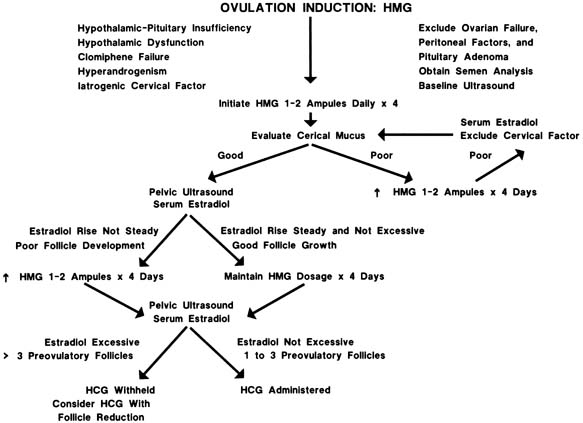
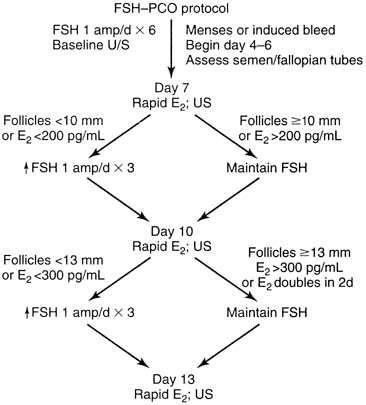
Because of the widespread use of gonadotropins, the potential for seeing
a significant adverse effect within one's professional career is
increased. In addition, it is becoming increasingly common for groups
of physicians to work together either in one practice or in satellite
offices in which care of patients and the responsibility for them are
shared. As a result of this increased usage and of the fragmentation
of care, informed consent has become increasingly important.37 Ovulation induction should rightfully be viewed as a safe and reasonable
form of treatment for widespread application. However, serious side
effects or serious complications do uncommonly occur. In view of this
fact, and because our patient base consists largely of healthy young women, the
treating physician must realize that complications can and do
arise, and this information should be shared with patients and documented
in the record. On-call coverage must be knowledgeable and
available daily. Endocrine laboratory results and ultrasound capabilities
should also be available daily or certainly almost every day, and
the results must be reviewed and interpreted each day to prevent potentially
serious side effects. To help ensure that this occurs, I have
developed a consent form to summarize the information provided to patients
before ovulation induction (Figs. 3 and 4).  Fig. 3. Informed consent for the use of hMG/FSH. Fig. 3. Informed consent for the use of hMG/FSH.
|
 Fig. 4. Informed consent for the use of human chorionic gonadotropin. Fig. 4. Informed consent for the use of human chorionic gonadotropin.
|
The potential side effects are reviewed carefully with the patient and
ideally with her partner before initiating treatment. With increasing
numbers of patients self-administering medications, it is our responsibility
to provide simple instructions concerning injections. This
is necessary to prevent the injection from being administered too low
on the buttocks, which could increase the risk of sticking the sciatic
nerve or a large blood vessel. Careful documentation of the instructions
should be written in the chart. If the physician or nurse at the
office administers the injection, it is very useful to make a notation
on which side the injection was administered and the location (e.g., identify the upper outer quadrant of the left buttock). This type
of documentation is particularly helpful if a problem associated with
a particular site is involved and the medical record clearly states that
the injection was administered elsewhere. Patients must be informed that there is approximately a 20% risk
of multiple births with these medications, three quarters of which are
twins. However, patients must also be advised that higher-order
pregnancies are not rare, and there may be as many offspring as there
are developing follicles. If more than three follicles are present
before the administration of hCG, the follicle number can be reduced by
follicle aspiration (if the goal is simply to lower the number
of possible offspring) or in an operating room (if the goal
is to perform in vitro fertilization followed by cryopreservation). Selective reduction
of the gestational sacs can also be performed if the number of pregnancies
exceeds three. However, it goes without saying that it is preferable
to prevent the higher-order pregnancy that places the patient
at risk for losing all her pregnancies than to undergo a selective
reduction. Because these alternatives may be unacceptable to some patients, a
frank discussion should be included before treatment to monitor
her cycle appropriately. Patients must be warned of ovarian hyperstimulation; some degree of ovarian
enlargement occurs in most patients and mild ovarian hyperstimulation
occurs in more than 40% of patients.38 Symptoms consist of mild bloating without ascites and ovarian diameters
of 5 and 7 cm. In moderate ovarian hyperstimulation, the ovaries enlarge
to 7 to 10 cm in diameter with an increase in abdominal girth, bloating, and
abdominal discomfort. An ovarian diameter of more than 10 cm
is considered severe; it occurs in approximately 1% of patients
and is accompanied by a number of typical symptoms (Table 4). The peak ovarian size usually occurs approximately 10 days after
the ovulatory dose of hCG, and this is an excellent time to ask the
patient to return for a follow-up visit. Patients whose ovaries
contain 10 or more follicles per side at the time of hCG are most likely
to have severe ovarian hyperstimulation. Table 4. When to Call Your Physician
| Weight gain >3 lb in 1 day |
| Shortness of breath |
| Abdominal pain |
| Chest pain |
| Pants become tight |
| Unable to stand upright |
| Dizziness |
| Calf pain |
| Arm or leg weakness | An adnexal diameter of 10 cm or more in the presence of ascites constitutes
ovarian hyperstimulation syndrome. Its pathophysiologic basis stems
primarily from increased capillary permeability mediated by the ovarian
renin-angiotensin cascade, histamine, serotonin, vascular
endothelial growth factor, cytokines, and vasoactive endothelial substances.39,40,41,42 Other potential side effects associated with this syndrome include pleural
effusion and an increased risk of thrombosis as a result of hemoconcentration, a
sludging of the blood flow through the arteries. This
latter event appears to be occurring more often, primarily because of
the increased numbers of patients exposed to gonadotropins annually. Ovarian
hyperstimulation or ovarian hyperstimulation syndrome should be
viewed as a potentially life-threatening problem. Treatment of ovarian hyperstimulation syndrome can usually be achieved
conservatively through hospitalization. The patient should be monitored
for input and urinary output. Fluid replacement should be accomplished
with normal saline or lactated Ringer's solution, because a hypotonic
solution may increase third spacing. Daily weights are helpful
to assess fluid retention. A weight gain of more than 3 lb/day may
require treatment. This is usually not achieved with diuretics, which
lead to further hemoconcentration, but rather with hypertonic solutions
such as albumin or dextran to draw third-spaced fluid back into the vascular tree. Paracentesis
or culdocentesis of the ascitic fluid can lead to dramatic improvements,43 but this latter approach is rarely necessary. The number of examinations
should be limited to reduce the risk of ovarian rupture. Abdominal
girths measured daily with a tape measure allow objective quantifying
of size. Daily blood tests for hematocrit and electrolytes provide a basis
for assessing the degree of hemoconcentration and electrolyte imbalance. Rarely, the enlarged ovary undergoes torsion; this is a surgical emergency. Although
oophorectomy was once advocated as the only treatment, it
is now known that the ovary can be untorsioned and retained without
oophorectomy. Doing so does not increase the risk of thrombosis or embolus. The final short-term complications of gonadotropin therapy are related
to pulmonary embolus, stroke, and death. These events are not limited
to patients who have pre-hCG estradiol levels exceeding 2000 pg/mL; I am aware of cases of cerebrovascular
accidents in which the estradiol value was less than 1000 pg/mL at the time of hCG administration.44 Although complications can be prevented with high probability, they cannot
be prevented with absolute certainty, even if one is cautious. For
this reason, all patients must be made aware of the potential risks of
gonadotropin therapy, and the conversation must be documented in the
chart. The final area of informed co.sent involves the long-term risks
of gonadotropin therapy. A study published in 1992 suggested that fertility
drugs may cause ovarian cancer.45 In that report, however, only ovarian cancer patients were studied. Of 620 women
with ovarian cancer, 3% reportedly had taken fertility
drugs, compared with only 1% of 1000 noncancer controls. The
findings in this study were based on only 31 women who had used infertility
medications. The study failed to report what dosage of the fertility
drugs the women had used, how long they received the medications, or
what drugs were used. The study did suggest that pregnancy, breastfeeding, and
oral contraceptive use may decrease a woman's chance
of ovarian cancer. These latter epidemiologic data are in accord with
the current information relevant to ovarian cancer. Subsequent studies
have been performed retrospectively looking at women who received gonadotropins. The
bulk of the evidence available today suggests that women
who have infertility have an increased risk of ovarian cancer over
those who are fertile. However, infertile women who use ovulation-inducing
agents do not appear to be at increased risk of ovarian
cancer over women who do not conceive and who do not take fertility drugs.46 Therefore, based on the results of these most recent data, patients must
be advised as to the potential risks that exist but reassured that
the medications appear to add little to the risk over and above that of
the infertility per se. Even so, most infertility patients appear willing
to tolerate a slightly increased risk of ovarian cancer, if such
a risk were the case.47 Although no amount of preparation, information, or careful monitoring can
eliminate all complications and side effects, patient education is
time well spent. Not only will she and her partner be less likely to be
angry if a complication occurs but also will she also be able to notify
the physician before a problem becomes serious. |