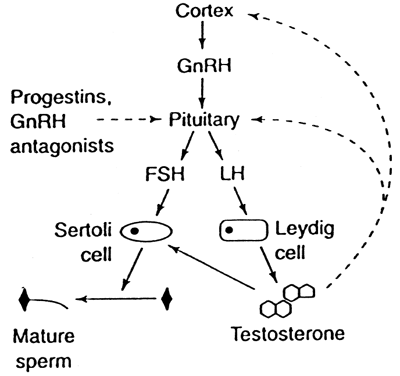
Hormonal Male Contraceptives Because of the drawbacks of existing methods of male contraception, efforts
have been made to develop a hormonally derived contraceptive analogous
to estrogen/progesterone birth control pill for women. Such a hormonal
contraceptive has the potential to be safe, easy to use, and reversible. Testosterone, when administered in slightly supraphysiologic
doses, can function as a contraceptive by suppressing the secretion of
the pituitary gonadotropins LH and FSH. Low levels of LH and FSH deprive
the testis of the signals required for spermatogenesis, leading to
markedly decreased sperm counts in most, but not all, men. Sperm counts
uniformly rise after the cessation of testosterone administration. Surveys
conducted in several countries suggest that such a hormonally
derived male contraceptive administered either by daily pills or periodic
injections would be welcomed by a large percentage of men and women.24,25 In general, hormonal contraceptives do not incapacitate existing sperm; they
block the initiation of sperm production. Given that sperm take
an average of 72 days to reach maturity, it is likely that any contraceptive
based on manipulation of the hormonal axis must be associated with
some delay in the onset of full contraceptive effect. In normal men, sperm
counts vary from 20 to 200 million sperm per milliliter of ejaculate. The
absence of spermatozoa in the ejaculate, a condition termed azoospermia, renders fertilization impossible and is therefore the ultimate goal of
hormonal male contraceptives. Most studies to date, however, demonstrate
that some men sustain partial but incomplete reduction of their sperm
counts, a condition called oligozoospermia.26 Sperm counts below 3 million sperm per milliliter of ejaculate are associated
with decreased rates of pregnancy.27 Severe oligozoospermia (counts less than 1 million sperm per milliliter) decreases
the chances of conception even further and is therefore considered
a reasonable short-term goal for male contraceptive research. Ethnic differences must be considered in interpreting results of contraceptive
trials. Study volunteers in Asia are more susceptible to testosterone-induced
suppression of spermatogenesis, with rates of azoospermia
in the 90% to 100% range. Men studied in Europe, North America, and
Australia, however, have rates of azoospermia closer to 60% to 80% on
the same regimens.27,28 Although the explanation for this difference is inconclusive, it is important
in the interpretation of trial results and complicates extrapolation
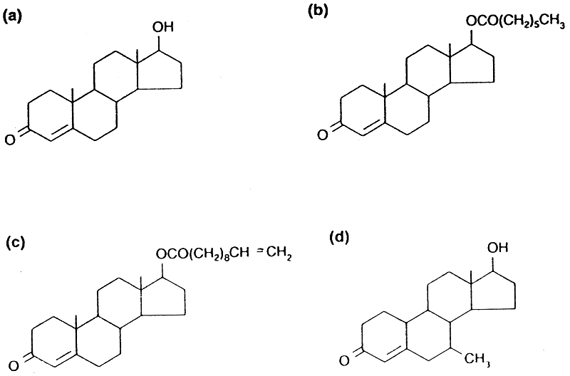
of data to different populations. Testosterone as Contraceptive Administration of unmodified testosterone (Fig. 2
A) is impractical because when given orally or by injection it is quickly
degraded by the liver. Therefore, most hormonal contraceptive regimens
have used longer-acting injectable testosterone esters such as testosterone
enanthate (TE) (see Fig. 2 B) given by intramuscular injection on a weekly to fortnightly basis. On
such a regimen, sperm counts approach 0 between 2 and 3 months, with
recovery of normal sperm counts 3 to 4 months after the injections are
discontinued. Two multicenter trials of TE as a male contraceptive have been conducted
by the World Health Organization (WHO). The first study enrolled 271 subjects
who were given 200-mg TE intramuscularly weekly.28 Of these men, 60% achieved azoospermia, and an additional 30% were rendered
severely oligozoospermic. Moreover, 119 of the men who became azoospermic
were instructed to discontinue other birth control and to continue
on TE injections. These couples were followed up for 1 year. During
that time, only 1 pregnancy occurred, thereby demonstrating testosterone-induced
azoospermia to be an effective contraceptive. The second WHO study examined the fertility of both the men who became
azoospermic and the men who achieved severe oligozoospermia with TE injections.27 In total, 399 men were enrolled in this study. Of these, 98% became severely
oligospermic (less than 3 million sperm per milliliter) or azoospermic. There
were no pregnancies caused the men who became azoospermic, and
in men who became severely oligospermic, fertility was reduced
to 8.1 pregnancies per 100 person-years. The overall failure rate (including
men who failed to suppress their sperm counts) was 3.4%, for an
overall contraceptive efficacy of 96.6%. In both groups, sperm counts
returned to normal after the cessation of testosterone injections, and
there were no major side effects. These studies demonstrated that injected TE is safe, fully reversible, and
effective as a contraceptive in most men; however, a proportion of
men fail to suppress below 3 million sperm per milliliter and therefore
presumably remain fertile. In addition, the necessity of weekly intramuscular
injections is a deterrent. Twelve percent of patients in the
second WHO study discontinued involvement for personal or medical reasons
or due to dislike of the injection schedule. Finally, high-dose
TE has been shown to decrease serum HDL cholesterol, which could accelerate
atherosclerosis.29,30 Because of poor acceptability of weekly injections, newer methods of sustained
testosterone delivery suitable for use in a contraceptive regimen
are being pursued. Testosterone undecanoate (TU) (see Fig. 2 C), is a long-chain ester that is absorbed from the intestine through the
lymphatics and therefore escapes first pass hepatic metabolism.31 It can be given orally two to four times a day, or by intramuscular injection, where
it results in normal serum T levels for at least 6 weeks
in hypogonadal men.32,33,34 Recently, a small trial of TU injections in normal men was conducted in
China.35 Volunteers received monthly injections of 500 or 1000 mg TU. Eleven of 12 in
the 500-mg group and 12 of 12 in the 1000-mg group became azoospermic, with
the 1000-mg group achieving azoospermia more quickly. Importantly, no
significant changes in serum HDL or serious side effects
were found, proving this to be a promising androgen for future studies. Of
particular interest will be studies to determine whether white men
are equally susceptible to TU-mediated suppression of spermatogenesis. The testosterone ester 7α-methyl-19-nortestosterone (MENT) (see Fig. 2 d) is also of considerable interest.36 This compound is ten times more potent than testosterone and is not converted
into dihydrotestosterone, the androgen implicated in prostatic
hypertrophy, acne, and male-pattern baldness. To date, MENT has not been
used in contraceptive trials, but its potency and tissue selectivity
could be advantageous in the long-term safety and side effect profile
of a hormonally derived male contraceptive. MENT (and other new tissue-selective
androgens) have the exciting potential of improving general
health by decreasing the likelihood of the development of prostatic
hypertrophy and prostate cancer in men in addition to providing safe
and effective contraception. Transdermal testosterone patches have been in use for the last several
years for the treatment of male hypogonadism but have only recently been
tested for male contraception. A contraceptive study combining 5.4-mg
testosterone daily delivered through transdermal patch with the progestin
levonorgestrel was recently described.37 This combination, unfortunately, resulted in azoospermia in only 2 of 11 men, with
counts below 3 million sperm per milliliter in 3 others. This
low success rate was probably due to insufficient testosterone delivery
through the transdermal route. Transdermal delivery of testosterone
is also hampered by the frequent complaints of skin irritation caused
by the patches, which has lead to high discontinuation rates in clinical
practice. Testosterone with GnRH Analogues and Progestins Because of the failure of testosterone-only regimens to suppress sperm
production completely in all men, compounds such as GnRH analogues and
progestins that also suppress pituitary gonadotropins are being studied
in combination with testosterone to optimize its contraceptive efficacy. The
combination of testosterone with the widely used GnRH agonists, such
as leuprolide, has proven disappointing, but research into the
use of GnRH antagonists as contraceptives remains promising. GnRH antagonists
can potently suppress FSH and LH production within hours of
administration, and their inhibition of gonadotrophin secretion is more
complete than that produced by agonists. Three human trials have been conducted using the GnRH antagonist Nal-Glu
with testosterone. The first two trials showed promise, with 7 of 8 subjects
in one study achieving azoospermia by 6 to 10 weeks of treatment.38,39 A third trial, however, demonstrated no difference in azoospermia when
compared with TE alone.40 The time required to reach azoospermia was roughly 7 to 10 weeks, and
normal sperm counts returned in roughly the same amount of time, demonstrating
the reversibility of this approach. Newer GnRH antagonists, such
as cetrorelix and acyline (Fig. 3), have recently been introduced for experimentation. Administration of
cetrorelix to cynomolgus monkeys safely reduces serum gonadotropins and
androgens by 80% within 16 days.41 Similar results were obtained in trials of healthy male volunteers.42 These agents are obvious choices for future testing in male contraceptive
trials.  Fig. 3. Amino acid composition of gonadotropin-releasing hormone (GnRH) and some
GnRH agonists and antagonists. Fig. 3. Amino acid composition of gonadotropin-releasing hormone (GnRH) and some
GnRH agonists and antagonists.
|
Currently available GnRH antagonists have some drawbacks. As peptides, they
are expensive to manufacture and must be injected subcutaneously
to avoid degradation in the intestine. Administration of these compounds
can induce mild burning sensations at the injection site and occasional
nontender, subcutaneous nodules, which resolve within weeks. Because
they are effective, however, additional work is being undertaken with
these compounds. Recently, the discovery of nonpeptide, orally active GnRH antagonists has
been published.43,44 Although not yet tested in humans, these compounds have immense potential
in male contraception as well as the treatment of many other conditions. The idea of using a progestin synergistically with testosterone to block
sperm production has been extensively tested.45 Combinations of testosterone and depot injections of medroxyprogesterone
acetate (DMPA) (Fig. 4 A) were shown to induce azoospermia in half of study subjects with some
degree of oligozoospermia in most others. The contraceptive efficacy
of these combinations, however, was poor, with several couples conceiving
while receiving therapy despite simultaneous use of other contraceptives.46 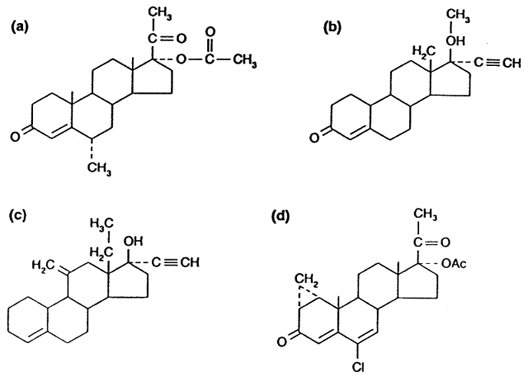 Fig. 4. Progestins used in male contraceptive research: ( a) medroxyprogesterone acetate, ( b) levonorgestrel, ( c) desogestrel, ( d) cyproterone acetate.(Amory JK, Bremner WJ: Newer agents for hormonal contraception in the male. Trends
Endocrinol Metab 11:61, 2000.) Fig. 4. Progestins used in male contraceptive research: ( a) medroxyprogesterone acetate, ( b) levonorgestrel, ( c) desogestrel, ( d) cyproterone acetate.(Amory JK, Bremner WJ: Newer agents for hormonal contraception in the male. Trends
Endocrinol Metab 11:61, 2000.)
|
Recent studies of progestins have focused on newer compounds, such as the
potent oral progestin, levonorgestrel (LNG) (see Fig. 4 B). A trial of LNG (500 μg orally daily) with TE (100 mg intramuscularly
per week) showed the LNG-TE combination was superior to TE alone
in terms of azoospermia (67% versus 33%) by 6 months.47 In addition, 94% achieved either severe oligozoospermia or azoospermia
in the LNG-TE group compared with 61% of the TE-alone group. Drawbacks
to the LNG-TE regimen included greater weight gain and decreases in
HDL cholesterol when compared with the TE-alone group. Subsequently, lower
doses of LNG have been demonstrated to be as effective at achieving
azoospermia with less weight gain and smaller reductions of HDL cholesterol.48 Other progestins, such as desogestrel (see Fig. 4 C), have been tested in male contraceptive regimens. When combined with 50- and 100-mg
doses of weekly TE, 18 of 23 patients became azoospermic
after 24 weeks and all but one suppressed to less than 3 million sperm
per milliliter.49 Another study has shown that 100-mg TE weekly with 150-μg desogestrel
daily may be more effective than TE/LNG at suppressing sperm counts
without causing greater weight gain or larger drops in HDL cholesterol
than have been seen with TE/LNG.50 Progestins with anti-androgenic effects such as cyproterone acetate (CPA) (see Fig. 4 D) have also been tested as potential male contraceptives. CPA suppresses
FSH and LH production at the pituitary level and also functions as
an antiandrogen by blocking the binding of testosterone and dihydrotestosterone
to androgen receptors.51 In a promising trial of this compound, two groups of men received CPA
at either 50- or 100-mg orally daily in combination with 100-mg TE intramuscularly
weekly, whereas a third group received weekly TE alone.52 All men receiving CPA became azoospermic, whereas only 3 of 5 in the TE-alone
group attained azoospermia (Fig. 5). In addition, the time required to achieve azoospermia in the CPA groups
was half that needed in the TE-alone group, implying that in addition
to its gonadotropin-lowering effects, CPA may also block later stages
of sperm maturation. No major adverse side effects, such as changes
in HDL cholesterol, liver function, libido, or sexual potency were noted
in this small sample of men. The sole drawbacks noted were slight
decreases in body weight and serum hemoglobin level that were dependent
on the dose of CPA. Subsequently, CPA has been combined with oral testosterone
undecenoate in a fully oral potential male contraceptive regimen.53 Of the 8 study subjects, 1 became azoospermic, 5 were suppressed below 3 million
sperm per milliliter, and the 2 remaining study subjects were
suppressed to 4 and 6 million sperm per milliliter. Alterations in
this regimen may lead to more complete and reliable spermatogenic suppression
and the eventual availability of a true “male pill.” 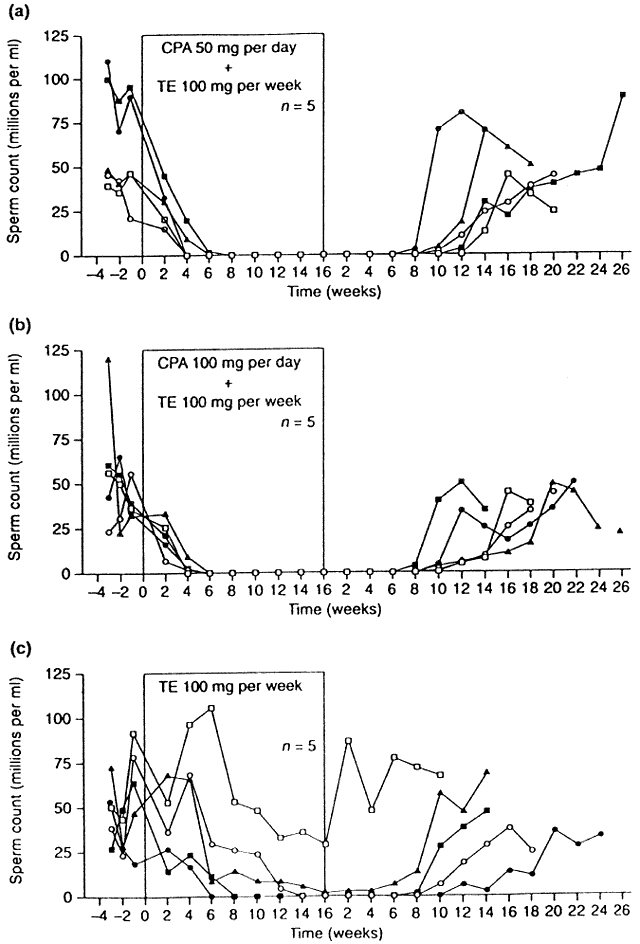 Fig. 5. Sperm concentrations in individual subjects during the control period, throughout 16 weeks
of hormone administration and during 26 weeks of recovery
phase.(Meriggiola MC, Bremner WJ, Paulsen CA et al: A combined regimen of cyproterone
acetate and testosterone enanthate as a potentially highly effective
male contraceptive. J Clin Endocrinol Metab 81:3018, 1996.) Fig. 5. Sperm concentrations in individual subjects during the control period, throughout 16 weeks
of hormone administration and during 26 weeks of recovery
phase.(Meriggiola MC, Bremner WJ, Paulsen CA et al: A combined regimen of cyproterone
acetate and testosterone enanthate as a potentially highly effective
male contraceptive. J Clin Endocrinol Metab 81:3018, 1996.)
|
|