Hormone Assessment Derangements of the hypothalamic-pituitary-gonadal axis are associated
with abnormalities in spermatogenesis and in sexual function. Men with
abnormalities on semen analysis (especially sperm concentration less
than 10 million per milliliter), decreased libido, or other clinical manifestations
of endocrinopathy should undergo hormone evaluation.25 Initial testing includes determination of serum testosterone and FSH. If
abnormalities are detected, serum LH and prolactin should also be determined. Figure 2 depicts normal hormone production and negative feedback inhibition within
the hypothalamic-pituitary-gonadal axis. The relation between serum
hormone values in an individual patient is characteristic of a specific
diagnosis (Table 3). Men who are azoospermic secondary to testicular failure often present
with small, soft testes measuring less than 10 cm3 with a small, flat epididymis. In the setting of primary testicular failure, decreased
testosterone production produces diminished negative
feedback inhibition on the pituitary, which in turn is stimulated to increase
FSH secretion (hypergonadotropic hypogonadism). These men will
often have excess conversion of testosterone to estradiol (aromatase
activity) and respond to aromatase inhibitor therapy with normalization
of testosterone levels and improved sperm production. Men with azoospermia
secondary to obstruction have normal FSH levels, as testosterone
production is normal, and the entire homone profile reflects the normal
state. Although a significantly elevated FSH is consistent with spermatogenic
failure, not all men with abnormal spermatogenesis have an
elevated FSH. The combination of physical examination and hormone evaluation
is extremely useful for differentiating whether azoospermia is
caused by obstruction or by testicular failure. Of men with obstructive
azoospermia, 96% have been found to have FSH levels of 7.6mIU/mL
or less or testicular long axis greater than 4.6 cm, whereas 89% of
men with nonobstructive azoospermia had FSH levels greater than 7.6 mIU/mL
or testicular long axis of 4.6 cm or more.26 Men with primary testicular failure, small, soft testes, elevated FSH, and
typically low ejaculate volume secondary to low androgen levels should
be advised to undergo genetic evaluation to rule out chromosomal
abnormalities such as Klinefelter syndrome and microdeletion of the Y
chromosome. 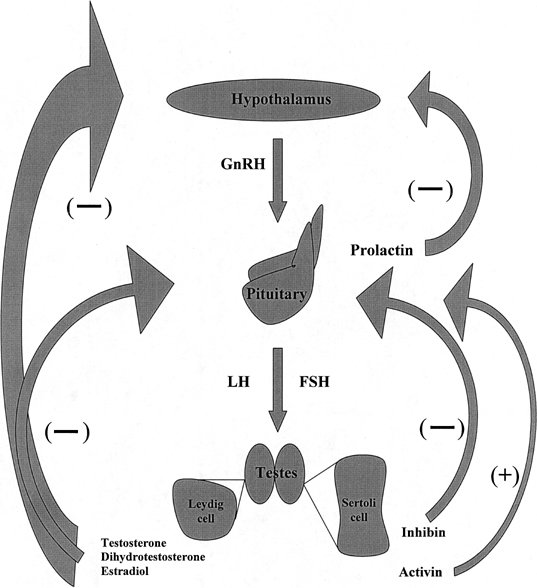 Fig. 2. Hypothalamic-pituitary-gonadal axis. GnRh, gonadotropin-releasing hormone; LH, luteinizing
hormone; FSH, follicle-stimulating hormone. Fig. 2. Hypothalamic-pituitary-gonadal axis. GnRh, gonadotropin-releasing hormone; LH, luteinizing
hormone; FSH, follicle-stimulating hormone.
|
TABLE 3. Correlation Between Serum Hormone Levels and Diagnosis
Diagnosis | T | FSH | LH | Prolactin |
Normal | Normal | Normal | Normal | Normal |
Primary Testicular | Low | High | Normal→High | Normal |
Failure (Hypergonadotropic hypogonadism) | | | | |
Hypogonadotropic hypogonadism | Low | Low | Low | Normal |
Functional pituitary adenoma | Low | Low→Normal | Low | High |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; T, testosterone.
Low testosterone, low FSH, and low LH are typical findings in hypogonadotropic
hypogonadism wherein the pituitary does not produce adequate FSH
resulting in low testosterone production. Hopogonadotropic hypogonadism
may be caused by Kallman syndrome, a disorder of the hypothalamus
in which sufficient GnRH is not produced, or by a pituitary defect, including
pituitary adenoma. Kallman syndrome is also associated with midline
abnormalities such as anosmia27 and less commonly synkinesia, unilateral renal agenesis and high, arched
palate. Men with this hormone profile should undergo cranial MRI to
evaluate the sella turcica for the presence of a pituitary adenoma. Functional adenomas of the pituitary may produce high levels of prolactin, which
in turn causes negative feedback inhibition on the gonadotropes
of the pituitary gland with subsequent decrease in FSH, LH, and testosterone. Decreased
libido may precede the subsequent establishment
of a hypoandrogenic state that results in inadequate virilization. Cranial
MRI is indicated to assess the pituitary gland. Although medical
treatment for microadenomas and macroadenomas is usually the same, sizeable
macroadenomas that may cause significant morbidity from mass effect
should be detected for follow-up and possible neurosurgical consultation
if the mass enlarges or causes other neurologic signs. Recent investigation has shown that many men with nonobstructive azoospermia
have an elevated testosterone:estradiol (T:E2) ratio when compared with the fertile population.28 Aromatase inhibitors decrease the conversion of testosterone and androstenedione
to estradiol and estrone, respectively, thereby increasing
serum testosterone levels. Administration of aromatase inhibitors has
been found to not only restore the T:E2 toward normal, but also to significantly improve semen parameters including
sperm concentration and motility in oligospermic men.28,29 An E2 level may be obtained in infertile, oligospermic men to determine the
T:E2 ratio, although these data were not available for review and inclusion
in the current recommendations of the Male Infertility Best Practice
Policy Committee.25 Postejaculatory Urinalysis Low-volume or absent ejaculate suggests bladder neck dysfunction and retrograde
flow of ejaculate into the bladder. This may be seen in patients
with diabetes mellitus or in men who have undergone surgical procedures
of the urogenital tract such as bladder neck reconstruction or transurethral
resection of the prostate. Men with ejaculate volume less
than 1.0 mL who are not found to have hypogonadotropic hypogonadism or
CBAVD should undergo postejaculatory urinalysis.25 Fluid production within the seminal vesicles and the prostate is dependent
on androgen stimulation, and the hypoandrogenic state of hypogonadotropic
hypogonadism does not support normal contribution of these glands
to the semen. CBAVD is associated with hypoplasia of the seminal
vesicles, also resulting in low-volume ejaculate that is acidic and often, but
not always, lacks fructose. Postejaculatory urinalysis determines the number of sperm that are present
within the bladder after centrifugation of the specimen and careful
examination of the pellet with light microscopy under 400× magnification. If
any sperm are noted within the urine of a patient with
azoospermia, then retrograde ejaculation is diagnosed. Patients with
oligospermia are difficult to assess for retrograde ejaculation, as the
sperm found in the urine may reflect sperm that are washed out of the
urethra with urination, and the results must be interpreted cautiously. Ultrasound Transrectal ultrasonography (TRUS) is indicated for those patients with
palpable vasa who have an acidic, low-volume ejaculate that contains
absent or minimal fructose to assess for ejaculatory duct obstruction. In
partial or unilateral ductal obstruction, the semen may contain fructose
and the pH may be only subtly acidic. On ultrasound, the seminal
vesicles can appear dilated (more than 1.5 cm in anteroposterior diameter) and
the ejaculatory ducts may be visualized as well with obstruction.30 In an azoospermic patient thought to have ejaculatory duct obstruction, the
seminal vesicles may be aspirated under ultrasound guidance,31 and intraoperative microscopic analysis of the aspirate is done. If present, motile
sperm should be cryopreserved for use in IVF with ICSI. Definitive
treatment is provided with transurethral resection of the ejaculatory
ducts after which sperm may be seen to return to the ejaculate
in 50% to 75% of cases with pregnancy occurring in 25% of
couples.32 If viable, but poor-quality sperm return to the ejaculate, IVF with ICSI
is recommended. Unilateral vasal agenesis may be associated with contralateral segmental
atresia of the vas deferens or seminal vesicle.33 TRUS can be done for evaluation of the ampullary portion of the contralateral
vas and seminal vesicle. Furthermore, approximately 25% of
men with congenital unilateral absence of the vas deferens and 10% of
men with CBAVD have unilateral renal agenesis; abdominal ultrasound
is indicated for this population for assessment of the kidneys.34 Transscrotal ultrasound is rarely necessary because the majority of scrotal
pathology can be palpated on physical examination. Clinically significant
varicoceles, congenital absence of the vas deferens, and testis
tumors are most commonly diagnosed with palpation alone. Scrotal ultrasound
is not indicated unless physical examination cannot be adequately
accomplished or findings on physical examination are equivocal. Genetic Analysis Chromosome abnormalities are clearly associated with male infertility. The
best characterized genetic anomalies are mutations within the CF transmembrane
conductance regulator (CFTR) gene, the sex chromosome abnormality
Klinefelter syndrome (47,XXY), and microdeletions of the Y chromosome. MUTATIONS WITHIN THE CYSTIC FIBROSIS TRANSMEMBRANE CONDUCTANCE REGULATOR
GENE. CBAVD, congenital unilateral absence of the vas deferens, congenital bilateral
partial absence of the vas or epididymides, and congenital epididymal
obstruction comprise the spectrum of vasal aplasia. At least 80% of
men with CBAVD are found to carry mutations within at least
one allele of the cystic fibrosis transmembrane conductance regulator (CFTR) gene,35 located on the short arm of chromosome 7. The gene encodes a protein that
acts as an ion channel but also affects formation of the distal two-thirds
of the epididymis, the vas deferens, seminal vesicle, and ejaculatory
duct. As greater numbers of mutations within the CFTR gene are
discovered, the percentage of men with CBAVD who are found to harbor
CFTR mutations increases. Possibly, all men with CBAVD carry mutations
within the CFTR gene, and failure to detect these mutations represents
both limitations within current testing methodologies and also practical
issues with regard to the number of mutations that should be tested
in a given patient, because some mutations carry a low frequency. Approximately 4% of
Caucasians are carriers of CFTR gene mutations. Because
men with evidence of vasal agenesis can be assumed to be
carriers of CF mutations, the female partner must be evaluated for CF
gene mutations prior to attempting ART to determine the risk for transmitting
CF or CBAVD to the offspring. The penetrance of the CF carrier
state in causing CBAVD appears to be low.36 KLINEFELTER SYNDROME. Up to 10% of men with nonobstructive azoospermia have karyotypic
abnormalities. The most common chromosome abnormality is Klinefelter
syndrome (47,XXY or 46,XY;47,XXY mosaicism). The clinical spectrum of
Klinefelter syndrome ranges from varied degrees of impaired spermatogenesis
with complete absence of spermatogenesis on one end of the spectrum
to severe oligospermia in rare cases. Testicular spermatozoa may be
recovered in most men with Klinefelter syndrome using microsurgical
testicular sperm extraction (TESE). Thus far, all children from our center
born after ICSI with use of retrieved sperm have had normal karyotype. Other
karyotypic abnormalities in infertile men include autosomal
translocations.37 Preimplantation diagnosis (evaluation of fertilized embryos by biopsy
during IVF) using fluorescence in situ hybridization for identification of normal chromosome composition may
be considered when using sperm from men with known chromosomal abnormalities.38 MICRODELETIONS OF THE Y CHROMOSOME. Y chromosome microdeletions are identified in up to 7% of infertile
men.39 Relevant microdeletions may be detected on three nonoverlapping regions
of the long arm of the Y chromosome, designated as AZF (AZoospermic
Factor) a, b, and c.40 Because they are too small to be identified by karyotype analysis alone, deletions
must be identified by polymerase chain reaction-based technique
utilizing multiple sequence-tagged–sites. The presence of
a deletion cannot be predicted by phenotype. Deletions within the different
regions are associated with different sperm retrieval rates. Men
with deletions of the AZFc region may have sufficient spermatogenesis
to produce spermatozoa within the ejaculate,41 but if not, sperm may be retrieved by microdissection TESE for most of
these men. Men with deletions of the AZFa or AZFb regions, however, have
a poor prognosis for successful sperm retrieval.42,43,44 Deletions of the recently elucidated AZFd region are associated with normal
spermatogenesis, and the clinical significance of deletions within
this region remains to be determined.45 Given that the Y chromosome will be passed on to all male children, concern
for transmission of impaired spermatogenesis to all male offspring
exists, and vertical transmission of deletions has been demonstrated.46,47,48 Because of the prognostic value and implications for offspring, all azoospermic
men considered for ICSI should be screened for both chromosomal
abnormalities with karyotype analysis and Y chromosome microdeletions.25 The couples should be counseled not only regarding the possibility of
passing infertility to the offspring, but also that genetic abnormalities
may exist that have not yet been elucidated and are not detectable
by current genetic testing methodologies. Analysis of Sperm Function Although sufficient sperm with adequate motility and normal morphology
are found on semen analysis, the sperm may be unable to fertilize an oocyte. For
this reason, additional analysis of sperm function may be indicated
to identify specific deficiencies in some of the multitudinous
components involved in normal sperm action, although utility in this
effort is realized only if the findings will affect treatment options. LEUKOCYTOSPERMIA. Excess leukocytes within the semen are detrimental to sperm function and
motility. Infertile men have been found to contain a greater number
of white blood cells in their ejaculates when compared with fertile men.49 On routine semen analysis, several cell types appear similar and cannot
be differentiated from one another, including epithelial cells, prostate
cells, immature germ cells (round spermatids, spermatocytes, spermatogonia), and
leukocytes. These cells are collectively referred to as
round cells. If the number of round cells in a semen analysis exceeds 5 million
per milliliter, then the percentage of these cells represented
by leukocytes should be determined to assess the likelihood of genital
tract infection. The histochemical peroxidase stain using ortho-toluidine
or the immunocytochemical pan-leukocyte monoclonal antibody
test, which has the advantage of detecting activated polymorphonuclear
granulocyes as well as leukocytes that do not contain peroxidase, can
be performed to determine the concentration of white blood cells within
the semen. A concentration of white blood cells greater than 1 million
per milliliter warrants semen culture for Mycoplasma hominis, Ureaplasma urealyticum, aerobic and anaerobic bacteria, and testing for Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis. Prior to submitting a semen specimen for culture, the patient should carefully
wash the penis with Betadine to diminish the likelihood of specimen
contamination with skin flora. ANTI-SPERM ANTIBODIES. The blood-testis barrier created by tight junctions between Sertoli cells
normally isolates sperm from immune recognition. When the barrier is
disrupted and sperm are exposed to blood, however, an antigenic response
is elicited. ASA may be found within the serum, semen, or bound to
sperm. Those that are bound to sperm are most clinically significant, because
they may disable sperm either by impeding transport through
the cervical mucous50 or by altering interaction between the sperm and oocyte such that fertilization
cannot occur.51 Antibodies found in semen are predominantly IgA and IgG, both of which
may diffuse into the genital tract, and IgA is also secreted in the male
reproductive tract. ASA are found in 80% of men who have undergone
vasectomy52 and are also associated with vasoepididymostomy or vasovasostomy, testicular
biopsy, infection, varicocele, cryptorchidism, and testicular torsion
or trauma.53 Pregnancy rates are clearly lower in men with ASA than in men without, and
among those men with ASA, significantly higher pregnancy rates are
achieved if fewer than 50% of the sperm are bound by antibodies.54 Men with semen analyses that show clumping or agglutination of sperm or
asthenospermia should be considered for ASA assessment. Either the immunobead
test or the mixed antiglobulin reaction test can be used to
screen for ASA. A fresh semen sample is used, and at least 200 sperm must
be available for evaluation.15 SPERM VIABILITY. Sperm viability is critical to ICSI success, and nonmotile sperm may not
be viable. Clinical scenarios in which this is relevant include the
use of testicular sperm, asthenospermia, or cryptozoospermia (sperm found
only after centrifugation of the semen specimen within the pellet). Men
with nonobstructive azoospermia who undergo microdissection testicular
sperm extraction typically have low numbers of sperm that are found, and
few may be motile. In this case, it is impossible to ascertain
which sperm are viable for use in ICSI without conducting a sperm viability
test. Viability testing is also important for cryopreserved specimens
after the thaw process. Cryopreservation has been shown to decrease
the number of viable sperm by 50%,55 and for specimens that contain few sperm prior to cryopreservation, rare
sperm may be available following thaw. Viability testing may be necessary
to select nonmotile, viable sperm for ICSI. In patients who are
not azoospermic, viability testing will differentiate viable, nonmotile
sperm from dead sperm, and if a large number of sperm on routine semen
analysis are nonmotile, but viable, then defects of microtubule action (immotile
cilia syndrome) must be considered. Viability testing is indicated if less than 50% of sperm are motile.15 Two different types of testing modality are available: dye exclusion with
eosin Y or trypan blue and hypo-osmotic swelling (HOS). With dye exclusion, the
dead sperm with damaged plasma membranes allow dye to fill
the cell, whereas viable cells exclude the dye. After the dye exclusion
test, sperm do not remain viable and cannot be used for ICSI. In hypo-osmotic fluid, water follows the solute concentration gradient
and diffuses into viable sperm, causing the plasma membrane to bulge and
the tail to curl,56 which can be easily observed with phase-contrast microscopy. A nonviable
cell cannot maintain the osmotic gradient and does not swell. Sperm
that react in the HOS test may be selected for use in ICSI. It is not
clear that routine use of the HOS test improves ICSI results. SPERM-CERVICAL MUCUS INTERACTION. To achieve fertilization, the sperm must pass through the cervical mucus
to enter the uterus and finally the fallopian tube. Just prior to ovulation, the
cervical mucus changes so that it is receptive to spermatozoa
and effectively filters out abnormal sperm, allowing those that are
fit to pass into the uterus. The mucus also serves as a nutritive, protective
reservoir to supply sperm to the uterus following intercourse. The
postcoital test (PCT) is a microscopic evaluation of the cervical
mucus after intercourse as close to ovulation as possible.57 The presence of motile sperm with adequate forward progression is a good
indicator that the interaction between cervical mucus and sperm is
not hostile.58 The routine application of the PCT has not been shown to confer benefit
to infertile couples,59 and an absence of consensus exists as to the clinical value of this test. Although
the significance of the test relative to fertilization potential
is vague, the test may be useful to ascertain the efficacy of
intercourse and proper deposition of semen within the vaginal vault. SPERM PENETRATION ASSAY (ZONA-FREE HAMSTER OOCYTE TEST). Removal of the species-specific zona pellucida from hamster oocytes allows
sperm from a different species to fertilize one oocyte. This process
requires sperm capacitation including the acrosome reaction, fusion
with the oolemma and incorporation of sperm into the oocyte. Because
multiple interactions are required, failure of an adequate number of sperm
to penetrate one oocyte, or failure of sperm to penetrate an adequate
percentage of oocytes, cannot be attributed to one specific defect. For
example, this artificial test relies on the occurrence of spontaneous
in vitro or unnaturally induced acrosome reactions.60 An absence of penetration is somewhat difficult to interpret, as the defect
may lie within the acrosome reaction or within an indeterminate
mechanism of penetration itself. Couples for whom the sperm penetration
assay (SPA) test was negative have achieved success with IVF, and therefore
extrapolation of SPA results is controversial.61 Variability also exists in the scoring of test results. Some laboratories
assess the percentage of oocytes that are penetrated, while others
assess the number of penetrations per oocyte. The sperm capacitation index, or
mean number of sperm to penetrate an oocyte, was proposed to
increase sensitivity of the test.62 Despite this change in result analysis, correlation of SPA results with
pregnancy remains unclear, and the test should be considered only for
those patients for whom the outcome of the test will influence treatment
decisions. COMPUTER-ASSISTED SEMEN ANALYSIS. Computer-assisted semen analysis (CASA) was introduced as a technology
intended to provide a precise, automated, reproducible, objective assessment
of spermatozoon characteristics, specifically movement pattern
and concentration. Computer analysis of digitized images procured with
a video camera to identify specific characteristics of sperm motion may
predict fertility potential. For production of meaningful, reproducible
results, CASA requires standardization of sample preparation, frame
rate and sperm concentration63 in addition to strict guideline adherence for use of the instruments. Widespread
use and application of CASA is a complex endeavor, and unequivocal
predictability of kinematic pattern effect on conception has not
been demonstrated. Whether this technology offers additional information
of clinical benefit to the infertile couple over routine manual
semen analysis remains to be established. ACROSOME REACTION. The acrosome is a membrane-bound structure derived from the Golgi apparatus
located most anteriorly in the head of the sperm, appearing as a
fluid-filled nuclear cap, the outer acrosomal membrane located subjacent
to the plasma membrane and the inner acrosomal membrane located anterior
to the nuclear membrane. The sperm binds to the zona pellucida, the
acrosome reaction occurs when the outer acrosomal membrane fuses with
the plasma membrane, and acrosomal enzymes responsible for penetration
of the zona pellucida are released. A high level of spontaneous acrosome
loss and a lack of response to calcium ionophore A23187, which
causes artificial induction of the acrosome reaction, are observed in
men with infertility.64 Both fluorescent-labeled lectins and monoclonal antibodies can be used
to assess the outer and the inner acrosomal membrane as well as acrosomal
contents after artificial induction of the acrosome reaction. In vitro acrosome reaction dysfunction can be detected by this assay, but the clinical
relevance of this observation is elusive. HUMAN ZONA PELLUCIDA BINDING TESTS. The hemizona assay is one of the tests that evaluates the ability of sperm
to bind to the zona pellucida. The zona pellucida from a nonviable
human oocyte (obtained from autopsy, surgical specimens, or most commonly
from an IVF center) is divided in half. One half is incubated with
patient sperm and the other with fertile control sperm at the same concentration. The
number of patient sperm bound is assessed relative to
the number of control sperm bound. Other tests differentially tag patient
and control sperm allowing assessment of the general ability of
the patient sperm to accomplish binding to the zona pellucida. Absent
or low-frequency binding of sperm suggests a defect in this discrete step
in fertilization. The test may have specific utility in assessing
sperm that fail IVF with normal results on SPA, wherein the oocytes lack
the zona pellucida. Widespread use of this testing is not possible
due to extensive experience required with micromanipulation techniques
and limited availability of human oocytes. BIOCHEMICAL TESTING. Reactive Oxygen Species. Metabolism of oxygen can result in the formation of reactive oxygen species (ROS) that, when
present in abundance, are toxic to aerobic cells. The
detection of ROS formation in the semen of 40% of infertile
men, but not in azoospermic and fertile men,65 suggested that ROS was a major potential cause or mediator of idiopathic
infertility. ROS are normally produced by sperm and are necessary for
normal sperm function. Low superoxide anion scavenging capacity in
seminal plasma may be responsible for the accumulation of high levels
of ROS.66 In fertile and infertile men, leukocytes are the major producers of ROS, but
in oligospermic men, sperm may produce up to 167 times more ROS
than in a corresponding fertile group.67 Motility, midpiece abnormalities and sperm-oocyte fusion are affected
by ROS through peroxidation of sperm lipid membranes.68 Chemiluminescent probes can be used to detect the amount of ROS produced
by sperm cells. Creatine Phosphokinase. Creatine phosphokinase is a key enzyme in the generation, transport, and
use of energy within spermatozoa. Clinical evidence has suggested that
elevated total creatine phosphokinase and perturbation in isozyme forms
is associated with infertility,69 but others have demonstrated that total creatine kinase activity and isozyme
distribution are not predictive of male fertility.70 Investigation is ongoing in biochemical tests of sperm function. |