Endometritis
Cultures obtained at hysterectomy indicate that the endometrial cavity is normally sterile. Endometrial infections may follow procedures that alter the usual protective role of the cervix, such as cervical conization or procedures associated with the introduction of contaminated cervical mucus into the uterus. Endometrial biopsy, hysterosalpingography, and the insertion of an IUD may predispose to endometritis and ascending genital tract infection. Secondary infections of the endometrium may follow primary invasion with C. trachomatis or N. gonorrhoeae. Uterine infections are more likely to occur in postpartum women when decreased host resistance and surgical trauma act synergistically to make the uterine cavity more susceptible to infection. Factors that tip the balance in favor of bacterial invaders are prolonged labor, premature rupture of the membranes, and operative delivery. Prophylactic antibiotics appear to decrease the incidence but not the severity of infections in cesarean section patients. In managing patients with secondary infertility, it is especially important to elicit the details of past cesarean section or postpartum endometritis.
Acute endometritis, especially as observed postpartum or after abortion, is a misnomer, because the infection is unlikely to involve the endometrium alone. Usually, there is an associated inflammatory reaction of the myometrium, parametrium, and in some cases, adnexal structures. Patients with endometritis usually have a decrease in lochial flow for 12 to 24 hours before becoming febrile. It is important to establish prompt uterine drainage and to remove any retained infected tissue. The infections are almost invariably polymicrobial except in cases with β-hemolytic streptococcal endometritis in which rapid tissue invasion and bacteremia usually produce less pronounced local signs of pelvic infection. Broad-spectrum coverage for the most frequently recovered aerobic and anaerobic organisms includes the use of single extended-spectrum drugs, such as cephalosporins (cefoxitin or cefotetan) or penicillins (mezlocillin or piperacillin) or the combination of an amino glycoside and clindamycin or metronidazole. Triple antibiotic therapy with the addition of ampicillin is usually reserved for the critically ill patients, whereas a single drug or the combination of two drugs that provide activity against anaerobes is often used in less serious situations. The principles of management and antibiotic therapy of major gynecologic sepsis are discussed in detail elsewhere in these volumes.
It is generally acknowledged that the prognosis for future fertility is improved if the initial response to antibiotics is prompt. The patients requiring operative intervention for postpartum sepsis are at greater risk for developing pelvic adhesions and subsequent infertility. For most patients, endometritis after cesarean section infrequently interferes with tubal morphology and function unless a pelvic abscess develops.17 However, secondary infertility was more common among women who underwent primary cesarean section (6%) than among those who delivered vaginally (2%).18
C. trachomatis salpingitis is not uncommon in infected women after induced abortion or vaginal delivery.19 The organism presumably ascends from the cervix, usually producing mild or no symptoms 2 weeks (range, 1 to 6 weeks) postpartum. Salpingitis occurs in 15% of women with C. trachomatis who undergo an induced abortion.20 Between one fourth and two thirds of women with tubal infertility have been pregnant before becoming infertile.21 Because C. trachomatis is usually present in 5% to 10% of pregnant women, the impact of chlamydial salpingitis after pregnancy and subsequent infertility may be substantial. Identification and treatment of C. trachomatis and N. gonorrhoeae during pregnancy is recommended to reduce postdelivery salpingitis and its sequelae. Obstruction of the uterotubal junction may accompany septic abortion or streptococcal infection. As a practical matter, it is difficult to relate any particular organism causing endoparametritis to unique structural reproductive damage.
Endometritis in nonpregnant women can be classified into acute, chronic, and fibrotic stages (Table 3). Endometritis is present in 40% of women with cervicitis.12 C. trachomatis and, to a lesser extent, N. gonorrhoeae infections are closely associated with endometritis. Because many women have neither organism (although testing by the more sensitive PCR technique is not often used), it seems likely that other bacteria also cause endometritis in nonpregnant women. After the acute inflammatory process has subsided, an endometrial biopsy should be obtained to exclude persistent inflammation. Foreign bodies, retained products of conception, infected polyps, chronic salpingitis, and uterine cancer can also lead to chronic endometritis. Although the causative agents in the chronic condition may vary as indicated in Table 3, the histopathologic features are similar. The characteristic picture consists of a diffuse infiltration of plasma cells in the endometrial stroma.
TABLE 3. Classification of Endometritis
Acute
Chlamydia trachomatis
Neisseria gonorrhoeae
Bacterial
Chronic
C. trachomatis
Bacterial (nontuberculous)
Tuberculous
Nonspecific
Other (Mycoplasma, viral, toxoplasmosis, rickettsia)
Fibrotic
Intrauterine (Asherman's syndrome)
C. trachomatis infection in particular should be considered as a cause of plasma cell endometritis. The presence of plasma cells is also highly correlated with salpingitis. Moreover, women diagnosed clinically with salpingitis but found to have normal fallopian tubes at laparoscopy frequently demonstrate endometritis by biopsy.22 Bacterial vaginosis has been associated with plasma cell endometritis23 and with endometritis or salpingitis.24 The association of bacterial vaginosis with endometritis is strengthened by the finding that plasma cell endometritis is linked to the recovery of bacterial vaginosis-associated microorganisms from the endometrium23 and with the recovery of anaerobic gram-negative rods from the endometrium even after statistical adjustment for gonorrhea and chlamydial infection.25
Histologic dating of the endometrium may be inaccurate because chronic endometritis is frequently associated with a mixed proliferative and secretory endometrium or inactive cyclically dilated glands. The usual clinical presentation includes discharge, pelvic pain, and dysfunctional uterine bleeding.
In contrast to other types of endometritis, the response of the endometrium to tuberculosis is much more specific. The typical lesion is the noncaseating granuloma composed of epithelial cells, giant cells, and peripheral lymphocytes. Genital tuberculosis is rare, but it should be considered when the endometrium shows signs of inflammation. It is nearly always secondary to a focus elsewhere in the body.
Many of the agents implicated in chronic endometritis have also been implicated in spontaneous abortion, including C. trachomatis, U. urealyticum, toxoplasmosis, cytomegalic inclusion virus, Rickettsia, and Listeria monocytogenes. Women with serologic evidence of C. trachomatis infection had a significantly higher occurrence of spontaneous abortion than other women.26 In one study, chlamydial infection was the second most frequent cause for recurrent fetal losses.27 However, systematic studies for toxoplasmosis, Listeria, and U. urealyticum have not provided convincing data that these organisms are common causes of recurrent abortion.
There is increasing evidence that an endometritis can interfere with implantation of the embryo or that spermatozoa are removed more quickly from the uterine cavity in the presence of a chronic inflammatory reaction. In laboratory animals, a single intrauterine injection of glycogen induces a marked leukocytic response and effectively terminates pregnancy before and during the implantation period. Transfer of viable leukocytes to the uterine lumen during early pregnancy causes a marked reduction in fertility. Inflammatory cells and their products have been shown to be toxic to preimplantation embryos in vitro.28
Although still controversial, a large number of studies indicate an adverse effect of prior chlamydial infection (as determined by positive chlamydial serology or heat shock protein-60 [HSP60] antibodies) on in vitro fertilization (IVF) outcome.16,29 Because pregnancy normally induces a TH2 (antibody-dominated) immune response, it has been postulated that an embryo toxic effect or a disruption of endometrial receptivity occurs as a result of the induction of a TH1 (cell-mediated immunitydominated) immune response. Endometrial infection may induce macrophage activation and proinflammatory cytokine production. The latter mechanism is supported by other studies that demonstrate that inflammatory hydrosalpinges have an adverse effect on endometrial receptivity, which in some cases may be overcome by surgical treatment of the hydrosalpinges.30 Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and other bioactive substances present in hydrosalpinx fluid reflux into the uterine cavity, thereby altering endometrial stomal and epithelial cell integrin expression that interferes with the “window of implantation.”
Traumatic damage to the endometrium may cause hypomenorrhea, amenorrhea, and intrauterine adhesions (i.e. signs of Asherman's syndrome). The extent of intrauterine adhesions correlates with the degree of menstrual insufficiency. The adhesions are sequelae of uterine trauma, almost always related to pregnancy. It is likely that infection plays a contributory role in their pathogenesis. Intrauterine adhesions may develop with a tuberculous endometritis, lending further support to the idea that Asherman's syndrome has an infectious basis. Fertility is severely impaired in this entity and may be caused by interference with implantation or to changes in endometrial metabolism. In the event of conception, potential complications include abortion, premature delivery, and problems with separation of the placenta. The diagnosis of intrauterine adhesions depends on hysterosalpingography or hysteroscopy. The prognosis for this syndrome with reference to fertility varies with the severity of the adhesions. In Valle's study of 47 patients with severe intrauterine adhesions, only 55% conceived, and less than one third of the patients had term pregnancies.31
Whenever possible, the cause of chronic endometritis should be determined. C. trachomatis and N. gonorrhoeae should be sought, and treatment should be given, as discussed in the following section on PID. The bacterial origin for non-STD organisms is difficult to prove, because endometrial cultures taken by the transcervical route are contaminated with cervical organisms. The tissue diagnosis of chronic nonspecific endometritis is best made during the follicular phase to avoid the normal inflammatory changes that occur premenstrually. Conversely, if tuberculosis is being considered, the granulomas are best recognized on days 24 to 26 of the cycle or within 12 hours after the onset of menstruation. The diagnosis of tuberculous endometritis may be aided by creating a pseudopregnancy without menses for 2 to 3 months, followed by a thorough curettage. The curettings are divided into two portions, one for histologic examination and one for culture. If these are positive for Mycobacterium tuberculosis, prolonged treatment with antituberculous agents is necessary, and the prognosis for fertility is poor.
Nonspecific chronic endometritis can be selflimited and is not uniformly influenced by therapy, but it may respond to curettage and cyclic estrogen and progestin therapy. Conjugated estrogens (2.5 mg/day for 30 days) with medroxyprogesterone acetate (10 mg/day for the last 10 days) and doxycycline (200 mg/day for an entire cycle) are recommended. A posttreatment biopsy is useful to determine whether therapy has been helpful.
Treatment of Asherman's syndrome is primarily surgical. In some cases, cervical and isthmic adhesions respond to transcervical dilatation and lysis. Adhesions can be resected with a hysteroscope under direct vision. In more severe instances, the dangers of perforating the bladder or uterus are best avoided by approaching the adhesions with a transfundal hysterotomy. If the vaginal approach is chosen, it is useful to be prepared for diagnostic laparoscopy in the event of a uterine perforation. An IUD is left in situ for 6 weeks postoperatively to prevent apposition of raw surfaces. The patient receives broad-spectrum antibiotics during this time and is maintained on large doses of conjugated estrogens and progestin cyclically for 2 months.32
Pelvic Inflammatory Disease
PID is a common but vaguely defined complex of signs and symptoms resulting from the spread of pathogenic microorganisms from the vagina and endocervix to the uterus, body of the endometrium, and fallopian tubes. It is a common complication of STDs and has reached epidemic proportions in the United States. Of the estimated 1 million women who annually develop PID, an average of 200,000 enter hospitals each year. According to statistics from the Centers for Disease Control and Prevention, the cost of PID measured in lost earnings and money spent for health services was estimated at $4.2 billion in 1990.33. The long-term consequences of PID include chronic pelvic pain, infertility, and ectopic pregnancies that are increased several-fold.
The best data on involuntary infertility after salpingitis are found in large Swedish studies,1,34 in which the initial diagnosis was confirmed by laparoscopy. Tubal infertility occurs in approximately 11% of women who have one episode, in 23% of women who have two episodes, and in 54% of women who have three or more episodes of salpingitis (Table 4).
TABLE 4. Factors Influencing the Frequency of Tubal Occlusion After Salpingitis
Clinical Findings | Tubal Occlusion |
Degree of acute inflammation at laparoscopy* |
|
Mild | 6% |
Moderate | 13% |
Severe | 30% |
Number of episodes of salpingitis* |
|
One | 11% |
Two | 23% |
Three or more | 54% |
Type of salpingitis† |
|
Gonococcal | 9% |
Nongonococccal | 16% |
* Westrom L: Incidence, prevalence and trends of acute pelvic inflammatory disease and the consequences of industrialized countries. Am J Obstet Gynecol 135:880, 1980.
† Westrom L: Effects of acute pelvic inflammatory disease on infertility. Am J Obstet Gynecol 121:707, 1975.
Acute salpingitis with or without oophoritis often coexists with various degrees of pelvic peritonitis. Infertility results from tubal occlusion, peritubal adhesions, or adhesions encasing the ovary in any combination. Tubal infertility is directly related to a number of factors present during the initial episode of salpingitis, which include (besides the number of episodes) the initial severity of tubal inflammation, the organisms responsible, and the occurrence of a subsequent ectopic pregnancy. The best predictor of subsequent infertility is the degree of tubal inflammation observed through the laparoscope during the acute phase (Table 4). The estimation of severity was based on direct observation of the tube and not on the severity of clinical symptoms and signs such as pain, fever, tenderness, or leukocytosis. Tubal infertility was subsequently found in 6% of women with mild, 13% of women with moderate, and 30% of women with severe tubal changes. Women with a pelvic abscess have had the highest (85% to 90%) rate of subsequent infertility.35
Approximately one half of the women with an ectopic pregnancy have grossly visible tubal damage or a partial occlusion of the tubes. About 7% to 10% of pregnancies that occur after an episode of salpingitis are in an ectopic location, and women with salpingitis have a 10-fold higher rate of ectopic pregnancy than does the general population. Ectopic pregnancy provides a poor prognosis for fertility. Approximately 40% of women who have had an ectopic pregnancy are not able to achieve an intrauterine pregnancy subsequently.36
To establish the diagnosis of salpingitis, other diseases, such as acute appendicitis, endometriosis, ovarian cysts, ectopic pregnancy, urinary tract infection, and gastrointestinal disease, must be excluded. The clinical diagnosis of acute salpingitis is confirmed by laparoscopy in fewer than two thirds of the patients. In the remaining patients, one fifth have normal pelvic findings, and other diagnoses are established in the others.37 The combination of lower abdominal discomfort with pain on motion of the cervix and bilateral adnexal tenderness was present in most patients who had salpingitis, but these findings were also common in the other women. Salpingitis is usually bilateral, but an 8% incidence of unilateral disease is reported; this manifestation may be more common in women using IUDs.38
Prompt recognition and vigorous treatment reduce subsequent severe complications of salpingo-oophoritis, such as generalized pelvic peritonitis, abscess formation, and adnexal destruction. It deserves reemphasis that salpingitis often produces minimal clinical signs. Approximately 60% to 80% of women with acute salpingitis have a normal temperature or no white blood cell elevation. This finding correlates with the observation that most women with tubal infertility have never been treated for a recognized episode of salpingitis. Epidemiologic studies support the concept of silent PID wherein a strong link exists between serum antibodies to C. trachomatis and tubal factor infertility or ectopic pregnancy in patients without a history of clinical PID.16 There seems to be no correlation between traditional indicators of severe clinical infection (e.g. tenderness, fever, leukocytosis) and the degree of tubal damage.
Physicians should be willing to treat women with mild symptoms for salpingitis. If the patients with mild symptoms had only cervicitis or cervicitisendometritis and not salpingitis, prompt treatment before the onset of salpingitis would have a major impact on preventing tubal occlusion. Inadequate treatment may predispose the patient to recurrent pelvic infection with the sequelae of hydrosalpinx, infertility, ectopic pregnancy, and chronic pelvic pain. So-called chronic salpingitis is often caused by indolent infection in patients who have received suboptimal antimicrobial therapy or to recurrent infection. Failure to use doxycycline or azithromycin to inhibit C. trachomatis may contribute to chronic salpingitis.39 Recurrent PID is a distinctly common event; the timing of recurrences, however, suggests that many are attributable to reinfection rather than relapse.
A population-based study of fertility in women with human immunodeficiency virus type 1 (HIV-1) infection in Uganda demonstrated that fertility is greatly reduced in HIV-1-infected women because of a lower rate of conception and increased rates of miscarriage and stillbirth.40 Numerous epidemiologic studies have demonstrated that there is an synergy among bacterial and viral STDs. Bacterial STDs have been implicated in the enhancement of HIV transmission. Conversely, the immunosuppression caused by HIV worsens the clinical course of other STDs. The low prevalence and incidence of pregnancy among HIV-infected women could reflect preexisting tubal factor infertility and higher clinical and subclinical fetal losses resulting from HIV-1 infection.
Salpingitis caused by M. tuberculosis, parasites, or fungi is uncommon in developed countries. The incidence of genital tuberculosis is higher in Europe, Israel, and South America, where it may be present in 5% to 10% of women seeking help in infertility clinics. In the United States and Australia, an incidence of less than 1% is reported. Nontuberculous salpingitis can be divided into gonococcal, chlamydial, and nongonococcal-nonchlamydial disease based on the results of endocervical or peritoneal fluid cultures.
GONOCOCCAL INFECTION.
When endocervical cultures are routinely employed, N. gonorrhoeae is recovered from approximately 30% of untreated patients with acute salpingitis. The frequency of gonococcal disease varies with the socioeconomic status of the population studied. In Swedish populations, the gonococcus was isolated in 10% to 30% of patients, whereas at an American city hospital, N. gonorrhoeae was recovered from most of the indigent women seen.1,41 Gonococcal PID is still a major cause of infertility in women in developing Asian and African countries.42
The recovery of N. gonorrhoeae from tubal or peritoneal fluid in acute salpingitis patients with endocervical gonorrhea ranges from 6% to 70%.38 Approximately one third of patients have N. gonorrhoeae as a sole isolate, one third have N. gonorrhoeae plus a mixture of aerobic and anaerobic bacteria, and one third have a mixture of aerobic and anaerobic bacteria in the cul-de-sac only.41 Aerobic and anaerobic streptococci and Bacteroides species constitute most of the nongonococcal isolates. The variable correlation between positive endocervical gonococcal cultures and specimens from peritoneal fluid has several possible explanations. Gonococci that invade the upper genital tract have different auxotrophic types and are less susceptible to antibiotics than are gonococci from uncomplicated anogenital gonorrhea.41 Although N. gonorrhoeae preferentially infects nonciliated tubal cells, the gonococcal toxin can destroy the cilia of adjacent cells. Not only is the organism difficult to isolate from pus, but the recovery of N. gonorrhoeae depends on the stage of infection. The gonococcus is most frequently isolated within 2 days of the onset of symptoms and is rarely isolated if symptoms are present for 7 or more days.38 Most symptomatic gonococcal PID cases have their onset during or just after the menses. These observations are consistent with the view that the gonococcus initiates the infection and, if the infection is not promptly treated, sets the stage for a mixed aerobic-anaerobic infection, involving pathogens that originate in the cervix and vagina.
CHLAMYDIAL INFECTION.
C. trachomatis is an intracellular bacterium that proliferates in columnar epithelial cells, where it remains protected from host immune defenses by a cell membrane. It takes a longer time for C. trachomatis to divide (24 to 48 hours) than for classic bacteria (1 to 4 hours). There is a characteristically long time between infection and the onset of symptoms among women with C. trachomatis, and only mild symptoms usually occur. Widespread or systemic symptoms are unusual, although infection of the endosalpinx can produce generalized peritonitis by contiguous spread, including perihepatitis (Fitz-Hugh-Curtis syndrome).
C. trachomatis causes the same spectrum of disease (e.g. urethritis, cervicitis, endometritis, salpingitis) as the gonococcus. C. trachomatis causes salpingitis more frequently than the gonococcus. The importance of chlamydiae has been recognized as women with mild symptoms or asymptomatic women have been included for study. The lower rate of C. trachomatis isolation in earlier studies may have been related to relatively mild symptoms and signs caused by chlamydiae compared with gonococci or the lack of a sensitive detection assay. It is apparent, however, that the degree of acute tubal damage among women with chlamydial infection equals or exceeds that observed with gonococcal infection.43 Women with chlamydial infection may have gonorrhea and vice versa.
C. trachomatis is inhibited in vitro by doxycycline and azithromycin but not by cephalosporins. Women with salpingitis should be treated with tetracyclines or other antibiotics that inhibit C. trachomatis, because cephalosporin therapy alone does not eradicate C. trachomatis.44
Chlamydia appears to be a particularly important organism in infertility. There are multiple published reports in which women with tubal infertility have a 25% to 70% higher incidence of C. trachomatis antibody than do infertile women with normal tubes.45 In the United States, C. trachomatis infections are now clearly the leading cause of tubal infertility.
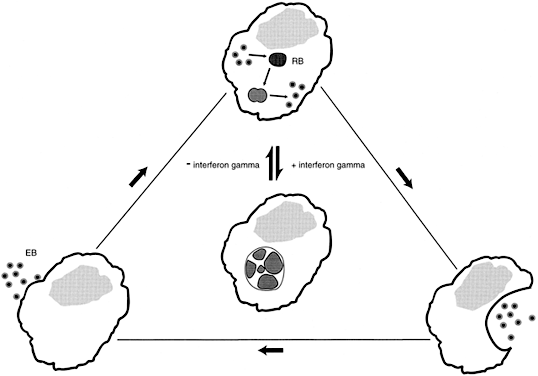
Women with asymptomatic C. trachomatis infections are less likely to seek medical attention than are women with genital tract symptoms. The undetected C. trachomatis are able to ascend from the lower to the upper genital tract, evade the host's immune response and persist for long periods of time.46 The mechanisms leading to chlamydial persistence and subsequent damage to the fallopian tubes have only begun to be elucidated. Experiments in vitro have established that interferon-γ (IFN-γ) produced in response to the chlamydial infection, blocks the intracellular life cycle of this organism, and results in the formation of large aberrant reticulate bodies. However, once IFN-γ is removed, as would occur when an extracellular chlamydial infection is cleared, the aberrant forms revert to normal reticulate bodies, and the typical chlamydial life cycle resumes (Fig. 1). The reticulate bodies differentiate into elementary bodies, the infected cell lyses, and neighboring epithelial cells are infected.47 A similar intracellular chlamydial persistence may occur after treatment with some antibiotics.48 Each cycle of chlamydial growth and inhibition damages the fallopian tube epithelia by an immunologic mechanism, resulting in an increasing extent of fibrosis and eventual tubal occlusion.49
In an in vitro fallopian tube organ culture, C. trachomatis does not cause any visible damage.50 It has become increasingly evident that the immune response to a C. trachomatis infection, not the infection per se, induces fallopian tube occlusion. A single antigen, the HSP60, has been implicated in initiating a proinflammatory immune response after a C. trachomatis upper genital tract infection. HSP60 is a highly conserved protein present in organisms ranging from bacteria to man. The amino acid sequence of the chlamydial and human HSP60s have almost a 50% homology.51 This protein functions as an intracellular chaperone, aiding protein assembly and transport. Under conditions of cell stress, such as an increase in temperature or exposure to free oxygen or nitrogen radicals, HSP60 gene transcription greatly increases in an attempt to prevent protein denaturation and maximize cell survival. In a quiescent but persistent chlamydial infection, synthesis of the major structural antigens ceases or is greatly reduced; however, synthesis of HSP60 is increased.47 Microbial HSP60 is a potent inducer of proinflammatory cytokines. In guinea pigs52 and monkeys53 previously sensitized to Chlamydia, introduction of purified chlamydial HSP60 initiated a localized inflammatory response. A number of investigations have demonstrated a correlation between immunity to the C. trachomatis HSP60 and recurrent episodes of salpingitis, tubal occlusion, and ectopic pregnancy.54,55,56,57 In women with a recent chlamydial cervical infection, immunity to chlamydial HSP60 is rarely observed.56 This suggests that repeated infections or chlamydial persistence in the upper genital tract is needed for sufficient HSP60 to be released to initiate an immune response in the host.
The homology between the chlamydial and human HSP60s also suggests that immune sensitization to conserved HSP60 epitopes may result in autoimmunity to human HSP60. Evidence of sensitization to HSP60 epitopes shared between C. trachomatis and humans has been reported.58,59 In women sensitized to conserved HSP60 epitopes, expression of human HSP60 in the fallopian tubes (in response to cell damage or past infection by other microorganisms) reactivates HSP60-sensitized lymphocytes and induces an inflammatory response. This may explain the sometimes puzzling observation of tubal inflammation in the apparent absence of infection.
Women with tubal factor infertility seek to become pregnant by assisted reproductive technology. However, evidence suggests that sensitization to HSP60 may also interfere with reproductive success after IVF.16,60 The early-stage embryo61 and epithelial cells in the decidua62 express HSP60. A murine hybridoma specific for HSP60 also was shown to react with the surface of human and mouse trophoblasts.63 HSP60 expression during pregnancy may reactivate HSP60-sensitized lymphocytes. The resulting proinflammatory immune response may directly interfere with embryo development or may disturb the balance of immune regulatory mechanisms needed to prevent rejection of the semiallogeneic embryo. Women undergoing IVF who had cervical IgA antibodies to chlamydial HSP60 had an increased rate of transient implantation after embryo transfer and a significantly poorer outcome than did antibody-negative women.16 Further analysis revealed that cervical immunity to a shared human HSP60 epitope and C. trachomatis was similarly correlated with IVF failure60 (Table 5). Circulating systemic humoral immunity to human HSP60 has also been associated with a history of spontaneous abortion.60 An association between IVF failure, humoral immunity to C. trachomatis, and expression of human HSP60 in ovarian follicle fluid has been reported.64
TABLE 5. Chlamydia trachomatis Infection and in Vitro Fertilization (IVF) Outcome
IVF Outcome | No. of Patients | No. (%) with Anti-Ct Cervical IgA |
Not pregnant | 99 | 17(17.2) |
Biochemical pregnancy | 21 | 4(19.0) |
Spontaneous abortion | 10 | 3(30.0) |
Live birth | 68 | 1(1.5) |
*Endocervical samples obtained at the time of oocyte aspiration were assayed for IgA antibodies to C. trachomatis by ELISA (Savyon Diagnostics).
Adapted from Witkin et al. Unsuspected Chlamydia trachomatis infection and in vitro fertilization outcome. Am J Obstet Gynecol 171:1208, 1994.
NONGONOCOCCAL-NONCHLAMYDIAL INFECTION.
Nongonococcal-nonchlamydial salpingitis may also arise de novo as a primary infection. Approximately 25% of women with PID have a nongonococcal-nonchlamydial cause.65 Patients with nongonococcal PID have the onset of pain distributed evenly throughout the cycle and less frequently associated with menses. There is less fever, vaginal discharge, and liver tenderness than with gonococcal PID. Despite these differences, the clinical presentation does not adequately distinguish between the two, and reliance on culture is necessary. Except for the presence of N. gonorrhoeae or C. trachomatis, no difference in vaginal or cervical flora exists between patients with gonococcal or chlamydial and nongonococcal-nonchlamydial salpingitis. As shown in Table 1, the cervix and vagina of healthy women contain an abundance of aerobic and anaerobic microorganisms. There may be a critical number of organisms needed to overwhelm local host defense mechanisms in the cervix, allowing an infection to ascend to the upper genital tract. There is probably a continuum from bacterial vaginosis to endometritis and salpingitis, because women with bacterial vaginosis are significantly more likely to be diagnosed with PID.10 The substantial isolation rate of bacteria other than gonococci or C. trachomatis from tubal fluid of these PID patients has shown that bacterial vaginosis organisms can cause acute salpingitis without antecedent chlamydial or gonococcal infection.66 Peritoneal or tubal cultures have yielded a mixed aerobic and anaerobic flora in 35% to 50% of patients, anaerobes alone in 15%, and aerobes alone in approximately 30% to 40% of patients. Between 4% and 17% of women with PID have had M. hominis, and 2% to 20%, have had U. urealyticum recovered from the fallopian tubes.66 Genital mycoplasmas probably play an infrequent role in PID, based on isolation rates, serologic data, and the observation that they produce little or no damage in human oviductal tissue cultures.67
PREDISPOSING FACTORS.
Previous gonorrhea, use of an IUD, frequent douching, and uterine instrumentation predispose to the development of nongonococcal PID.68 It is possible that unrecognized tubal damage impairs normal defense mechanisms even in the absence of clinically overt PID. When patients who have had PID subsequently acquire gonorrhea, more than one third develop acute onset PID, in contrast to the 10% to 17% rate in general.3
The use of an IUD is associated with approximately a threefold to fivefold increased risk of PID, which appears to exist for as long as the IUD is in place.21 The IUD may be a greater risk factor in nongonococcal than in gonococcal PID and is associated with an increased frequency of adnexal masses.21 Several reports have indicated the possible association between pelvic infection caused by Actinomyces israelii, an anaerobic gram-positive bacterium, and the use of an IUD.69 The use of any type of tail in an IUD provides a potential route for infection to the uterine cavity. Oral contraceptives may decrease the risk of developing PID, although they have less protective effect than barrier contraceptives. It is logical that women who have used IUDs suffer more tubal infertility and that women who used oral contraceptives have less infertility than women who have used neither method.21,70
Hysterosalpingography is commonly used in a complete infertility investigation. The introduction of water-soluble contrast media has eliminated the complications of oil embolism and has reduced the risk of granuloma formation, but inflammatory reactions continue to be serious complications of this procedure. The frequency of serious infection after hysterosalpingography varies from 0.3% to 3.1% of patients.71 It is possible that these episodes are caused by reactivation of preexisting disease rather than a de novo infection. High-risk patients for post-hysterosalpingography infections include those with prior pelvic infection or prior adnexal tenderness, a mass, or dilated fallopian tubes. Antibiotic prophylaxis with doxycycline (100 mg twice daily for a total of 7 days) reduces the incidence of post-hysterosalpingography infections.72
Pathogenesis
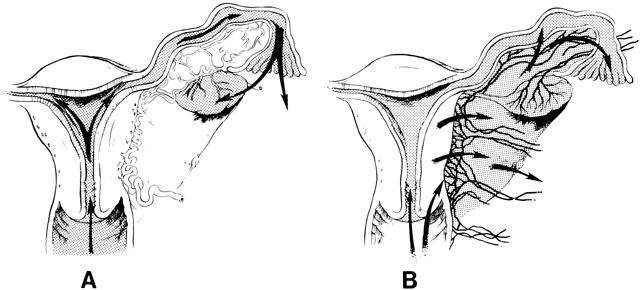
The pathways of spread of genital tract infections are shown in Figure 2. In gonococcal and chlamydial salpingitis, the microorganisms ascend by surface extension from the lower genital tract through the cervical canal by way of the endometrium to the fallopian tubes (Fig. 2A). Microscopically, the endosalpinx is inflamed and edematous. There can be adhesion of the mucosal folds, destruction of cilia, occlusion of the infundibulum, and production of a pyosalpinx. The gonococcal infection may spread beyond the endosalpinx, with possible focal abscess formation and perisalpingitis. In some cases of nongonococcal salpingitis, particularly with M. hominis,73 the pathogens may enter through lesions in the cervix or endometrium and spread to the parametria and tubes through lymphatics and blood vessels (Fig. 2B). The inflammatory swelling that affects the parametria and the tubes is more pronounced than in gonococcal salpingitis, but the endosalpinx is usually intact.
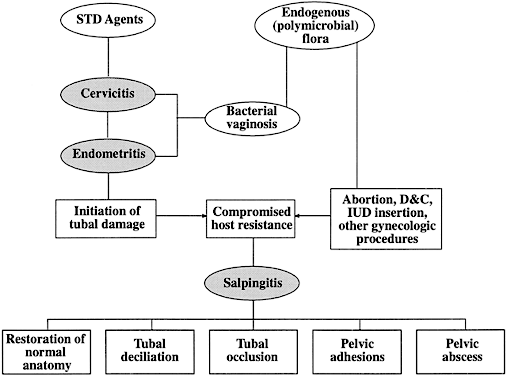
The sequelae of PID that are responsible for infertility include chronic interstitial salpingitis, hydrosalpinx, salpingitis isthmica nodosa, and periadnexal adhesions. Infertility may also occur because of abnormal secretory, ciliary, and peristaltic function of the fallopian tube. The postulated interrelationships of STDs and endogenous organisms in the pathogenesis of tubal infertility secondary to PID are depicted in Figure 3.74
Therapeutic Considerations
Early recognition and proper treatment of upper genital tract infection are mandatory to prevent permanent damage to the female reproductive tract and subsequent infertility. There is controversy over the issue of outpatient versus inpatient treatment of patients with acute salpingitis. For economic and logistical reasons, most women are treated on an outpatient basis. The decision for hospitalization is usually based on the clinical severity of the illness, although criteria vary. It seems reasonable to treat major pathogens such as N. gonorrhoeae and C. trachomatis in every patient. An antibiotic regimen that takes into account the polymicrobial nature of the cause of acute salpingitis must be used. However, after treatment with different antibiotics, similar infertility rates have been found.75 This could be interpreted to indicate that the ideal antibiotic has not been found or, more likely, that most tubal damage occurs before the patient presents for treatment. Women treated after 3 or more days of symptoms had significantly more infertility than those treated earlier.76 Better recognition and treatment of cervicitis and endometritis before salpingitis develops is even more important in the prevention of infertility than the treatment of salpingitis per se. Recommended treatment schedules for uncomplicated salpingitis are shown in Table 6.
TABLE 6. Recommended Therapy for Salpingitis
Parenteral Regimen A
Cefotetan 2 g, IV every 12 hours,
or
Cefoxitin, 2 g, IV every 6 hours,
plus
Doxycycline, 100 mg, IV or orally every 12 hours
Parenteral Regimen B
Clindamycin, 900 mg, IV every 8 hours,
plus
Gentamicin loading dose IV or IM (2 mg/kg of body weight), followed by
a maintenance dose (1.5 mg/kg) every 8 hours. Single daily dosing may
be substituted.
Regimen A
Ofloxacin, 400 mg, orally twice each day for 14 days,
plus
Metronidazole, 500 mg, orally twice each day for 14 days.
Regimen B
Ceftriaxone, 250 mg, IM once,
or
Cefoxitin, 2 g, IM plus Probenecid, 1 g, orally in a single dose concurrently
once,
or
Other parenteral third-generation cephalosporin (e.g., ceftizoxime, cefotaxime),
plus
Doxycycline, 100 mg, orally twice each day for 14 days. (Include this regimen
with one of the above regimens.)
Centers for Disease Control and Prevention: 1998 Guidelines for treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep 1998;47(RR1):82–82.
For outpatients, all women with suspected PID should have an initial parenteral antibiotic to inhibit N. gonorrhoeae. In many areas with high rates of penicillin-resistant gonorrhea, cefoxitin or ceftriaxone should be given. However, single-agent therapy is not appropriate for PID. Tetracyclines or doxycycline given alone no longer reliably inhibits N. gonorrhoeae. Tetracyclines, however, should be given for 10 to 14 days to inhibit C. trachomatis. Patients with suspected abscesses or severe illness that may indicate the presence of organisms other than gonococci or chlamydiae should be hospitalized. Recommended treatment regimens inhibit not only N. gonorrhoeae and C. trachomatis but also a wide variety of aerobic and anaerobic bacteria. For instance, parenteral clindamycin is effective against C. trachomatis and anaerobes. One review indicates that few hospitalized women receive the recommended antibiotic regimens.39
The concomitant use of steroids with antibiotics has been thought to reduce the sequelae of salpingitis, but in a prospective study, Falk77 could show no beneficial effect as judged by hysterosalpingography findings or subsequent laparotomy. Prevention of PID recurrence and its adverse effects on fertility also requires treatment of asymptomatic male sexual partners.
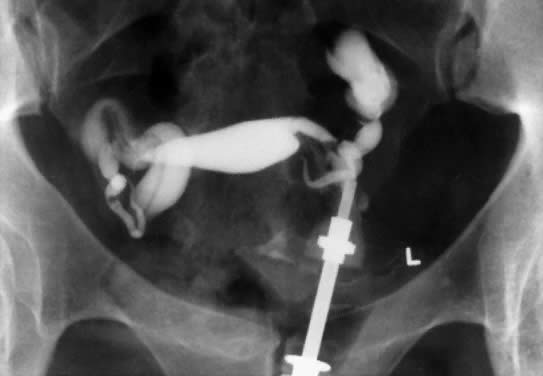
In patients with postinflammatory tubal disease, pregnancy outcome has been correlated with the presence or absence of fallopian tube rugae on hysterosalpingograms (Fig. 4). Pregnancy occurred in 61% of patients with moderate to excellent rugal patterns, whereas only 7% of patients with no demonstrable rugae conceived postoperatively.78 Laparoscopic and salpingoscopic evaluation of the endosalpinx provides another means to assess prognosis for fertility.79 However, visualization of the tubal mucosa by salpingoscopy provides even more reliable data on which to classify and score the extent of tubal disease.
Management of Tubal Infertility
Today and in the foreseeable future, assisted reproductive technologies (ART), endoscopic surgery, and microsurgery have an important place in the management of infertility that results from tubal disease. There are some tubal causes of infertility for which surgery can offer little or no chance of success, such as after severe bilateral hydrosalpinx, multisite tubal obstruction, or in patients with extensive and dense pelvic adhesions. At the other end of the spectrum are patients who can achieve a 50% to 65% intrauterine pregnancy rate after microsurgical or laparoscopic adhesiolysis when the fimbriae are spared from disease and a male factor is not encountered.79 In choosing between IVF and tubal surgery, the physician must compare success rates (which can are best defined by the birth of a live baby) and take into account the patient's age, presence of a male subfertility factor, the personal priorities of the couple, and the availability of expertise.
Differentiating the cause of tubal occlusion by history and ancillary tests (e.g. chlamydial serology) can contribute to the assessment of prognosis. Severe male factor combined with tubal disease in the female partner is an indication for advanced laboratory techniques in assisted reproduction such as intracytoplasmic sperm injection.
In patients with mild tubal disease (stage I hydrosalpinx) the prognosis is good with term pregnancy rates of 39% to 59% and ectopic pregnancy rates of 4% to 10% after microsurgical neosalpingostomy.79 Patients with moderate disease (stage II hydrosalpinx) make up about one third of the total and have an intermediate prognosis for a term pregnancy, but the risk of ectopic pregnancy is at least 10%.
The surgical prognosis for pregnancy is uniformly poor in patients with flat tubal mucosa or a fibrotic and thick-walled hydrosalpinx (stage III or IV); IVF is advised for this group of patients. However, patients with large hydrosalpinges can benefit from prophylactic salpingectomy before undergoing IVF to improve implantation rates and to reduce the likelihood for ectopic pregnancy.30,80
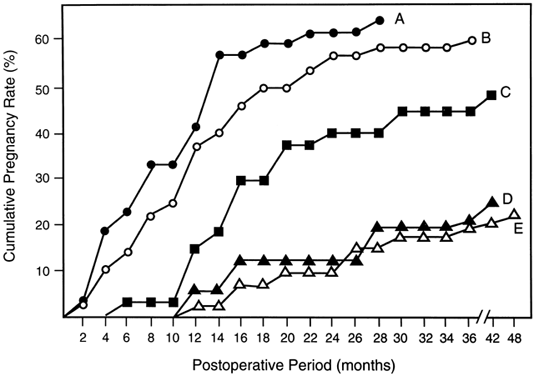
It is widely acknowledged that pregnancy outcome after tubal surgery is time dependent81 (Fig. 5). Most physicians advise their patients to opt for IVF at 12 to 18 months after unsuccessful surgery. In contrast to salpingoneostomy, the cumulative pregnancy rate increases rapidly after fimbrioplasty (i.e. deagglutination of visible fimbriae) and results in intrauterine pregnancy rates of 60% or better (Fig. 5). Although less common than distal fallopian tube disease, proximal tubal obstruction (PTO) occurs in 10% to 20% of hysterosalpingographies performed to evaluate infertility.82 The diagnostic and therapeutic options for managing PTO have been expanded by the introduction of fluoroscopic or hysteroscopic fallopian tube catheterization, which results in tubal patency in about 85% of patients with PTO.82 Microsurgical resection and tubal cornual anastomosis is the preferred surgical option for women with persistent occlusive disease in the proximal oviduct not opened by transcervical catheterization.82 A review of the world literature indicates a mean intrauterine pregnancy rate of 58% after tubocornual anastomosis, with an ectopic pregnancy rate of 4%.83 It is reasonable to expect a continued improvement in pregnancy outcome for IVF procedures (reported in the United States for the year 1995 as 22.5% delivery rate per oocyte retrieval).84 Data from large U.S. centers indicate a 40% to 70% cumulative delivery rate after three IVF cycles in younger women with tubal disease and without associated male-factor infertility.85,86 These results compare very favorably with the best outcomes after tubal reconstructive surgery.
 106 white blood cells/1 ml of semen.
106 white blood cells/1 ml of semen.