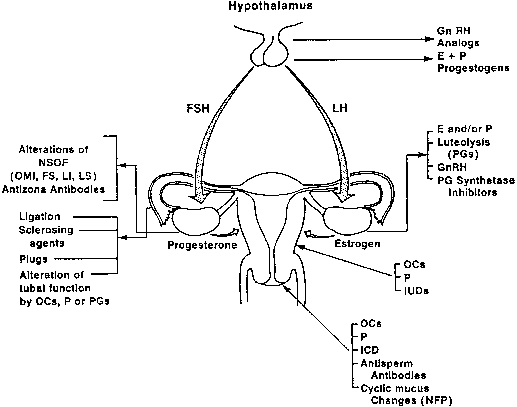
Because the ovary is the site of gamete development and release in the
female, it is an important target for contraceptive manipulation. Furthermore, the
development and function of the corpus luteum are essential
for the survival of the conceptus in the first trimester of pregnancy. Synthetic
sex steroids may affect the ovaries indirectly by their
inhibitory action on gonadotropin release or directly by altering the
follicular environment. Suppression of midcycle surge of LH and FSH by
estrogens, estrogen progestogen combination, or progestogens alone has
already been alluded to. The suppression of midcycle peaks of LH and
FSH, however, does not lead invariably to anovulation. Corpora lutea
have been found in the majority (85%) of women receiving small daily doses
of progestogens such as norgestrel, norethindrone, and quingestauol
or megestrol acetate.7 These corpora lutea, however, are functionally abnormal, producing subnormal
amounts of progesterone. Two mechanisms may be involved in the
suppression of endogenous production of progesterone in the luteal phase
in women treated with progestogens. First, the suppression of the gonadotropins
during the follicular phase at midcycle may interfere with
the normal stimulation and function of the corpus luteum. Second, progestogens
may have a direct effect on steroidogenesis (luteolytic) in
the corpora lutea. Ovarian Follicle The ovarian follicle plays a central role in reproductive function. The
development of a healthy and normal-functioning ovarian follicle is in
fact prerequisite to processes leading to ovulation and conception. The
ovarian follicle is actively involved in the process of steroidogenesis
and biosynthesis of a number of proteins and peptides, such as inhibin
and activin. Follicular fluid is also a rich source of many substances. It contains
a low concentration of serum proteins less than 1,000,000 kd molecular
weight, as well as many other substances, such as LH, FSH, estrogens, progestins, androgens, sex hormone binding protein, ovulating enzymes (plasminogens, proteases), and nonsteroidal ovarian factors. Follicular
fluid is rich in steroid hormones, and steroid concentration in follicular
fluid greatly exceeds that in blood. Preovulatory follicles contain
high levels of estrogens. Progesterone is found in fairly low levels
in the nonovulating follicle, but its concentration rises in the
late follicle. Follicles also contain measurable amounts of FSH, LH, and
prolactin, always at concentrations lower than are found in the blood. FSH
is found in all follicles that have started to form an antrum, whereas
LH and progesterone appear together in preovulatory follicles. Prolactin
and androgen levels fall with follicular maturation. Thus, follicles
have their own hormonal environment, and it appears that the
diffusion of steroids from the follicular fluid into blood is restricted, as
evidenced by the very high estrogen levels maintained in the
follicular fluid. Movement of steroids into follicular fluid from nearby
follicles is also restricted, as can be seen by the low progesterone
levels detected in follicles during the luteal phase, in the presence
of an ipsilateral corpus luteum.11 Prostaglandins, which are believed to be involved in ovulation, are also
found in follicular fluid of preovulatory follicles after LH surge. In recent years, much research has been devoted to the identification and
possible function of nonsteroidal ovarian factors. These substances
play an important role in modulating intraovarian function and in fine-tuning
gonadotropin action.10,11,12 Oocyte maturation inhibitor is a protein factor that can prevent oocyte
maturation in vitro. Luteinization inhibition and stimulation factors are concerned with the
process of luteinization. Various animal studies suggest that small
follicles might contain a luteinization inhibition factor, whereas large
follicles might contain a luteinization stimulation factor. These
factors seem to be responsible for the development of LH receptors (or
lack of them) and luteinization. Other putative intraovarian regulators
may be involved in subtle in situ modulation of the growth of follicular structure and with intercompartmental
communication, allowing for a tighter linking of different cellular
populations. Potential intraovarian intercellular communication may
take place by paracrine or autocrine action.13 Among putative intraovarian regulators, several have received considerable
attention and may conceivably be a target for fertility regulation.10,11,12 Insulin-like growth factor-I (IGF-I) is a polypeptide involved in amplification
of gonadotropin hormonal action. Recent information indicates
that IGF-I, which is produced by granulosa cells, binds to the interstitial
cells that are not a site of IGF-I gene expression but are endowed
with receptors. Thus, it appears that IGF-I may engage in intracompartmental
communication in the interest of coordinated follicular development.11,12 Another polypeptide, transforming growth factor-x, has proved to be a potent
inhibitor of gonadotropin-supported granulosa cell differentiation. Transforming growth factor-3T is yet another polypeptide that is produced
both by granulosa and theca interstitial cells and has been shown to
alter profoundly the proliferation and differentiation of rat granulosa
cells. At this time, however, the potential significance of transforming
growth factor β1 to human ovarian physiology remains unknown. Mention should also be made
of basic fibroblast growth factor, interleukin-1, and tumor necrosis
factor-2, which are thought to be involved in granulosa cell development
and regulation of luteal cells.12,13 Finally, the existence of the renin-angiotensin system (RAS) has been shown
in the ovary. High levels of renin and angiotensin are found in preovulatory
follicular fluid, and these substances are believed to be
involved in the maturation of the oocyte and in ovulation, either directly
or through other ovarian regulators. It has also been suggested that
angiotensin II may play a role in the formation of the corpus luteum
and steroid secretion by luteal cells.11,12 Contraceptive Implications The dominant preovulatory follicle has a precise hormonal milieu. It is
conceivable that a disturbance of this environment might lead to follicular
atresia or anovulation. Both combination oral contraceptives and
progestogen-only formulations might well bring about an alteration of
the follicular environment in addition to their other effects. The specificity of the factors discussed and their presumed localization
of action suggest a number of new targets for contraception.11 For example, if the LH surge were prevented from inducing withdrawal of
oocyte maturation inhibitor from the oocyte in the dominant follicle, all
of the endocrine events of the cycle would occur, but the oocyte
would not be viable. Injection of oocyte maturation inhibitor to reach
the oocyte during the time when the surge is occurring could prevent
resumption of meiosis. Follistatin injected into several animal species has been shown to prevent
FSH secretion and follicular growth. Similarly, inadequate formation
or maintenance of corpora lutea might result from local overproduction
of luteinization inhibition factor. Currently, the factors influencing
the control of synthesis and release of follicular peptide are not
well understood, but considerable efforts are being made to obtain such
information. The ovarian GnRH receptors may be considered an excellent potential target
for contraception. GnRH and its agonists may affect the ovarian function
in two different ways: - Chronic stimulation of pituitary gonadotropes by potent GnRH agonists may
cause cellular desensitization, with a subsequent decrease in circulating
gonadotropins.
- GnRH may directly suppress ovarian steroidogenesis, ovulation, and corpus
luteum function.
Similarly, the GnRH antagonists can block pituitary gonadotropin secretion; thus, timed
administration of GnRH agonists or antagonists can block
ovulation, follicular development, or corpus luteum function, depending
on the phase of the cycle when the drug is administered. Interference With Corpus Luteum Function Corpus luteum function is essential for implantation of the conceptus and
its further development in the first 8 weeks of gestation. Thereafter, the
hormonal burden for the maintenance of pregnancy is shifted to
the placenta. The corpus luteum is, therefore, an attractive target for
fertility regulation. Disruption of luteal function may prevent implantation. After
implantation, induction of luteolysis is likely to cause
an arrest of fetal development and abortion. Studies in experimental
animals and in humans have shown that GnRH analogues may exert luteolytic
activity. For example, administration of GnRH agonists to Rhesus
monkeys 3 to 5 days postovulation has been found to cause a significant
shortening of the luteal phase and decrease in serum progesterone
values. Administration of human chorionic gonadotropins (hCGs) prevented
the shortening of the luteal phase in animals treated with the analogue. In
rats, administration of GnRH antagonists interferes with pregnancy. In
monkeys, however, initial attempts to alter early pregnancy
with GnRH antagonists have not been successful. In humans as well, GnRH
analogues induce luteolysis when given 5 to 8 days after the LH peak. The
early corpus luteum seems to be refractory to luteolytic activity
of the agonists. Unfortunately, the luteolysis induced by GnRH-agonist
treatment is prevented by exogenous hCG. In addition, simultaneous
administration of GnRH analogues and hCG does not cause luteolysis. These
studies suggest that rising levels of hCG in early pregnancy may oppose
the GnRH induced luteolysis. Interference With Follicular Rupture The precise sequence of events leading to follicular rupture and extrusion
of oocytes has not been clarified. In several animal species, including
subhuman primates, prostaglandin F2α may play an important role. Prostaglandin synthetase inhibitors, such
as indomethacin and ibuprofen, have been shown to prevent follicular rupture
and ovulation after hCG administration, while the ovaries continue
to undergo luteinization. Furthermore, prostaglandins have luteolytic
activity, so their administration in the early luteal phase may interfere
with the function of corpus luteum and prevent survival of the
conceptus. Another potential intraovarian factor target for control of
follicular rupture is RAS. RAS involvement in oocyte maturation and
ovum release has been suggested by several studies. In fact, the administration
of saralasin acetate, an RAS antagonist, in animal studies has
been shown to block the reproductive process. Clinical studies are
required to determine the feasibility of such an approach in a human model. |