Schally and Guillemin's group.7,26 Like other neuropeptides, TRH is derived from a much larger 30-kd precursor (Fig. 5) and specifically stimulates release of thyroid-stimulating hormone (TSH). TRH
also has prolactin-releasing properties, because lactotropes
have receptors for TRH. TRH may play other roles besides its physiologic
role in TSH release. It has been localized by immunocytochemical techniques
to the cerebral cortex, spinal cord, paraventricular nucleus, pineal
gland, neurohypophysis, and ventral horn motor cells.27 A diagrammatic view of the circumventricular hypothalamic nuclei is shown
in Figure 6. Neuropharmacologic studies implicate TRH as a neuromodulator with general
stimulant activity. Experimentally observed properties of TRH are
summarized in Table 2.28 TABLE 2. Central Nervous System---Mediated Actions of Thyrotropin-Releasing
Hormone
Hypothermia
Reversal of spinal shock
Increased spontaneous motor activity
Alteration in sleep patterns
Anorexia
Inhibition of avoidance behavior
Reversal of depression
Neuromodulator of classic transmitter action Releases norepinephrine and dopamine from synaptosomal preparations
Enhances disappearance of norepinephrine from nerve terminals
Potentiates excitatory actions of acetylcholine on cerebral cortical neurons
Vale W, Rivier C, Brown M: Regulatory peptides of the hypothalamus. Ann
Rev Physiol 39:473, 1977.
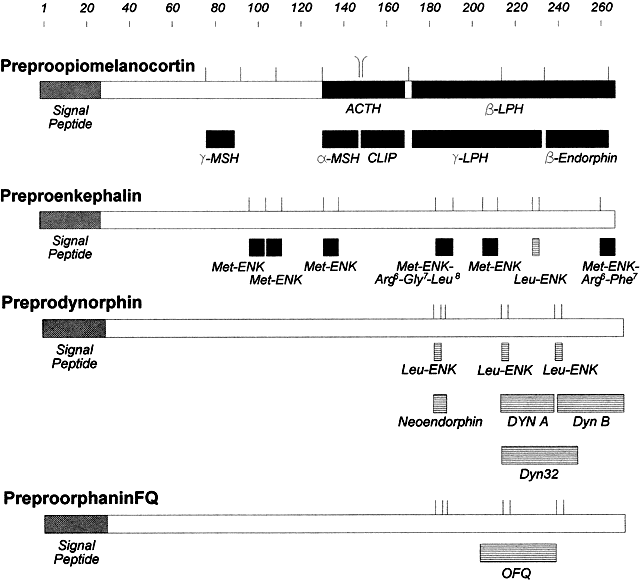
 Fig. 5. Amino acid sequence of thyrotropin-releasing hormone (TRH), gonadotropin-releasing
hormone (GnRH), GnRH analogs, somatostatin (SRIF), and the
SRIF analog octreotide. Fig. 5. Amino acid sequence of thyrotropin-releasing hormone (TRH), gonadotropin-releasing
hormone (GnRH), GnRH analogs, somatostatin (SRIF), and the
SRIF analog octreotide.
|
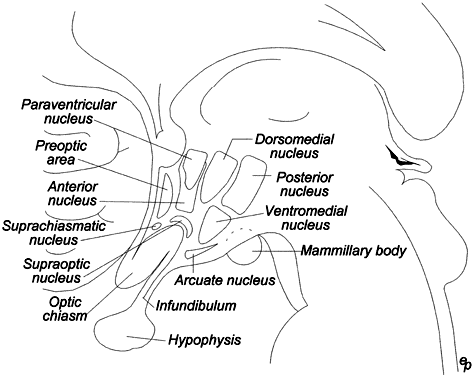 Fig. 6. Distribution of circumventricular hypothalamic nuclei. Fig. 6. Distribution of circumventricular hypothalamic nuclei.
|
Transgenic mice deficient in TRH have been generated.29 These mice develop normally and are fertile. The affected mice are hypothyroid
with elevated TSH levels (i.e. tertiary hypothyroidism). The mice also display hyperglycemia and abnormal
insulin secretion.29 Because of its ability to reverse spinal shock in animals, TRH has been
used experimentally in patients with spinal cord trauma as an adjunctive
treatment with promising results. Because of a short half-life, additional
clinical applications will require development of long-acting
analogs. Gonadotropin-Releasing Hormone GnRH is a decapeptide that was also isolated and characterized by Schally
and Guillemin's laboratories in 1971 from porcine and ovine hypothalami.6,7 The structure of this peptide is identical in all mammals, including humans (Fig. 5). GnRH has been localized primarily to the hypothalamus in the interstitial
nucleus (medial preoptic area) and has its highest concentrations
in the arcuate nucleus adjacent to the median eminence30 (Fig. 6). Small amounts of GnRH are also found in the circumventricular organs
of the brain. There are an estimated 1,500 GnRH neurons in the hypothalamus. GnRH, like many other neuropeptides, is synthesized as part of a much larger
precursor peptide containing 92 amino acids.31 GnRH is cleaved from the precursor, leaving a 56-amino acid peptide known
as GnRH-associated peptide (GAP).32 GAP has been shown to have potent activity to inhibit the release of prolactin. The
placenta, which also secretes GnRH, forms a different precursor
protein through the use of a different promoter sequence.33 Because of the importance of GnRH in the control of reproduction, intensive
efforts have been made to determine its physiologic role. When administered
to humans, GnRH stimulates a rapid and large release of LH
and a smaller release of FSH. In the absence of GnRH, as in patients with
Kallmann's syndrome, there is virtually no secretion of LH and FSH, emphasizing
the importance of GnRH in the reproductive axis. As with
other knockouts, the unexpected finding of anosmia in these patients
led to the discovery that GnRH neurons arise in the olfactory placode
and migrate to the hypothalamus.34 The half-life of GnRH in the peripheral circulation is 2 to 4 minutes.35 Because GnRH is rapidly degraded by peptidases, analogs have been synthesized
that have much longer half-lives (Fig. 5). By trial and error testing of thousands of GnRH analogs, it was discovered
that substitution of amino acids at the 2 or 3 position results
in analogs with antagonistic properties, whereas modifications at the 6 or 10 position
result in analogs with agonist action. Two unique functional properties of GnRH have permitted its widespread
clinical application. When GnRH is administered as a continuous infusion, pituitary
LH response is initially brisk but is followed by a decrease
in LH secretion and decrease in GnRH receptors (i.e. downregulation). Agonistic analogs of GnRH promote this downregulation
phenomenon and can be used to induce "medical castration" by shutting
down the pituitary-gonadal axis. Several GnRH analogs are available clinically
and are widely used for a number of diseases, including endometriosis
and uterine leiomyomas. If GnRH is administered in a pulsatile
fashion at 60- to 90-minute frequencies, LH receptors and LH response
increase. Continuation of this mode of administration for prolonged
periods results in stimulation of a normal menstrual cycle36 or normal testosterone production and spermatogenesis in patients with
a deficiency or disorder of GnRH secretion. A summary of the clinical
applications for GnRH and its analogs is provided in Table 3. TABLE 3. Clinical Application of Gonadotropin-Releasing Hormone and Its
Analogs
Activation of pituitary-gonadal axis Dynamic testing of pituitary
Ovulation induction
Induction of spermatogenesis
Cryptorchidism
Downregulation of pituitary-gonadal axis Contraception
Gonadal steroid-dependent tumors Prostate cancer
Breast cancer
Endometrial cancer
Endometriosis
Polycystic ovarian disease
Uterine leiomyoma
Premenstrual syndrome
Precocious puberty
Controlled ovarian hyperstimulation
Besides its role in the control of reproduction, GnRH also has intriguing
behavioral effects. When administered to ovariectomized, estrogen-primed
female rats intracerebrally, GnRH induces lordotic posturing and
mating behavior.37 In contrast, administration of GnRH antibodies has suppressed sexual behavior.38 This effect is a unique property of this peptide. From a phylogenetic
perspective, these behavioral effects appear similar to a peptide that
has been isolated from yeast, the α-mating factor. The α-mating
factor has sequence homology with GnRH and can stimulate LH release
from gonadotrophs.39 The role of GnRH as a true neurotransmitter is not established. GnRH has
been shown to elicit a slow excitatory synaptic potential in frog sympathetic
ganglia.40 Whether GnRH plays a modulatory role on other neurotransmitter systems
remains to be determined. Somatostatin During the screening of multiple fractions from hypothalamic extracts, fractions
with the ability to inhibit secretion of growth hormone (GH) from
anterior pituitary cells were isolated. One factor subsequently
isolated and characterized by Guillemin's group was a 14-amino acid peptide
named somatostatin (Fig. 5).8 This peptide is derived from a 210-amino acid precursor. Clinical studies of this peptide demonstrated that somatostatin inhibited
GH, TSH, prolactin, glucagon, and insulin secretion in humans.41 Subsequently, somatostatin was found to be widely distributed in the gastrointestinal
tract, the delta cells of the pancreas, and the thyroid-parafollicular
cells. In the central nervous system, somatostatin has
been localized to the hypothalamus, limbic system, septum, hippocampus, cortex, and
medulla.42 The secretion of hypothalamic somatostatin is episodic and probably plays
a role in the regulation of episodic GH secretion.43 Because somatostatin is localized adjacent to other hormone-secretory
cells, control of secretion can be affected by paracrine action. In the
case of the thyroid, parafollicular cells that contain calcitonin and
somatostatin regulation may occur through autocrine action. In general, somatostatin injected intraventricularly in animals acts to
decrease spontaneous motor activity and displays a sedative effect. The
half-life of somatostatin in the peripheral circulation is only 2 to 4 minutes.40 Mice overexpressing somatostatin have been generated.44 These mice displayed motor hyperactivity, especially in females, supporting
a role for somatostatin as a neurotrophic factor. Mice deficient
in somatostatin receptor type 2 do not respond to the negative feedback
of GH45 and have high gastric acid secretion.46 Corticotropin-Releasing Hormone CRH was the first hypothalamic-releasing hormone to be recognized. However, it
was not until 1981 that Vale and his colleagues first reported
the isolation and characterization of ovine CRH as a 41-amino acid peptide.47 Through genetic engineering techniques, the structure of human CRH was
determined to be a 41-amino acid peptide that differed from the ovine
hormone in 7 positions and was derived from a 196-amino acid precursor.48 Using immunocytochemical techniques, CRH was found to be highly concentrated
in the hypothalamus (i.e. paraventricular nucleus and parvocellular neurons) and pituitary stalk. Immunoreactive
CRH was found in the posterior pituitary, thalamus, cerebral
cortex, cerebellum, pons, medulla oblongata, and spinal cord.49 CRH neurons are also capable of synthesizing and storing oxytocin and
vasopressin. There is evidence that CRH, like GnRH, is released in regular
episodic pulses from a pulse generator intrinsic to the hypothalamus (Fig. 7).50 The physiologic significance of the pulsatile nature of CRH remains unknown. 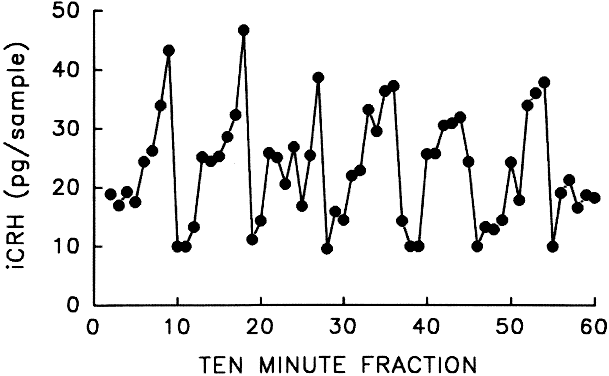 Fig. 7. Pulsatile release of corticotropin-releasing hormone (CRH) from the hypothalamus of the cynomolgus monkey. The regular pulses, occurring
at 90-minute intervals, are reminiscent of the gonadotropin-releasing
hormone pulse generator. (Adapted from Mershon JL, Sehlhorst
CS, Rebar RW, Liu JH: Evidence of a corticotropin-releasing hormone
pulse generator in the macaque hypothalamus. Endocrinology 130:2991, 1992.) Fig. 7. Pulsatile release of corticotropin-releasing hormone (CRH) from the hypothalamus of the cynomolgus monkey. The regular pulses, occurring
at 90-minute intervals, are reminiscent of the gonadotropin-releasing
hormone pulse generator. (Adapted from Mershon JL, Sehlhorst
CS, Rebar RW, Liu JH: Evidence of a corticotropin-releasing hormone
pulse generator in the macaque hypothalamus. Endocrinology 130:2991, 1992.)
|
In initial studies, intracerebroventricular injection of CRH in rats activated
the sympathetic nervous system, which increased plasma levels
of epinephrine, norepinephrine, glucose, glucagon, and insulin.51 These responses suggest that CRH is a principal mediator of ACTH/cortisol
secretion during stress in addition to its ability to activate the
sympathetic nervous system. CRH may also play a role in depression, because
elevated concentrations of CRH-like peptides have been reported
in the cerebrospinal fluid of depressed patients.52 CRH also can suppress secretion of GnRH-LH release in vitro53 and in vivo.54,55 This effect can be reversed by a CRH antagonist53 or naloxone.56 This effect remains controversial, because other studies have found no
effect of CRH on LH pulsatility.57 Outside the central nervous system, CRH has been localized to pancreas, stomach, duodenum, liver, lung, placenta, and adrenal gland. The molecular
weight of immunoreactive CRH from these tissues suggests that it
is similar to hypothalamic CRH.49 The physiologic role of CRH in these tissues is unknown. In clinical studies, the half-life of human CRH is 5 minutes when injected
into the circulation.58 When injected into humans, the ACTH-releasing effects of CRH are synergistically
potentiated by vasopressin.17 These responses suggest that ACTH release may be modulated by peptide-peptide
interaction. Transgenic technology has been used to generate mice that overexpress CRH
and mice deficient in CRH and its receptor. Mice overexpressing CRH
show elevated ACTH and glucocorticoid and display the signs of Cushing's
syndrome, including female infertility.59 The CRH knockout mice are viable, reproduce, and have normal longevity
despite severe glucocorticoid deficiency.60 Vasopressin and oxytocin expression were not increased. Knockout mice
have a decreased glucocorticoid response to stress, with males much more
severely affected. The most interesting finding in CRH-deficient mice
was the effect of CRH deficiency on the offspring; lack of lung maturation
led to neonatal death within 12 hours of life. The mice could
be rescued by the administration of glucocorticoid to the maternal drinking
water.61 This is reminiscent of the beneficial effect of glucocorticoid on lung
maturation to women in preterm labor. Mice deficient in CRH receptor
type 1 also show glucocorticoid deficiency but display increased exploratory
activity and decreased anxiety, suggesting a role for CRH in stress
and the anxiety response. CRH administration is limited to research and diagnostic applications. CRH
may be useful in the differentiation between pituitary and nonpituitary
Cushing's syndrome.62 The therapeutic potential for CRH lies in its involvement in memory, obesity, and
Alzheimer's disease.63 Additional diagnostic and therapeutic applications await development of
long-acting analogs. A new member of the CRH family, urocortin, was cloned from rat brain.64 It was later described in humans, sharing 95% identity with the rat molecule.65 This 40-amino acid peptide shares 45% identity with CRH and stimulates
ACTH release in vitro and in vivo.66 Urocortin has greater ability to bind and activate type 2 CRH receptors
than CRH, suggesting that it may be the endogenous ligand for the type 2 CRH
receptor.64 When administered intravenously to rats, urocortin produces a significant
reduction in blood pressure,67 similar to the activity of urotensin, a closely related, vasoactive hormone
in fish. Growth Hormone-Releasing Hormone The most recent hypothalamic-releasing factor to be characterized, sequenced, and
synthesized is growth hormone-releasing hormone (GHRH). Because
many brain peptides have been localized outside the central nervous
system, it was not surprising that GHRH was isolated from two patients
with pancreatic tumors that induced acromegaly. From these tumors, two
peptides (40 and 44 amino acids long) were isolated that possessed
GH-releasing properties.68,69 Both have been shown to be equally potent and specific for GH release
that is inhibited by somatostatin.70,71 Subsequent reports indicate that both peptides are derived from a 108-amino
acid precursor and are localized predominantly to the arcuate nuclei. Studies in patients with acromegaly have demonstrated no significant different
responses to GHRH,72 whereas in patients with hyperprolactinemia and galactorrhea responses
to GHRH are blunted.73 The discovery of this peptide has obvious clinical applications. Studies
that use GHRH for the treatment of GH-deficient children are already
in progress.74 As with other hypothalamic peptides, long-acting analogs of GHRH may be
an alternative to the use of synthetic human GH in the treatment for
this condition. There are now several nonpeptide analogues of GHRH, such
as MK-677, in clinical development for the stimulation of the GH axis.75 Neuropeptide Y, Galanin, Substance P, and Angiotensin II A number of other neuropeptides that are localized to the circumventricular
nuclei have been shown to modulate release of prolactin, GH, and
GnRH. To review the interaction of these peptides is beyond the scope
of this chapter; however, four of the better-known neuropeptides are discussed. Neuropeptide Y (NPY) is the most abundant neuropeptide in the brain. It
is one of several gastrointestinal peptides that are also localized in
the CNS. NPY shares many similarities with the pancreatic family of
peptides, showing a high degree of homology to peptide YY and pancreatic
peptide (Table 1). There is considerable evidence that NPY plays a significant role in
the regulation of appetite, body weight, and reproduction. In this capacity, NPY
may be an important factor in the complex interplay between
nutrition and reproduction. NPY is a 36-amino acid peptide and is the only member of the pancreatic
peptide family with localization in the CNS. NPY has a CNS distribution
that is similar to somatostatin and is localized to the many hypothalamic
nuclei, including the arcuate nucleus, median eminence, amygdala, septum, cortex, and
the hippocampus.76,77 NPY receptors are also widely distributed in the CNS, especially the hypothalamus
and median eminence.77 Pharmacologic and molecular biologic studies have confirmed the presence
of at least four subtypes of NPY receptor.78 Considerable evidence supports a major role for NPY in the control of reproductive
function. Initial studies demonstrated that NPY stimulated
LH release from intact rats but inhibited LH release from ovariectomized
animals. The stimulatory effect of NPY on LH release was later shown
to be mediated by an increase in the hypothalamic release of GnRH, in
estrogen-treated animals or tissues. NPY is secreted in an episodic
manner that corresponds to GnRH pulsatility. There is evidence that NPY
is involved in the pulsatile secretion of GnRH and in the burst of
GnRH seen at the time of the LH surge.79 There is also evidence that NPY influences LH secretion at the pituitary
level. NPY has a pronounced inhibitory effect on sexual behavior.80 NPY is recognized as the most potent appetite-stimulating substance in
the brain. Injection of NPY into the brains of satiated rats consistently
stimulates ongoing feeding behavior and the development of obesity. Evidence
that NPY is an endogenous appetite stimulator came from the
neutralization of NPY by administration of NPY antibody, which eliminated
the normal feeding pattern of rats. It is likely that NPY interacts
with other neurosecretory peptides, such as galanin and β-endorphin, to
regulate feeding behavior.79 It was expected that a mouse deficient in NPY would display alterations
in feeding behavior and body weight, but this is not the case. NPY-deficient
mice maintain a normal body weight and have normal reproductive
function.81 In addition, the response of these animals to the newly discovered obesity
hormone, leptin, is normal. Surprisingly, NPY-deficient mice have
an increased propensity for seizure activity.81 It is likely that other neuropeptides, such as galanin, play a compensatory
role in feeding and reproductive function in these animals.82 Galanin is an important neurosecretory peptide. Originally isolated in 1983 in
extracts of porcine intestine, galanin is composed of 29 amino
acids in all species except humans, in whom it is 30 amino acids long.83 The first 14 amino acids, which are associated with biologic activity, are
fully conserved across 13 species in which galanin has been characterized.83 The name galanin was derived from the first and last amino acids of the sequence: glycine
and alanine. Galanin is recognized as a neurosecretory peptide, with
wide distribution in the central nervous system. Most important is its
secretion from the hypothalamus, which also has a high concentration
of galanin receptors. There is evidence that hypothalamic galanin has
physiologic roles involving feeding, cognition, gastrointestinal function, and
regulation of hypothalamic and pituitary hormone secretion. The galanin gene was cloned in 1987 from rat pituitary and hypothalamus.84 Analysis of the galanin peptide suggests that it is unrelated to any other
known families of neuropeptides, making it unique among neurosecretory
peptides. Like all other neurosecretory peptides, the galanin receptor (GALR) is
a member of the G protein-coupled receptors with seven
transmembrane domains.85 Galanin receptors are found predominantly in the brain in three regions: cerebral
cortex, amygdala, and hypothalamus. The first GALR was cloned
from a human melanoma in 1994.86 There are three known GAL receptors, designated GALR1, GALR2, and GALR3, which
differ considerably in primary structure and distribution.85 Based on the effects of different galanin agonists and antagonists, it
is likely that there are other galanin receptors.83 Galanin has a well-recognized role in reproductive function. One of the
first indications of this action was the demonstration that galanin expression
is markedly stimulated by estrogen.87 There is evidence that galanin is co-released with GnRH and that it modulates
LH secretion from the pituitary. In this regard, galanin secretion
is modulated by estrogen and testosterone levels and varies with
the estrus cycle, peaking in proestrus with the LH surge.88,89 Galanin stimulates the release of GnRH from the hypothalamus in a dose-dependent
manner.90 Based on these findings, it has been proposed that galanin expression
may modulate the pulsatility of GnRH release.88 The pituitary gland is another important site of galanin secretion, with
expression stimulated by estrogen.84 Galanin was also shown to stimulate prolactin release and proliferation
of prolactin-secreting cells (i.e. lactotrophs).91 Galanin, in conjunction with NPY and other hypothalamic neuropeptides, has
a well-characterized role in the regulation of feeding and body weight.92,93 Hypothalamic galanin secretion is modulated by circulating hormones, such
as insulin and leptin, and nutrient ingestion. There is evidence that
galanin secretion from one particular site within the hypothalamus, the
anterior paraventricular nucleus, mediates these effects.94 Galanin responds to these metabolic signals by regulating energy balance
through its effects on behavior, hormone secretion, and metabolism.95 Galanin has orexigenic action, or stimulation of eating behavior. In this
regard, galanin may act through β-endorphin, which also stimulates
appetite.96 Galanin is specifically linked to ingestion of fats, in contrast to NPY, which
stimulates the ingestion of fats and carbohydrates.95 Galanin not only stimulates feeding behavior, but also has effects on
metabolism, acting to reduce energy expenditure. These effects tend to
increase body weight, especially of adipose tissue.93 A knockout mouse for the galanin gene has been developed and characterized.97 The mice develop normally and can reproduce, indicating that galanin does
not play critical roles in these physiologic processes. The mice have
a normal body weight compared with wild-type littermates. The mice
do demonstrate a significant reduction in pituitary prolactin content, supporting
an important role of galanin in the development or maintenance
of the lactotroph cell. Mammary development was also delayed. Affected
mothers cannot lactate, so that the pups die shortly after birth
if not transferred to a foster mouse.91 The stimulatory response of the lactotroph cell to estrogen is also abolished. The
mice also demonstrate several neurologic abnormalities, including
important changes in the responses of sensory neurons to injury
and pain.97 NEUROSECRETORY PEPTIDES AND THE LINK BETWEEN NUTRITION AND REPRODUCTION. Two neurosecretory peptides, NPY and galanin, play major physiologic roles
in nutrition and reproduction, suggesting that these peptides may
be involved in the reproductive changes seen in disorders such as anorexia
nervosa and the chronic anovulation of obesity. The most important
part of this puzzle was the discovery of the obesity hormone leptin.98 This important discovery provided the missing piece of the puzzle: how
does the hypothalamus know how much fat tissue is present in the organism? Produced
by fat tissue, circulating levels of leptin correlate directly
with the amount of adipose tissue present.99 Receptors for leptin are located in the hypothalamus, where leptin acts
to regulate energy expenditure and body weight. In this model (the "lipostat" hypothesis
of body weight regulation), leptin serves as the
feedback signal to the hypothalamus that allows the animal to maintain
body weight in a narrow range, despite daily fluctuations in food consumption
and energy expenditure.100 It is likely that the primary mechanism by which leptin modulates appetite
and energy balance is through its effects on NPY, galanin, and possibly
other neurosecretory peptides, such as melanin-concentrating hormone (MCH), opiates, and
CRH.100,101 Leptin receptor is coexpressed in NPY neurons,102 where leptin acts to decrease NPY mRNA and neural signaling.103,104 Leptin also inhibits the expression of galanin in the hypothalamus.101 Although not yet proved, it is likely that these effects of leptin on
NPY, galanin, and other neurosecretory peptides modulate the reproductive
axis.90 The requirement for both NPY and galanin is demonstrated in studies showing
markedly decreased GnRH pulsatility when the activity of both neuropeptides
is inhibited but only partial effect when only one is blocked.105 This study helps to explain why mice deficient in either neuropeptide
have normal weight. Taken together, the interaction of leptin, NPY, galanin, and
other neuropeptides is beginning to shed light on the long-recognized
link between nutritional status and reproductive function. Substance P was one of the first nonhypothalamic-releasing hormones shown
to affect pituitary function. It is related to the tachykinin family
of neuropeptides and has been localized to the brain stem, amygdala, cortex, medulla, and
hippocampus.106 Receptors for substance P-like peptides are found in brain, pituitary
lactotrophs (i.e. prolactin-releasing cells), and gonadotrophs.107 In animal studies, administration of substance P to rats stimulates prolactin
secretion and inhibits GH release.108 Mice deficient in substance P or its receptor are viable and fertile but
do show altered responses to pain.109 Although angiotensin II is well known for its regulation of salt-water
balance, this octapeptide is also found in the paraventricular and supraoptic
nuclei. It is often colocalized with another osmoregulating hormone, vasopressin. With
regard to its modulatory role on pituitary function, angiotensin
II has been shown to stimulate LH release and inhibit
prolactin release.110 Mice deficient in angiotensin II are fertile and show the predicted alterations
in salt balance and blood pressure.111 Male mice lacking angiotensin-converting enzyme show altered sperm mobility
and decreased fertility.112 |