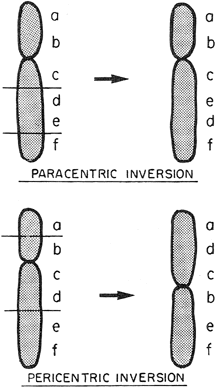
Luteal Phase Defects DIAGNOSIS AND VALIDATION. Implantation in an inhospitable endometrium is an entirely plausible explanation
for spontaneous abortions. Progesterone deficiency in particular
could result in the estrogen-primed endometrium being unable to sustain
implantation. Luteal phase deficiency (LPD) describes the condition
in which the endometrium manifests an inadequate response to progesterone, irrespective
of reason. Progesterone secreted by the corpus
luteum is necessary to support the endometrium until the trophoblast produces
sufficient progesterone. The pathogenic mechanisms postulated
as explanations for LPD include decreased secretion of gonadotrophin-releasing
hormone (GnRH), decreased follicle-stimulating hormone (FSH), decreased
luteinizing hormone (LH), inadequate ovarian steroidogenesis, endometrial
receptor defects, or deficiencies of any of the gene products
induced by progesterone (e.g., glycodelin, integrins, or MMPs). Once almost universally accepted by gynecologists as a common cause for
fetal wastage, LPD is now generally considered an uncommon explanation
for clinical pregnancy loss. One major problem is lack of reproducible
diagnostic criteria. Another is that endometrial histology identical
to that observed with luteal phase “defects” exists in fertile
women. When regularly menstruating fertile women having no history
of abortions underwent endometrial biopsies in serial cycles, LPD
was found in 51.4% in any single cycle and 26.7% in sequential cycles.99 Not only is the diagnosis of LPD not specific, but interobserver variation
in reading endometrial biopsies is considerable. Biopsies read by
five different pathologists resulted in one third of patients having
differences of interpretation sufficient to alter management.100 Pathologists reading coded endometrial biopsy slides a second time agreed
with their own initial diagnosis in only 25% of cases.101 With the possible exception of daily serial progesterone assays in research
settings, measuring serum hormone levels shows little improvement
in sensitivity and specificity over that of endometrial biopsy. A single
low serum progesterone value in the luteal phase is only 71% predictive
of a luteal phase defect as defined on the basis of an abnormal
endometrial biopsy.102 Soules and colleagues103,104 have long attempted to characterize gonadotropin and progesterone secretion
in LPD. This group believes that diagnosis is best made on the basis
of a single assay of three pooled blood samples; a level of more
than 10 ng/mL defines LPD.105 Combining Doppler ultrasound and hormone assays has been considered as
an alternative to endometrial biopsy, but this approach was not verified
as reliable by Sterzik and associates.106 Li and coworkers107 showed a correlation between low plasma progesterone levels (less than 30 nmol/L) and
endometrial dating 2 days behind that expected on the
basis of LH surge; the biopsy was taken 7 days after the LH surge. Of 24 women
with recurrent abortions whose plasma progesterone level was
less than 30 nmol/L, 8 showed endometrial delay; of 62 with a progesterone
level of more than 30 nmol/L, only 7 showed a delay. The same study
further concluded that the prevalence of endometrial defects was higher (27%) than
in a control group of 22 fertile women with no prior abortions (11%). Like
similar studies, voluminous endocrine data were gathered, but
relatively few other potential confounding variables (e.g., maternal
age) were taken into account. Endometrial abnormalities due to hormonal deficiency could be the explanation
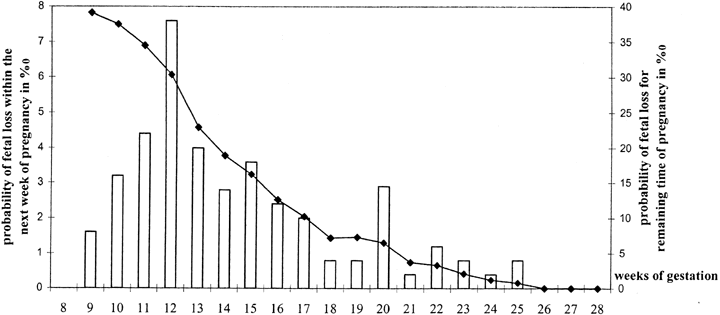
for the finding by Wilcox and coworkers5 of progressively increased loss rates beginning on day 9 after ovulation; no
pregnancies occurred beyond day 12. The other explanation could
be late implantation of slower-growing genetically abnormal embryos. Molecular analysis of markers such as endometrial receptors or factors
genes one day might prove useful, provided their perturbation is indeed
the explanation for LPD. However, no such explanation for LPD has been
proved. It is possible that LPD is a secondary effect of abnormal oocytes
and nature's method of preventing abnormal concepti from developing
into abnormal babies. TREATMENT. The efficacy of treatment is unproved, principally because no randomized
studies have validated LPD as a genuine entity. Studies by Tho and associates108 and by Daya and Ward109 have been cited as providing some evidence of efficacy, but their experimental
designs can be criticized because concurrent control groups were
not recruited. Li and coworkers110 identified 21 women with three or more consecutive first-trimester losses. Following
their previously published protocol,111 they obtained blood or urine daily for 9 days until the LH surge; 7 days
later, timed endometrial biopsy was obtained to detect LPD, diagnosed
as more than 2 days behind chronologic dating. Among 25 subsequent
pregnancies by the 21, 13 conceived without and 12 conceived with ovarian
stimulation (hMG followed by hCG). The two groups were apparently
not randomized. Of 13 pregnancies conceiving with ovarian stimulation, 11 continued
to at least 24 weeks; of 12 conceiving without ovarian stimulation, only 5 continued to at least 24 weeks. Results were statistically
significant by chi-square testing but there was no correction (Yates) for
small numbers and no adjustment for potential confounding
variables (e.g., maternal age). Meta-analysis by Karamardian and Grimes112 showed no beneficial effect of progesterone treatment. The consensus is
that LPD is either an arguable entity or cannot be proved to be treated
successfully with progesterone or progestational therapy. Using the St. Mary's (London) Early Pregnancy Assessment unit cohort, Rai
and colleagues113 found that 11% (53/486) of unexplained recurrent aborters had elevated
LH levels. However, neither Carp and coworkers114 nor Tulppala and associates115 observed elevated LH levels in women experiencing recurred losses. Bussen
and colleagues116 failed to find elevated LH levels among 42 women with recurrent losses, despite
such women showing prolactin and androstenedione levels 40% higher
than those in 15 other women who showed normal LH and two losses. We
conclude that LH elevations by themselves are not significant but
could point to other underlying problems. Thyroid Abnormalities Decreased conception rates and increased fetal losses are associated with
overt hypothyroidism or hyperthyroidism. Subclinical thyroid dysfunction
has not generally been considered an explanation for repeated losses.117 However, thyroid perturbation could play a role. Bussen and Steck118 studied 22 women with recurrent abortions in comparison to a like number
of nulligravid and multigravid controls. Thyroid antibodies were increased
in the former. Stagnaro-Green and associates119 and Glinoer and coworkers120 concluded that antithyroid antibodies and mild thyroid disease were associated
with spontaneous abortions, and Singh and colleagues121 opined that thyroid antibodies were a useful marker for clinical losses
in the assisted reproductive technology population. Earlier, Pratt and
associates122 reported increased antithyroid antibodies in euthyroid women experiencing
first-trimester losses, but the same group123 concluded that the ostensible association was secondary to nonspecific
organ antibodies. Rushworth and colleagues124 prospectively studied 870 women in the St. Mary's (London) Early
Pregnancy Assessment Unit; all women had at least three spontaneous abortions. Of
these 1621, 19% had antibodies against thyroglobulin or thyroid
microsomal factors. However, subsequent outcomes did not significantly
differ whether women were positive or negative for thyroid antibody. Overall, asymptomatic thyroid antibodies would not seem to be a major cause
of early pregnancy loss. Diabetes Mellitus Women with poorly controlled diabetes mellitus clearly show an increased
risk for fetal loss. In the cohort best studied to address this question, women
whose glycosylated hemoglobin level was greater than four
standard deviations above the mean showed higher pregnancy loss rates
than either diabetic women showing lower glycosylated hemoglobin levels
or euglycemic controls.3 Total pregnancy loss rates were 16.1% (62/386) compared with 16.2% (70/432) in
controls. Almost all these losses were early in pregnancy, by 8 weeks. Similar
conclusions have been repeatedly reached in retrospective
studies.125 Poorly controlled diabetes mellitus should thus be considered one cause
for early pregnancy loss. However, subclinical or gestational diabetes
is probably not a major etiologic factor because the number of insulin-dependent
diabetic women who experience pregnancy loss and have poor
control is too small to exert a large attributable effect. Intrauterine Adhesions (Synechiae) Intrauterine adhesions could interfere with implantation or with early
embryonic development. Most often these adhesions arise after overzealous
uterine curettage during the puerperium or intrauterine surgery (e.g., myomectomy). Adhesions are most likely to develop if curettage is
performed 3 or 4 weeks postpartum. Women with uterine synechiae usually
manifest hypomenorrhea or amenorrhea, but 25% to 30% show repeated
abortions. Adhesions surely cause early pregnancy failure in rare individuals, but
the overall contribution to pregnancy loss is probably very
small. Incomplete Müllerian Fusion Müllerian fusion defects are well-accepted causes of second-trimester
losses and pregnancy complications. Low birthweight, breech presentation, and
uterine bleeding are also commonly accepted correlates. However, the
rate of incomplete Müllerian fusion in early (first-trimester) losses
is probably overstated. Most studies lack controls126,127,128,129,130,131,132 or uncritically pool early and later pregnancy losses. One major problem in attributing second-trimester losses to uterine anomalies
is that both phenomena occur so frequently that concurrent adverse
outcomes could be coincidental. Stampe-Sorenson126 found unsuspected bicornuate uteri in 2 of 167 (1.2%) women undergoing
laparoscopic sterilization; another 3.6% had a septate uterus and 15.3%showed
fundal anomalies. Simon and associates133 found Müllerian defects in 3.2% (22/679) of fertile women; 20 of
the 22 defects were septate. A more unbiased figure was found by Byrne
and colleagues,134 who performed ultrasound in women not undergoing imaging for gynecologic
reasons. The frequency was estimated to be 0.4%(8/2065). Some studies claim an increased rate of spontaneous abortions in women
with septate uteri132 or T-shaped uteri.129 In septate uteri, an increased risk of pregnancy loss could plausibly
reflect implantation occurring on an inhospitable fibrous surface. There
is less reason to believe early losses would be increased with bicornuate
uteri or T-shaped uteri, but poor vascularization could still play
a role. Other studies show no discernible differences among various
subtypes.128 Grimbizis and coworkers135 reviewed several series to divide abortion rate by subtype. Overall, the
authors concluded that uterine malformations were found in 13% of women
with recurrent losses and 5% of fertile women. The highest rates
were for untreated septate uterus (44.3%); rates were for bicornuate uterus 36.0%, untreated
arcuate uterus 25.7%, didelphys 32.2%, and unicornuate
uterus 36.5%. Losses later in pregnancy can more confidently be attributed to uterine
anomalies. Losses occurring in the first trimester after 8 weeks but
lacking confirmation of prior fetal viability are statistically more likely
to represent missed abortions in which fetal demise actually occurred
weeks earlier. Losses occurring before 8 weeks should in general
be attributed to other causes, although doubtless some are due to implantation
on septi. Quantitative estimates of the role that uterine anomalies
play in first-trimester losses cannot be cited with confidence. Leiomyomas Leiomyomas occur frequently and produce clinical problems well recognized
by gynecologists. Leiomyomas plausibly could also cause early pregnancy
loss, but analogous to uterine fusion anomalies, the coexistence
of uterine leiomyomas and reproductive losses need not necessarily imply
a casual relationship. The location of leiomyomas is more important than size, submucous leiomyomas
being most likely to cause abortion. Plausible mechanisms increasing
pregnancy loss rates might include thinning of the endometrium over
the surface of a submucous leiomyoma, predisposing to implantation
in a poorly decidualized site. Rapid growth of leiomyomas could occur
due to the hormonal milieu of pregnancy, compromising blood supply and
resulting in necrosis (“red degeneration”) that in turn leads
to uterine contractions or infections that eventually lead to fetal
expulsion. Clinically, it is best initially to assume that leiomyomas have no etiologic
relationship to pregnancy loss. Surgery for this indication alone
should thus be undertaken with reticence. Incompetent Internal Cervical Os A functionally intact cervix and lower uterine cavity are obvious prerequisites
for a successful intrauterine pregnancy. Characterized by painless
dilation and effacement, cervical incompetence usually occurs during
the middle of the second trimester or the early part of the third
trimester. This condition frequently follows traumatic events such as
cervical amputation, cervical laceration, forceful cervical dilatation, or
conization. There is little reason to postulate a relationship to
first-trimester losses. Intermittently the question arises as to whether prior induced abortion
is associated with subsequent loss. The long-term consensus is that little
if any relationship exists;136 however, controversy persists.137,138 Not all studies have taken into account obvious potential confounding
variables such as increasing maternal age in subsequent pregnancies. Indeed, an
increased loss rate is observed as the number of prior terminations
increases,139 but this increase merely parallels that with increasing numbers of spontaneous
losses. Infections Infections are accepted causes of late fetal losses and logically could
be responsible for early fetal losses as well. Among the many microorganisms
reported to have been associated with spontaneous abortion are
variola, vaccinia, Salmonella typhi, Vibrio fetus, malaria, cytomegalovirus, Brucella, toxoplasmosis, Mycoplasma hominis, Chlamydia trachomatis, and Ureaplasma urealyticum. Transplacental infection doubtless occurs with each of these microorganisms, and
sporadic losses could logically be caused by any. Proof that infections truly cause repetitive losses has been less forthcoming. One
line of indirect evidence is that certain organisms (e.g., U. urealyticum and M. hominis) have been isolated in midtrimester placentas and abortuses, but only
rarely from induced (control)midtrimester abortions.140 Other evidence consists of studies in which empiric antibiotic therapy
ostensibly benefits couples experiencing repeated losses. For example, repetitive
aborters treated for 4 weeks with tetracycline before pregnancy
showed a subsequent fetal loss in only 10%;141 aborters who chose not to take tetracycline showed a 38% loss rate. However, the
two groups (treated and untreated) were not randomized and
therefore were not necessarily comparable. Other studies have not found
any difference in outcome between women treated or not treated with
antibiotics.142 BACTERIAL VAGINOSIS. Some studies have suggested a relationship between bacterial vaginosis, presumed
due to Gardnerella vaginalis, and abortion. There are few data specific to early losses. Most literature
in this field has focused on the relationship to pregnancy complications
in the second and third trimesters. For example, Hay and associates143 found a 5.5-fold increased risk for losses at 16 to 24 weeks, and McGregor
and colleagues144 found an increased risk for losses before 22 weeks. Another study involved
Belgian women seen at obstetric registration earlier than 14 weeks.145 At the initial visit, an investigator knowledgeable in bacterial vaginosis
performed vaginal fluid microscopy, searching for clue cells and
leukocytes; vaginal and cervical cultures were initiated for G. vaginalis, U. urealyticum, and M. hominis, various other bacterial species, Chlamydia trachomatis, and herpes simplex. Of 218 pregnancies, 21 (10%) aborted, and in this
group bacterial vaginosis was five times more common (relative risk 5.5, 95%confidence
interval 2.9–10); gestational age at pregnancy
loss was usually less than 14 weeks (mean 11.3 ± 2.9 weeks). Relative
risk for pregnancy loss was also increased for U. urealyticum (1.58) and M. hominis (1.25) but not for herpes, chlamydia, or enteric bacteria. Regression
analysis led the authors to conclude that bacterial vaginosis was the
likely explanation. It is uncertain whether the above study took into
account key confounding variables such as maternal age, prior pregnancy
history, or gestational age. The magnitude of the attributable risk
found in this study also is at considerable odds with other studies. Findings
could be applicable only to this particular sample population, could
be explained on the basis of failing to take into account confounding
variables, or could be applicable only to pregnancies of later
gestational age (late first and early second trimester). Recall that all
but 3% of pregnancy losses have occurred by 8 weeks and all but 1% by 16 weeks. U. urealyticum AND M. hominis. Of the organisms implicated in repetitive abortion, these seem most plausibly
related to repetitive spontaneous abortions because they fulfill
several prerequisites: the putative organism can exist in an asymptomatic
state, and virulence is not universally severe enough to cause infertility
due to fallopian tube occlusion and, hence, preclude the opportunity
for pregnancy in which spontaneous abortions might occur. Kundsin
and associates146,147 have long contended that Ureaplasma is associated with recurrent abortions. From 46 women with histories of
three or more consecutive losses of unknown etiology, Stray-Pedersen
and colleagues148 recovered mycoplasma significantly more often among women with repetitive
abortions (28%) than among controls (7%). Infected women and their
husbands (n = 43) were then treated with doxycycline, with subsequent
cultures confirming eradication of mycoplasma. Nineteen of the 43 women
became pregnant; of the 19, 3 experienced another spontaneous abortion, whereas 16 had
normal full-term infants. Among 18 women with untreated
mycoplasma, there were only five full-term pregnancies. A recent
study showing a positive correlation between pregnancy loss earlier than 14 weeks
and U. urealyticumM. hominis has been discussed above.145 HERPES. Data are less compelling than for other organisms. Women with a history
of herpesvirus have shown a higher spontaneous abortion rate than controls, 34% versus 10.6% in
an early study by Nahmias and coworkers.149 Other reports substantiated this potential relationship.150 However, potential confounding variables were not taken into account in
the above studies. Donders and colleagues145 found no correlation, despite doing so for several other organisms. C. trachomatis. Chlamydial antibodies have been sought in the sera of women who experienced
repeated losses, and an association was claimed on the basis of high
antibody titers.151,152 Gronroos and associates150 studied a population of women with threatened abortions and concluded
that cervical IgA (but not IgG) antibody liters were increased in women
who actually aborted. Other data show no relationship.153,154 Rae and colleagues154 found no significant difference between the frequency of IgG anti-chlamydial
antibodies in the sera of women with recurrent abortion (n = 106) versus controls (n = 3890; antipodal gravidas), 24.5% versus 20.3%, respectively. Paukku
and associates155 reported no differences in the frequencies of C. trachomatis (IgG or IgA antibodies) in 70 Finnish women with histories of spontaneous
abortions, compared with 40 parous women and 94 asymptomatic sexually
active women. Donders and associates145 also found no relationship in their prospective Belgian study. In conclusion, most studies indicate no significant role for C. trachomatis. A caveat is the possibility that only certain strains of chlamydia could
confer embryotoxicity. The power to detect such an effect would be
limited. TOXOPLASMOSIS. Toxoplasmosis antibodies have been observed in Mexican and in Egyptian
women having repetitive losses.156,157 However, the ubiquitous nature of this organism makes it unclear whether
antibody frequencies are higher than in the general Mexican or Egyptian
populations in which toxoplasma is endemic. Most recent studies have
tended to conclude that toxoplasmosis is not a significant cause of
reproductive loss.158 IS INFECTION AN EPIPHENOMENON IN PREGNANCY LOSS? Do the infectious agents discussed above actually cause fetal losses, or
do they merely arise after fetal demise due to other etiologies? To
what extent do the two phenomena overlap? Cohort surveillance for infections
beginning in early pregnancy can help shed light on the true role
of infections in pregnancy loss. To this end, the frequency of infections
in pregnant women was prospectively determined using the multicenter
United States NICHD Diabetes in Early Pregnancy study alluded to
earlier.159 Data were collected prospectively on the frequency of clinical infections
in 386 diabetic subjects and 432 control subjects seen frequently (weekly
or every other week) during the first trimester. Infection occurred
no more often in 112 subjects experiencing pregnancy loss than in 706 having
successful pregnancies. This held true both for the 2-week
interval in which a given loss was recognized clinically as well as
in the prior 2-week interval. Similar findings were observed in both control
and diabetic subjects and further held true when data were stratified
into ascending genital infection only versus systemic infection
only. CONCLUSION. Infections surely explain some pregnancy losses, especially later in the
first trimester and subsequently throughout pregnancy. However, the
attributable risk is probably low for an early loss, even in sporadic
cases, and lower still among women experiencing repetitive abortions. Antiphospholipid and Anticardiolipin Antibodies An association between second- and third-trimester pregnancy loss and certain
autoimmune phenomena is accepted.160,161 Antibodies found in women with pregnancy loss are diverse, encompassing
nonspecific antinuclear antibodies (ANA) as well as antibodies against
such specific cellular components as phospholipids, histones, and double- or
single-stranded DNA. Antiphospholipid antibodies (aPL) in turn
represent a broad category that encompasses lupus anticoagulant (LAC) antibodies
and anticardiolipin antibodies (aCL). The consensus has
long been that midtrimester fetal death rates are increased in women with
LAC or aCL, perhaps dramatically so.162 Controversy centers on the role these antibodies play in first-trimester
losses. Descriptive studies initially seemed to show increased aCL levels in women
with first-trimester pregnancy losses. However, frequencies of various
antiphospholipid antibodies (LAC, aCL, aPL) soon were shown to be
similar in women who experienced and who did not experience first-trimester
abortions.163,164,165,166 A major pitfall in assessing the role these antibodies play in first-trimester
losses is the unavoidable selection bias in ascertaining and
studying couples only after they have presented with spontaneous abortions. That
antibodies did not arise until after the pregnancy loss cannot
readily be excluded. To address this pitfall, the multicenter NICHD
collaborative study cohort (Diabetes in Early Pregnancy study) was again
used, analyzing sera prospectively obtained from insulin-dependent
and nondiabetic women within 21 days of conception. A total of 93 women
who later experienced pregnancy loss (48 diabetic, 45 nondiabetic) were
matched 2:1 with 190 controls (93 diabetic, 97 nondiabetic) who
subsequently had a normal live-born offspring.167 No association was observed between pregnancy loss and the presence of
either aPL or aCL. Neither aCL nor aPL would thus seem to contribute
greatly, if at all, to first-trimester pregnancy losses in the general
population. The issue is, however, not closed in the opinion of some. It has been stated
that results would be different if more specific assays were performed, such
as antiphosphatidylethanolamine.168 The marked variation in antiphospholipid antibodies occurring during pregnancy
is another confusing factor.169 Could antibodies connote significance only in selected clinical subsets, such
as women attempting to achieve pregnancy by in vitro fertilization? This is plausible, given that some reports show an increased
prevalence of aCL170 in women requiring in vitro fertilization. The possibility of an effect restricted to the group experiencing
only many repetitive abortions cannot be excluded. Power calculations
are not adequate to exclude categorically an effect operative
only in the 1% to 3% of women with three or more repetitive losses, or
especially in the few having five or more. However, one third of women
in the cohort study by Simpson171 experienced at least one prior loss, and in that group the frequency of
aCL and aPL was still no greater than among women without prior losses.172 It has also been claimed that even if aCL and aPL are not present, antibodies
to β2 glycoprotein (aβ2 GP-1) might still be increased and relevant to repetitive aborters. However, Balasch
and coworkers173 found no such increase. Only 1 of 100 women having repetitive abortions
and not having LAC or aCL showed a β2 GP-1. Any possible association between a β2 GP-1 and spontaneous abortions is also unlikely to be independent of
the association with aPL and aCL. No relationship is likely to exist between
a β2 GP-1 and implantation failure in the in vitro population, despite earlier claims by Stern and associates.84 In conclusion, a relationship between first-trimester loss and aPL and
aCL seems unlikely to be generally applicable. Treatment regimens should
thus be embarked on with reticence and initiated only with relatively
nontoxic agents (e.g., aspirin and heparin, but not steroids). Specifically
giving aspirin to women having only prior first-trimester abortions
shows no improvement in outcome compared with those not given aspirin (68% [251/367]vs. 64% [278/438])113 These results contrast with the beneficial effect observed in women having
a previous second-trimester loss (65% vs. 49%). Administering intravenous
immunoglobulin is not recommended.174,175 Anti-sperm Antibodies Anti-sperm antibodies (ASA) are another group of antibodies in which a
relationship to fetal loss was once claimed. After vasectomy, approximately 50% of
men show ASA. In men, the presence of these antibodies connotes
difficulty in impregnating women even after vasectomy is surgically
reversed. Women manifesting ASA could have their fertilization adversely
affected. Several studies show an increased frequency of ASA among
women experiencing repeated abortions.176,177,178,179 Others reached opposite conclusions.180,181 The biologic basis for an association might be cross-reaction with paternally
derived whole-body antigens essential for embryonic survival. Again seeking to obtain prospective data on the relationship between presence
of ASA in maternal sera and first-trimester pregnancy losses, first-trimester
sera from the NICHD DIEP cohort were studied. Recruited
within 21 days of conception, a total of 111 women who experienced pregnancy
loss (55 diabetic, 56 nondiabetic) were matched 2:1 with 104 diabetic
and 116 nondiabetic women (controls) who subsequently had a normal
live-born infant.182 No differences were observed with respect to IgG, IgA, or IgM binding
when a positive ASA test was defined as 50% of sperm showing antibody
binding. At 20% binding, no association was found to IgG and IgM ASA antibodies, although
a significant difference was observed for IgA ASA. This
single positive finding probably reflects multiple comparisons. In conclusion, the presence of ASA contributes little to pregnancy loss
in the general population. A role in the selective subset of couples
experiencing repeated losses is not excluded. Hypercoagulopathies Any relationship between fetal loss and aCL or aPL could reflect placental
thrombosis. If so, other maternal hypercoagulable states could be
associated with increased fetal losses. Currently observed associations
include factor V Leiden (Q1691G→A), prothrombin 20210G→A, and
homozygosity for the 677C→T polymorphism in the methylene tetrahydrofolate
reductase gene(MTHFR). Studying sera from women previously experiencing repetitive losses, Rai
and associates183 found an association between activated protein C resistance and second-trimester
losses. The prevalence of factor V Leiden was 7.1% in women
with abortions184 versus 4% to 5% in the general population; no association was observed
between factor V Leiden and aPL or aCL. Women with histories of repetitive
abortions who had factor V Leiden and then became pregnant had a
lower likelihood (4/11 [30%]) of a live birth than women who
lacked factor V Leiden (77/177[60%]).185 This group also found thromboelastographic abnormalities to be more common
in repetitive aborters than in controls,186 offering a way to identify high-risk women. In this group's latest
contribution,187 they showed that acquired activated protein C resistance but not congenital (factor
V Leiden) activated protein C resistance was more common
in aborters. To elevate acquired activated protein C resistance, they
studied 904 women with three or more consecutive losses at less than 12 weeks
and 207 women with at least one loss after 12 weeks. Acquired
activated protein C resistance Leiden mutation was more common in both
groups (8.8% each, 87% late) than in 150 controls (3.3%). By contrast, the
rate of factor V Leiden (the congenital form of activated protein
C resistance) was similar among the three groups (3.3% of 1808 women
with recurrent early loss, 3.9% of 414 women with late loss, and 4.0% of 300 controls). In
Brazilian women, Souza and coworkers188 found factor V Leiden in 4 of 56 (7.1%) aborters versus 6 of 384 (1.6%)controls; factor
II G20210A (prothrombin) was found in 2 of the 56 (3.6%) aborters
versus 4 of 384 (1%) controls. Not all authors accept a relationship. Dizon-Townsend and associates,189 Preston and colleagues,190 Pauer and coworkers,191 and Balasch and associates192 failed to find a relationship between early losses and the presence of
factor V Leiden. Deficiencies of antithrombin, protein C, or protein
S were found by Kutteh and colleagues193 to be no more frequent in 50 women with three or more losses than in 50 controls. Coumans
and associates194 assessed 52 women with two or more losses before 12 weeks for markers
and found no relationship to any of these same hemostatic markers. An
increased frequency of hyperhomocystinemia was observed (6 of 35 patients
tested [17.1%])compared with controls (4.5%). Such a relationship
was also shown by Grandone and colleagues195 and Ridker and associates.196 In conclusion, hypercoagulable states, especially those confirmed by factor
V Leiden or prothrombin 20120G→A, seem plausible causes of repetitive
losses. Additional studies would be helpful. Anti-fetal Antibodies, Embryotoxic Antibodies, and Aberrant Th1 Cytokine
Production An otherwise normal mother may produce antibodies against her fetus on
the basis of genetic dissimilarities. Obstetricians are familiar with
late pregnancy loss caused by Rhesus-negative women having anti-D antibodies. More
relevant for early pregnancy loss is isoimmunization due
to anti-P antibodies, a phenomenon that adheres to the same principles
as Rh(D)isoimmunization. Most individuals are genotype Pp or PP, but
homozygosity for p exists (pp). If a woman of genotype pp has a Pp or
PP husband, her offspring may or must be Pp. If the mother develops anti-P
antibodies, pp fetuses will be rejected (aborted) early in gestation. Hill and colleagues197 proposed that aberrant cytokines can cause repetitive abortions in women
acting through T-helper cell perturbations. The rationale centers on
the belief that T-helper 1 (Th1) cytokines are deleterious, whereas
Th2 cytokines are not. The former include tumor necrosis factor (TNF), interleukin (IL) -2, and
interferon (IFN)-gamma; the latter include IL-4, IL-5, IL-6, and
IL-10, all secreted by activated T cells expressing
the CD4 phenotype. Natural killer cells expressing CD56 also produce
these salutary cytokines. In women with recurrent loss, immune cell
responsiveness is activated to produce increased IFN-gamma and TNF.197 In support of the hypothesis that Th1 cytokines are deleterious, downregulation
of Th1 cytokine in rodents improves pregnancy outcome.198,199 Progesterone therapy is sometimes stated to ameliorate against deleterious
effects. The presence of embryotoxic antibodies and aberrant cytokines are reasonable
hypotheses, but their existence before human recurrent embryonic
loss has not been conclusively established. Prospective population-based
studies in women without a prior history of abnormal outcomes are
needed. The contribution of this phenomenon to pregnancy loss in the general population
remains uncertain. Alloimmune Disease (Shared Parental Antigens) A long-standing biologic puzzle is why the fetus is not rejected by its
mother on the grounds of having foreign (paternal) antigens. The maternal
immunologic response must be militated against through blocking or
suppressive factors unique to pregnancy. Paradoxically, the protective
mechanism could involve maternal—paternal differences (i.e., compatibilities). PARENTAL HLA SHARING. Parental (and hence maternal—fetal) histoincompatibility has been
proposed as salutary (counterintuitively) for pregnancy maintenance. Evidence
in support of a beneficial effect for maternal—fetal incompatibility
can be cited. Increased placental size exists in mice arising
from matings in which paternal and maternal histocompatibility antigens
differ, with higher implantation frequencies occurring in histoincompatible (H2) murine
zygotes. It follows that human HLA antigens
shared between mother and father could lead to maternal—fetal homozygosity
for a given allele, potentially exerting a deleterious effect. Whether
human HLA sharing per se is a mechanism underlying pregnancy loss in humans is unclear. Initially
studies showed greater parental HLA sharing in aborters than in controls.200,201 However, couples sharing HLA-DR antigens may experience no spontaneous
abortions despite 10 or more pregnancies.202 Thus, the story was destined to be complex. Rigorous population-based studies have been conducted by Ober and colleagues,203,204 who studied the relationship of pregnancy losses and parental HLA-β sharing in the Hutterites. The Hutterites are a genetic isolate, inbreeding
producing a high rate of homozygosity by descent and, hence, opportunities
for manifesting recessive alleles. These studies involved high-resolution
HLA typing (alleles at 16 loci) in 31 Hutterite colonies
in South Dakota. Pregnancy outcome was followed through a calendar diary
and home pregnancy test kits, used if menses had not begun 1 month
after prior menstrual bleeding. Data were available on 251 pregnancies
in 111 couples.203,205 HLA genotyping was performed on surviving offspring to determine whether
losses selectively occurred with a specific genotype. Comparisons involved
offspring homozygous for the shared antigen and having inherited
the maternal allele, versus offspring heterozygous and having not inherited
the maternal allele shared with the spouse. Loss rates were greatest
if couples shared alleles at all 16 loci, presumably reflecting
inheritance of a common HLA haplotype (odds ratio 4.39). Sharing was
greater for HLA-B (odds ratio 2.54) than for either HLA-C (odds ratio 2.20) or
complement C4 (odd ratio 2.11); all pairwise comparisons to
controls were statistically significant, but only when couples sharing
the entire haplotype were excluded. No deficiency of homozygous children
was observed. If offspring were heterozygous yet identical to the
mother, 13.6% fewer than expected living children were observed (p = 0.095); however, the sample size for this comparison was small. PARENTAL SHARING AT LOCI OTHER THAN HLA. Genetic explanations other than HLA sharing per se could explain why only some couples who share HLA antigens show untoward
outcomes. Any deleterious effect could reflect maternal—fetal
histoincompatibility, but not for HLA. The causative locus could be closely
linked, specifically to HLA-B. This hypothesis would be consistent
with HLA-G being the only HLA antigen expressed on trophoblasts. Genes responsible for deleterious effects exerted through shared parental
alleles may not even be immunologically mediated. A lethal recessive
gene, again perhaps closely linked to HLA, could exist. Murine embryos
homozygous for certain alleles at the T/t locus die at early stages
of embryogenesis. A T/t-like complex in humans could help explain the
rare kindreds in which multiple family members have repeated pregnancy
losses. 206 Postulating a mutant gene in heterozygous form in parents implies autosomal
recessive inheritance. If only homozygous offspring were lethal, the
ratio of abortuses to live-borns should be 1:3 (25%:75%); however, in
families in which the mechanism might usually be presumed operative, the
clinically observed ratio seems closer to 1:0. IMMUNOTHERAPY. If fetal rejection occurs as a result of diminished fetal—maternal
immunologic interaction (alloimmune factors), immunotherapy to stimulate
beneficial blocking antibodies generated at the few potentially differing
loci is a reasonable hypothesis. The rationale was originally
based on observations that blood transfusions before kidney transplantation
decreased the rate of allograft rejection.207 Women lacking blocking antibodies but sharing HLA antigens with their
spouse were immunized with paternal leukocytes, third-party leukocytes, or
trophoblast membranes. The first prospective randomized trial yielded
impressive results,208 but later studies were universally less so. A multicenter U.S. effort
pooling the results of immunotherapy by injection of paternal leukocytes
showed only an 11% increased pregnancy rate in the immunized group.209 Meta-analysis by Fraser and colleagues210 found an odds ratio of only 1.3 in favor of a beneficial effect. The definitive study was reported in 1999 by Ober.204 This NICHD collaborative study involved six U.S. and Canadian centers, identifying
women with three or more spontaneous abortions of unknown
cause. Subjects were aged 40 years or younger, had no anti-HLA antibodies, and
had no evidence for known or suspected causes of spontaneous
abortion (parental chromosomal translocations, LPD, uterine anomalies, aCL, LAC). Women
randomized into one arm (n = 91) underwent immunization
with paternal mononuclear cells; women in the other arm were given
saline (controls)(n = 92). Pregnancy beyond 28 weeks occurred in 46% (31/68) in
the immunized group versus 65% (41/63) in the nonimmunized
group (p = 0.26). Of course, these findings were the opposite of what would be
expected if immunotherapy had been salutary. Adjustments for potential
confounding variables (e.g., maternal age, prior pregnancy) failed to
alter the conclusions. Significantly, the success rate was nearly identical
in immunized women who developed (31%) and failed to develop (30%) HLA
antibodies. The sole puzzle was the relatively higher frequency
of chromosomally abnormal abortuses or fetuses in the immunized group. This
was explained by the authors on the basis of losses in their treatment
group occurring later in pregnancy, a time when recovering products
of conception for cytogenetic analysis was easier. CONCLUSION. Parental HLA sharing leading to fetal rejection remains an attractive hypothesis, with
HLA-B the locus showing the strongest association. However, the
role of HLA sharing in pregnancy losses in the general population
is uncertain and probably low. The phenomenon could play a role
in a subset of repetitive aborters, but nonetheless immunotherapy cannot
be recommended. Drugs, Chemicals, and Noxious Agents Many exogenous agents have been implicated in fetal losses, but relatively
few have been accepted scientifically. The difficulty is that pregnant
women are frequently exposed to relatively low doses of ubiquitous
toxic agents. Outcomes are usually assessed through case-control studies conducted after
prior exposure to exogenous agents. case-control studies have considerable
power to detect any associations present but suffer the inherent
bias of control women having less incentive to recall antecedent events
than women experiencing an abnormal outcome (recall bias). Another
experimental pitfall is that exposure to potentially dangerous chemicals
is usually unwitting and, hence, poorly documented. Even when exposure
unequivocally occurs(e.g., industrial accidents or residence near
toxic waste sites), quantifying the exposure and enumerating the multiple
toxic agents present may be difficult if not impossible. Exposure
to many agents concurrently makes it difficult to attribute adverse
effects to a single agent. Given these caveats, one must be cautious about attributing pregnancy loss
to exogenous agents. On the other hand, common sense dictates that
exposure to potentially noxious agents be minimized. ENVIRONMENTAL CHEMICALS. Many chemical agents have been claimed to be associated with fetal losses,211,212 but consensus now seems to be settling around only a few.213 These include anesthetic gases, arsenic, aniline dyes, benzene, solvents, ethylene
oxide, formaldehyde, pesticides, and certain divalent cations (lead, mercury, and
cadmium). Workers at greatest risk are those
in the rubber industries, battery factories, and chemical production plants. Many
reports continue to be generated concerning specific agents, a
recent example being selenium, an element considered relevant to
fertilization.214 Scialli and coworkers215 and Shepherd216 have cataloged relevant animal and human references, and various on-line
listings are available. However, the real difficulty lies in defining
the effects of lower-level exposures and in quantifying a risk that
can be communicated to a given couple. Fortunately, most patient queries
can be answered with reassurance that the pregnancy is not at increased
risk. IRRADIATION. External irradiation and internal radionuclides in high does are proved
abortifacients. Of course, therapeutic x-rays or chemotherapeutic drugs
are used during pregnancy only in seriously ill woman whose pregnancies
often must be terminated for maternal indications. Pelvic x-ray exposure
up to perhaps 0.1 Gy (10 rads) places a fetus at little to no
increased risk. In fact, most exposures are usually far smaller, at 0.01 to 0.02 Gy (1 to 2 rads). The contribution of X-irradiation to clinically
recognized losses is thus small. CHEMOTHERAPEUTIC AGENTS. Similar to x-irradiation, chemotherapeutic agents in high doses are proved
abortifacients; however, high doses are given only in dire circumstances. Low
doses are sometimes medically important for nonneoplastic
conditions, and ambient exposures can occur. A potential for deleterious
effects on hospital personnel handling chemotherapeutic agents exists; thus, pregnant
hospital workers must minimize exposure. Caffeine Consensus has long existed that no deleterious effects exist with caffeine. Most
studies investigating pregnancy losses have been retrospective, and
cohort data showed the odds ratio for an association between abortion
and caffeine (coffee and other dietary forms) to be only 1.15 (95% confidence
interval 0.9 to 1.45).217 Additional data on women exposed to higher levels (more than 300 mg) of
daily caffeine would be useful, but in general reassurance can be given
concerning moderate caffeine exposure and pregnancy loss. Cigarette Smoking An association between smoking and spontaneous abortion is accepted, but
the effect is probably very modest. It could be explained entirely on
the basis of confounding variables. Kline and associates218 reported increased abortion rates in smokers, independent of maternal
age and independent of alcohol consumption. A modest dose-response curve
was found by Alberman and colleagues.219 Ness and associates220 compared tobacco use as assessed by urinary cotinine levels, comparing 400 women
with spontaneous abortions to 570 having ongoing pregnancies. Women
with urinary cotinine had an increased risk of abortion, but
the odds ratio reached only 1.8 (95% confidence interval 1.3 to 2.6). Alcohol An association between alcohol consumption and fetal loss was once well
accepted, but this claim now seems less certain. In 1980 Kline and coworkers218 compared 616 women experiencing spontaneous abortions with 632 women delivering
at less than 28 weeks' gestation. Among women whose pregnancies
ended in spontaneous abortion, 17% drank alcohol at least twice per
week; 8.1% of controls drank similar quantities. Harlap and Shiono221 also found an increased risk for abortion in women who drank in the first
trimester. However, Halmesmärki and associates222 later found that alcohol consumption was nearly identical in women who
did and did not experience an abortion. In that study, 13% of aborters
and 11% of control women drank on average three to four drinks per week. Parazzini
and coworkers223 reached similar conclusions, as did Ness and colleagues.220 Alcohol consumption should be avoided or minimized during pregnancy for
many reasons, but alcohol probably contributes little to the rate of
pregnancy loss. Given the high frequency of alcohol ingestion in the
general population, however, even a small effect could have epidemiologic
significance. Clinically, women more often need to be reassured, especially
after inadvertent drinking before realizing they are pregnant. Contraceptive Agents Contraception with an intrauterine device in place clearly increases the
risk of fetal loss; the second trimester is especially hazardous. If
the device is removed before pregnancy, there is no increased risk of
spontaneous abortion. Using oral contraceptives before or even during
pregnancy is not associated with fetal loss, nor is spermicide exposure
before or after conception.224,225 Trauma Women commonly attribute pregnancy losses to trauma such as a fall or a
blow to the abdomen. However, fetuses are actually well protected from
external trauma by intervening maternal structures and amniotic fluid. Any
contribution of trauma to early pregnancy loss is quite small. Psychological Factors Impaired psychological well-being has been claimed to predispose to early
fetal losses. The first investigations showing a benefit to psychological
well-being were those of Stray-Pedersen and Stray-Pedersen.226 One group consisted of 16 pregnant women previously experiencing repetitive
abortions who received increased attention but no specific medical
therapy. They proved more likely (85%) to complete their pregnancies
than 42 women not given such close attention(36% successful outcome). One
pitfall was that only women living “close” to the university
were eligible to be placed in the increased-attention group. Women
living further away served as “controls” by default; their
differing from the experimental group in ways other than geographic
proximity was not excluded. In 811 subsequent couples, the same high
success rate (86%) was observed in women given “tender loving
care.”227 Again, the expected background in this series is uncertain, making it
difficult to assess significance. Other studies have also reported a beneficial
effect of psychological well-being.26,27,228,229 The biologic explanation for any salutary effect remains obscure. Unanswered
is whether the ostensible positive effect of psychological well-being
is real or secondary to other factors. Confounding factors were
not taken into account, making it difficult to determine whether the outcome
was truly better than the expected background rate of 70%. Given
no ostensible harm, psychological support can be recommended, but not
at the expense of eschewing other potential causes. Severe Maternal Illness Many symptomatic maternal diseases show an increased frequency of spontaneous
abortion. In few disorders other than diabetes mellitus have potential
confounding variables been assessed. Nonetheless, Wilson disease, phenylketonuria, cyanotic heart disease, hemoglobinopathies, and inflammatory
bowel disease seem implicated in early abortion. Not every
study claims that a maternal disease is associated with increased fetal
loss rates; celiac disease is one example lacking a proven effect.230 The pathogenesis of losses in these disorders presumably involves one or
more of the mechanisms discussed previously, more often endocrinologic
or immunologic. Overall, relatively few fetal losses in the general
population will result from severe maternal disease. |