Prolactin-Secreting Pituitary Tumors
The characteristic features of prolactin-secreting pituitary tumors are galactorrhea and amenorrhea. In these patients, excessive production of prolactin is responsible for galactorrhea and may be the cause of amenorrhea. This may be a central mechanism, considering that infusion of GnRH in a pulsatile manner has been shown to induce full folliculogenesis and ovulation. This would imply that patients with hyperprolactinemia have an abnormality in GnRH pulsatility.
In reported studies, approximately 20% of patients with amenorrhea without galactorrhea exhibited elevated prolactin levels.35 These reports are generally composed of patients referred to medical centers and academic institutions. In a comparison study of screened (referred) and unscreened patients, the actual incidence of hyperprolactinemia was significantly reduced in a group of unscreened women. Similarly, the finding of galactorrhea in women with normal ovulatory function is poorly correlated with increased prolactin levels.36 Prolactin has many structural variances, which have been shown to fluctuate throughout the menstrual cycle. In addition, various forms of growth hormone have been detected in patients with galactorrhea and normal ovulatory function. Thus, this symptom alone cannot be used as a reliable marker of hyperprolactinemia. In these patients, a prior history of physiologic or pharmacologic stimulation of lactotropic cells can be elicited. It is unknown whether the transient hyperprolactinemia induced by these stimulatory influences is responsible for persistent or subsequent galactorrhea.
The diagnosis of a prolactin-secreting tumor is based primarily on the serum prolactin concentration and the radiologic appearance of the sella turcica. The upper limit of the normal range of serum prolactin is 20 to 30 ng/mL, depending on the individual laboratory and standards used. Prolactin values of approximately 50 ng/mL are associated with a 25% tumor incidence and values of 100% ng/mL with a 47% increased incidence; levels greater than 200 ng/mL are virtually diagnostic of a pituitary tumor. Pituitary tumors less than 1 cm, however, have been demonstrated in patients with prolactin levels less than 100 ng/mL. As with other hormonally active neoplasms, the absolute level of prolactin generally corresponds to the relative size of the lesion; however, it should be cautioned that large, inactive tumors are often associated with slightly elevated prolactin levels. Hyperprolactinemia in the absence of a demonstrable pituitary lesion suggests lactotrope hypertrophy, and these patients should be followed up at 6- to 12-month intervals.
The value of anterior pituitary function tests in the diagnosis of prolactin-secreting pituitary adenomas is limited and generally not used in establishing a diagnosis. In patients with a prolactin-secreting pituitary tumor, the prolactin response to TRH is blunted, probably as the result of maximal prolactin secretion by the tumor. Whether pituitary tumors function autonomously in their production of prolactin remains unclear. It appears that in most patients there is some degree of prolactin responsiveness to provocative stimulation, although not always of the same magnitude as that observed in normal persons. In addition, a blunted prolactin response occurs in hyperprolactinemic patients who have no radiologic evidence of a tumor. It is for these reasons that the predictive value of prolactin stimulation by TRH has not been established. Likewise, challenges with GnRH, levodopa infusion, and insulin-induced hypoglycemia will demonstrate altered responses in the presence of a prolactinoma, but they are not useful in establishing the diagnosis of these lesions.
The clinical management of prolactin-secreting pituitary adenomas varies according to the evaluation and the patient's needs. Treatment is directed toward eradicating aggressive pituitary tumor enlargement, which may be destructive to adjacent tissue and structures; restoring ovulation; and stopping lactation. Large pituitary tumors greater than 1 cm (macroadenomas) are usually associated with extracellular extension. These lesions should be treated with definitive surgery or radiation therapy, or both. Dopamine agonists (e.g., bromocriptine), however, have been shown to be highly effective as a primary treatment for large prolactinomas. Surgery may also be facilitated by preoperative administration of a dopamine agonist, which has been shown to reduce the size of most prolactin-secreting microadenomas. Some neurosurgeons, however, believe this increases fibrosis and actually makes surgery more difficult.
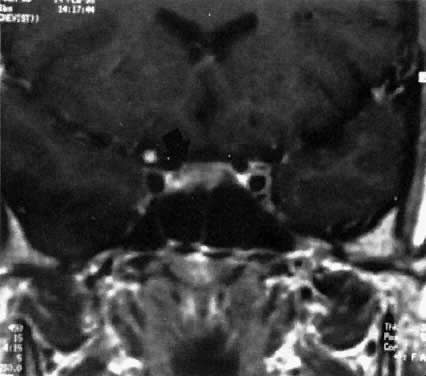
Intracellular lesions are usually less than 1 cm (microadenomas) and may be treated medically (Fig. 1).37 Surgery and irradiation therapy are seldom used as initial forms of treatment in these patients. Both bromocriptine administration and trans-sphenoidal resection are associated with a high degree of success.38, 39 Therapeutic effectiveness is assessed by the reduction of normalization of prolactin levels. Clinically, in approximately 70% of patients, restoration of normal endocrine function occurs within 3 months of treatment. The resolution rate of unwanted lactation, however, is less predictable. Bromocriptine administration is noninvasive and may reduce tumor size or may retard or prevent potential tumor growth; therapeutic benefits depend on its continued use. In most instances, within 2 weeks after therapy is discontinued, prolactin levels return to pretreatment values; at times even an overshoot may occur. Whether long-term administration of bromocriptine affects permanent tumor progression is not known, but patients have been followed for as long as 17 years on therapy without evidencing a tumor regrowth. Bromocriptine is available in the United States in a short- or long-acting oral form, and in Europe in a long-acting injectable form. Surgery has an advantage of actual tumor removal and minimum morbidity, but these benefits may be offset by the significant risk of recurrent hyperprolactinemia and in some cases recurrent tumor mass necessitating long-term therapy with drugs such as bromocriptine.
|
MICROADENOMAS AND PREGNANCY.
Over the years it has become apparent that patients with intrasellar microadenomas are at minimal risk for complications during pregnancy.40 Originally there was great concern about the possibility of sudden tumor growth and its attendant complications. Fewer than 7% of reported cases, however, have manifested evidence suggestive of tumor expansion (e.g., headaches, visual disturbances, diabetes insipidus). Recognition of an intrasellar tumor is important because complications arising from expansion of a documented microadenoma during pregnancy have been exceedingly rare. Once pregnancy is confirmed in patients with a microadenoma, bromocriptine administration should be discontinued. In regard to the issue of teratogenicity, exposure of a fetus to bromocriptine either during early pregnancy in association with the induction of ovulation, or in later pregnancy during the reinstitution of drug therapy, has not been associated with an increase in congenital defects, spontaneous abortions, multiple gestations, or deficiencies in mental or physical development up to 8 years of age. In the postpartum period, breastfeeding has been permitted in patients with no evidence of tumor-related difficulty. Despite the success of pregnancy outcome, a patient with an intrasellar microadenoma should be informed of the diagnosis and educated regarding potential complications. Of utmost importance is that the patient recognize visual field defects and changes in visual acuity that may accompany progressive tumor enlargement. Examination by gross confrontation may be useful. Formal evaluation of vision performed in women with microadenomas has not been productive. Measurement of serial serum prolactin levels during pregnancy to assess tumor growth has not been efficacious and should be discouraged.
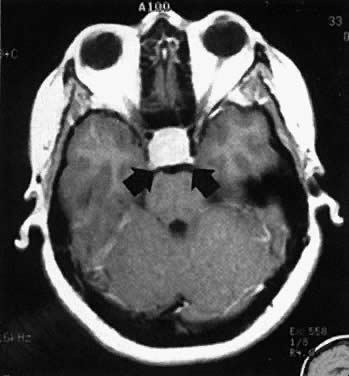
MACROADENOMAS AND PREGNANCY. In patients with macroadenomas, however, the occurrence of pregnancy has been associated with significant risk of complications (Fig. 2). Of untreated cases, the reported incidence of complications has been reported as approximately 17% by survey. Clinical symptoms related to possible tumor growth appear to occur throughout the entire length of gestation, but the onset of symptoms tends to be most common in the first trimester. The predominant symptom is headache, although nausea and vomiting frequently occur. Often it is difficult to determine whether these symptoms are a result of pregnancy or the tumor. Once visual field defects develop or visual acuity is compromised, however, substantial tumor enlargement may have already occurred. If untreated, this process can result in blindness and optic atrophy. The problems must be attended to at once by either medical treatment with bromocriptine or more frequently with operative intervention. A growing number of patients with macroadenomas have become pregnant after primary bromocriptine therapy, and several of these patients have experienced significant side effects related to extracellular tumor growth. In nearly all of these antidotal cases, the reinstitution of a drug resolved signs of an enlarging tumor and the remainder of the pregnancy was uneventful. Many patients with a history of a large tumor, however, have been followed throughout pregnancy without having a complication or requiring concurrent medical therapy. Those patients who have had tumor-related complications during pregnancy evidenced regression of lesions when bromocriptine administration was reinstituted, strongly suggesting that these lesions were actually prolactinomas. If significant reduction in tumor size has been demonstrated before an attempt at conception, then during pregnancy the likelihood exists that tumor-related complications would resolve by the reinstitution of bromocriptine. Therefore, it is generally recommended that patients with macroadenomas contemplating pregnancy undergo bromocriptine therapy (1) to reduce the size of their tumor, and (2) to achieve normal ovulation.
|
THERAPEUTIC CONSIDERATIONS IN PREGNANCY. Some patients with hyperprolactinemia and microadenomas may not harbor prolactin-secreting tumors, but rather nonfunctional neoplasms that do not respond to bromocriptine. In the absence of any measurable reduction of tumor size, the therapeutic advantage of bromocriptine may not be realized in women with an extracellular lesion. Thus, for bromocriptine to serve as a noninvasive therapeutic alternative or an adjunct in patients with microadenomas, should they become pregnant, the efficacy of the drug on tumor size must be demonstrated. In the absence of any significant effect of tumor shrinkage, then definitive therapy should be performed.
Growth Hormone-Secreting Pituitary Tumors
In children, when the epiphyseal plate is not yet closed, growth hormone excess results in gigantism. These subjects display marked increases in height, but remain eunuchoidal because of hypogonadotropism. Excessive secretion of growth hormone in adults causes acromegaly, the stigmata of which are usually mild (Table 1).
TABLE 1. Features of Acromegaly
Serum growth hormone levels > 10 ng/mL
Bone growth and soft tissue proliferation
- Nose
- Jaw
- Suborbital ridge
- Hands and feet
Carpal tunnel syndrome
Parasthesias of hands
Sweating
Thickening of skin
Increased mortality in patients more than 45 years of age
- Congestive heart failure
- Hypertension
Lethargy and weight gain
Organomegaly
- Liver
- Heart
- Kidney
Acromegalic changes characteristically occur in the face and extremities. Bone growth and soft tissue proliferation resulting from inappropriate secretion of growth hormone lead to enlargement of the nose, jaw, and suborbital ridges. In addition, the tongue may enlarge, and the teeth may become widely spread. Overgrowth of bone and soft tissue about the joints causes deformity of the hands and feet, which may hasten the onset of osteoarthritis. Carpal tunnel syndrome as well as weakness and paresthesias in the hands may also occur. The voice deepens as a result of vocal cord thickening. Excessive sweating, overproduction of sebum, and thickening of the skin are early subtle changes associated with acromegaly.
The manifestations of acromegaly are significant. After age 45, mortality from cardiovascular cerebrovascular and respiratory disease in these patients is twice that as of the normal population.41 Lethargy and weight gain occur in more than one half of these patients and hypertension in about one fourth. Enlargement of the heart, liver, and kidneys is a common finding. The cardiomegaly may be associated with congestive heart failure. Because acromegalic changes develop insidiously, significant neoplastic growth may occur before the disease is recognized. Consequently, in earlier reported series, the incidence of visual disturbance was high. Visual defects are currently encountered in approximately 20% of cases.42
The diagnosis of a growth hormone-secreting pituitary tumor is based on the basal level of serum growth hormone, the growth hormone response to hypoglycemia, and the radiologic appearance of the pituitary. A normal fasting serum growth hormone level is less than 5 ng/mL. Exercise and stress tend to increase growth hormone secretion, however, and should be considered in the interpretation of basal levels. Concentrations greater than 10 ng/mL are abnormal and occur in more than 90% of patients with acromegaly. Growth hormone levels between 5 and 10 ng/mL are inconclusive and require further evaluation. To test growth hormone response to hyperglycemia, a standard (100-g) glucose tolerance test is performed. In normal subjects, circulating levels of growth hormone are suppressed to less than 5 ng/mL by 1 to 2 hours after oral administration of glucose. If the level of growth hormone is not suppressed to less than 5 ng/mL, the diagnosis of acromegaly is established.43 This test is particularly useful in patients with borderline normal or indeterminate levels of serum growth hormone.
As mentioned above, the development of acromegaly is subtle and may go unnoticed for years. Therefore, it is not surprising that the pituitary is enlarged in nearly all patients with growth hormone-secreting tumors. Recently, immunocytochemical studies have demonstrated the presence of growth hormone and prolactin in the same secretory granule type.44, 45 Apparently, growth factors are secreted by these tumors and have been isolated by gel filtration.46 The normal pituitary gland secretes insulin-like growth factor-1 and -2, epidermal growth factor-2, basic fibroblast growth factor, transforming growth factor-α, and nerve growth factor, and some adenomas have been demonstrated to secrete chondrocyte growth factor. In 55 tumors taken from acromegalic patients, immunocytochemical analysis showed 45.5% to contain prolactin.47 Human pituitary tumors also have been shown to produce bioactive and immunoactive interleukin-6 and to express interleukin-6 messenger RNA.48
Growth hormone-secreting tumors have been shown to be influenced by other hormonal activities. For instance, somatostatin inhibits the secretion from these adenomas, and secretion is stimulated by growth hormone-releasing factor. Solution hybridization techniques to evaluate RNA have demonstrated two of the somatostatin-receptor subtypes (SSTR-3 and SSTR-4) in these tumors. Recently a third somatostatin receptor has been described, SSTR-5, which shows preference for mammosomatotrophic lineage. Finally, estrogen has been shown to induce anterior pituitary enlargement and arteriogenesis in Fisher 344 rats. This effect can be inhibited by treatment with bromocriptine.49, 50, 51, 52
The management of growth hormone-secreting pituitary tumors is directed toward suppressing excess growth hormone secretion and eliminating sequelae of gross tumor enlargement. Reduction of serum growth hormone levels to less than 10 ng/mL arrests progressive symptoms and in most instances results in the regression of acromegalic manifestations. Acromegalic changes caused by tissue proliferation appear to be the most responsive to therapy. Excess sweating is usually entirely eliminated, but bone and cartilage changes are irreversible.
Definitive therapy may be accomplished by surgery or irradiation. In properly selected cases, trans-sphenoidal removal has afforded good results with minimal complications.53 Conventional external and proton-beam irradiation have had similar success in eradicating these neoplasms.54 Radiation therapy may require 3 to 6 years before the total effect is realized, however. Evidence for hypopituitarism is low, but partial impairment of pituitary hormone reserve has been increasingly demonstrated with more sophisticated means of evaluating pituitary function. The use of bromocriptine mesylate to reduce growth hormone levels has met with good success, but whether it exerts an inhibitory regressive effect on tumor growth is unknown. This form of therapy may be helpful when patients respond poorly to other methods or when other methods are contraindicated.
Adrenocorticotropic Hormone-Secreting Pituitary Tumors
Pituitary ACTH secretion that arises from a neoplasm produces hypercortisolism (Cushing's syndrome). The clinical manifestations of hypercortisolism are listed in Table 2. A detailed description of these symptoms is given elsewhere in these volumes.
TABLE 2. Clinical Features of Cushing's Syndrome
Feature | Frequency (%) |
Obesity | 92 |
Moon facies | 90 |
Hypertension | 85 |
Glucose intolerance | 80 |
Menstrual and sexual dysfunction | 74 |
Hirustism and acne | 70 |
Striae | 70 |
Proximal muscle weakness | 67 |
Osteoporosis | 55 |
Easy bruising | 50 |
Emotional changes | 50 |
Edema | 45 |
ACTH-secreting pituitary tumors are characteristically small. In this regard, CT scanning or MRI has proved to be extremely useful: Abnormalities consistent with a small pituitary tumor have been detected in as many as 75% of patients with Cushing's disease.55 An ACTH-secreting pituitary tumor occasionally is found in patients without a discernible pituitary lesion. Recently Wilson and co-workers56 described an extracellular intercavernous sinus ACTH-releasing adenoma causing Cushing's disease.
The diagnosis established by the lack of cortisol suppression after administration of low-dose dexamethasone and partial suppression after administration of high-dose dexamethasone, normal or moderately elevated plasma ACTH levels, and an abnormal CT scan.57, 58 In normal subjects, plasma ACTH levels are less than 100 pg/mL. In patients with an ACTH-secreting pituitary tumor, the plasma ACTH level can range from 50 to 200 pg/mL.
Because ACTH-secreting pituitary tumors are small, selective removal of these lesions may be readily accomplished by trans-sphenoidal microdissection. In the hands of experienced neurosurgeons the risk of complications is low, and hypopituitarism is minimal. Another means of treatment is radiation therapy. Again, however, the total therapeutic effect may require several years.59 To achieve a reliable prediction of the integrity of the hypothalamic-pituitary axis, it has been recommended that close observation and careful monitoring with serum cortisol levels be performed after this type of surgery; routine glucocorticoid therapy is not needed.60
Nelson's syndrome is a specific clinical entity in which patients exhibit hypercortisolism and Cushing's syndrome. Nelson's syndrome occurs in 10% to 20% of patients who have Cushing's disease and who have been previously treated with a bilateral adrenalectomy. In most cases of Cushing's disease, serum ACTH levels are normal or moderately elevated, which results in hypercortisolism. Apparently the feedback from hypercortisolism partially inhibits potential pituitary ACTH secretion. When the hypercortisolism is eliminated with a bilateral adrenalectomy, negative feedback by cortisol is lost; pituitary ACTH secretion is then unrestricted. Nelson's syndrome is characterized by exceedingly high post-treatment levels of serum ACTH and MSH, which causes severe depigmentation of the skin and rapid enlargement of the pituitary. Because most cases of Cushing's disease result from a pituitary neoplasm, it is believed that these patients have a preexisting pituitary adenoma that sustains rapid growth after bilateral adrenalectomy. In fact, the growth rate often leads to impingement on vital surrounding structures. Hypopituitarism as a result of invasive tumor growth is common, and malignant transformation has been reported.61 Therefore, aggressive management is imperative. If the lesions is not amenable to surgical resection, radiation therapy should be used.
Thyroid-Stimulating Hormone-Secreting Pituitary Tumors
Pituitary tumors that actively secrete TSH are rare. The genesis of these tumors in humans in unknown, although longstanding hypothyroidism has been associated with the development of TSH-secreting pituitary tumors. In contrast, several isolated cases of TSH-secreting tumors have been reported in which patients had clinical evidence of hyperthyroidism.62 These patients exhibited elevated serum TSH levels, blunted TSH response to TRF, and an abnormal sella turcica x-ray. Of a total of 800 patients observed for a period of 15 years, Beckers and associates63 reported 7 patients with hyperthyroidism due to TSH-secreting macroadenomas. Serum TSH varied between 1.1 and 36.3 mIU/L. The serum α-subunit level was low in one case, whereas four others had elevated concentrations.64 Hyperthyroidism also has been reported due to pituitary adenomas composed of two different cell types, one apparently secreting the α-subunit alone and the other cosecreting the α-subunit along with TSH.65 These were evaluated with double-goal particle immunostaining, which showed that all cells contained secretory granules positive for the α-subunit, whereas very few were positive for β-TSH and the α-subunit.
Follicle-Stimulating Hormone-Secreting Pituitary Tumors
Like TSH-secreting tumors, those that release gonadotropins are extremely rare.66 In the few isolated cases reported, increased serum FSH or LH levels, or both, were not consistently associated with hypogonadism. Gonadotropin-secreting tumors have been misdiagnosed previously as “nonsecreting macroadenomas.” The majority of these could be recognized even in postmenopausal patients by the response of serum LH-β to TRH challenge. For instance, Daneshdoost and colleagues67 evaluated 16 women with apparent nonsecreting adenomas and treated them with TRH; 11 had significant responses. The administration of a GnRH agonist increased rather than decreased LH in α-subunit levels in a single patient with an LH-secreting pituitary tumor, and the administration of GnRH agonists have likewise been shown to reduce prolactin secretion when chronically administered to patients with prolactinomas.68, 69 Human pituitary tumors that secrete chorionic gonadotropin have been described, as have glycoprotein-producing pituitary tumors that exhibit pulsatile glycoprotein hormone secretion.70, 71 Gonadotropin-secreting tumors have been suppressed with the administration of dopamine agonists such as bromocriptine, and cabergoline has been found to decrease both FSH and prolactin secretion in macroadenomas.24, 72, 73, 74
Craniopharyngioma
Craniopharyngioma is the most common sellar neoplasm of childhood and adolescence; only one third of cases involve adult patients. Craniopharyngiomas also have been found in two siblings.75 These tumors arise from remnants of Rathke's pouch and are more commonly seen as suprasellar cystic lesions than as intrasellar tumors. The latter may be radiographically indistinguishable from nonfunctioning pituitary adenomas. The finding of suprasellar calcification in more than one half of patients suggests the diagnosis. Unlike nonfunctional tumors, craniopharyngiomas often demonstrate aggressive growth extending into the optic chiasm, hypothalamus, and third ventricle, causing visual defects, diabetes insipidus, and signs of increased intracranial pressure. Primary therapy consists of surgical resection of the tumor and drainage of the cystic lesions.76 The tumor occasionally invades surrounding vital structures and adheres to them so that complete removal of the tumor is not technically feasible. In these cases, postoperative radiation therapy is indicated and may decrease the rate of tumor recurrence.
Empty Sella Syndrome
The primary empty sella syndrome is a frequent cause of sellar enlargement, particularly in middle-aged, obese women who are often hypertensive.77 Headache is a common symptom. Associated conditions include those that result in increased intracranial pressure, such as pseudotumor cerebri. The primary empty sella develops from a congenital defect of the sellar diaphragm through which herniation of the arachnoid membrane occurs. Consequently, cerebrospinal fluid pressure is transmitted into the sellar space, thus enlarging it. In general, the sellar enlargement is uniform and symmetric, although asymmetry suggestive of an intracellular neoplasm may be observed. It has been speculated further that pituitary tumors may exist for an extended period of time, undergo infarction, and then give rise to the empty sellar syndrome.78 Antipituitary antibodies also have been described in patients with empty sella syndrome and may be part of the pathogenesis of this problem.79
The diagnosis of empty sella syndrome is usually made at the time of CT scanning or MRI by the demonstration of air in the sella. The sella may be partially or totally empty. In either situation, pituitary function is usually completely normal. Management of these patients is conservative and expectant, and surgical intervention is not indicated. Many of these patients, however, will have hyperprolactinemia with concurrent signs of hypoestrogenism, and these patients are generally treated with either replacement hormonal therapy or bromocriptine. As with patients who have a microadenoma of the pituitary, estrogen replacement therapy or oral contraceptives are not contraindicated in patients with empty sella syndrome. Prospective trials have demonstrated no effect of oral contraceptives on the growth of microadenomas, and the slight increase in prolactin secretion produced by oral contraceptives is not clinically significant.80, 81