A history of one abortion is associated in most studies with a nonsignificant, excess risk of ectopic pregnancy of approximately 30% (Table 1).1,2,3,4,5,6 These observed associations, although real, may be due to chance, as the 95% confidence intervals (CIs) for all the odds ratios (ORs) included 1.0. CIs in four studies were narrow enough to rule out a twofold excess risk. Investigations of the effect of two or more prior abortions experienced small numbers of exposed women. Although all CIs included 1.0, suggesting no significantly elevated risk, the CIs were wide, and point estimates ranged from a fivefold protective effect (OR, 0.2)1 to a fourfold excess risk (OR, 4.0).2
Table 1. Epidemiologic Studies of Induced Abortion as a Risk Factor for
Ectopic Pregnancy*
Study/No. of | No. of | No. of | Crude | Adjusted |
|
Abortions | Cases | Controls | Odds Ratio | Odds Ratio | 95% CI |
Pregnant Controls |
|
|
|
|
|
Levin et al2 |
|
|
|
|
|
USA, 1976-1978 |
|
|
|
|
|
0 | 56 | 396 | 1.0 | 1.0 | — |
1 | 20 | 86 | 1.6 | 1.3 | 0.6–2.7 |
| 9 | 16 | 4.0 | 2.6 | 0.9–7.4 |
Daling et al3 |
|
|
|
|
|
USA, 1975-1979 |
|
|
|
|
|
0 | 167 | 411 | 1.0 | 1.0 | — |
1 | 25 | 48 | 1.3 | 1.3 | 0.6–2.6 |
| 7 | 6 | 2.6 | 1.8 | 0.5–7.1 |
Skjeldestad and Atrash1 |
|
|
|
|
|
Norway, 1987-1990 |
|
|
|
|
|
0 | 121 | 97 | 1.0 | 1.0 | — |
1 | 44 | 14 | 2.5 | 1.3 | 0.9–1.8 |
| 9 | 4 | 1.5 | 0.2 | 0.04–0.9 |
Atrash et al4 |
|
|
|
|
|
USA, 1988-1990 |
|
|
|
|
|
0 | 121 | 678 | 1.0 | 1.0 | — |
1 | 43 | 268 | 0.9 | 0.9 | 0.6–1.3 |
| 18 | 110 | 0.9 | 0.9 | 0.5–1.7 |
Nonpregnant Controls |
|
|
|
|
|
Burkman et al5 |
|
|
|
|
|
USA, 1976-1978 |
|
|
|
|
|
0 | 352 | 2362 | 1.0 | 1.0 | — |
1 | NA | NA | 1.0 | 1.0 | 0.5–1.8 |
| NA | NA | NA | 0.9 | 0.8–1.1 |
Holt et al6 |
|
|
|
|
|
USA, 1981-1986 |
|
|
|
|
|
0 | 123 | 457 | 1.0 | 1.0 | — |
1 | 55 | 280 | 1.0 | 0.9 | 0.6–1.3 |
| 33 | 50 | NA | 1.2 | 0.6–2.4 |
Skjeldestad and Atrash1 |
|
|
|
|
|
Norway, 1987-1990 |
|
|
|
|
|
0 | 121 | 176 | 1.0 | 1.0 | — |
1 | 44 | 41 | 1.6 | 1.2 | 0.8–1.7 |
| 9 | 10 | 1.4 | 1.8 | 0.4–7.8 |
Last Pregnancy: Abortion vs. Birth |
|
|
|
|
|
Skjeldestad and Atrash1 |
|
|
|
|
|
Norway, | 29 | 166 | 1.6 | 0.5 | 0.2–1.0 |
1987-1990 |
|
|
|
|
|
CI, Confidence interval; NA, not available.
*An earlier version of this table, containing errors, appears in Hogue et al7.
Some would contend that the observed ORs reflect a truly elevated risk, not withstanding the statistical uncertainty of small sample sizes. However, two features in study design also affect OR estimates in these studies. These are the choice of comparison group and the control of competing risk factors. In addition, selective recall tends to bias the OR estimates. These issues are discussed next.
Choice of Comparison Group
Most epidemiologic studies of ectopic pregnancy are case-control studies, because ectopic pregnancy is a rare event. The alternative cohort design requires many participants, is more expensive, and is difficult to carry out. For a case-control study, there is no one correct way to select an appropriate group of women for comparison with the case series. A population-based sample includes never-pregnant women as well as women pregnant for the first time. By definition, these women cannot have been exposed to a prior induced abortion and may be excluded from the analysis.7 However, excluding nonpregnant and primigravid women may overestimate the probability of exposure of abortion in the population, because these women are likely to be more effective contraceptive users and less likely to have unintended pregnancies that might be terminated by abortion. Such an overestimate would bias the OR toward the null (i.e., find no association between abortion and ectopic pregnancy when, in fact, there is an association). In Table 1, the studies that use a nonpregnant control group tend to report no association (i.e., OR, approximately 1.0).
Conversely, studies that use women giving birth for a comparison group bias the point estimate away from the null. Women giving birth are on average less likely to have a prior induced abortion than a comparison group of pregnant women chosen to represent all pregnancy options (i.e., induced abortion of this pregnancy as well as delivery).8 For example, an Italian study reported an OR of 2.1 for the comparison group with women giving birth, but an OR of 1.2 for a comparison group of women hospitalized for nonobstetric reasons.9 The four studies summarized in Table 1 did strive to equate the likelihood of potential exposure to prior induced abortion as much as possible. The pregnant comparison groups included both women with induced abortions and women giving birth. Additionally, Daling and associates3 excluded—from both case and comparison groups—unmarried women and those not living in a stable relationship who might be more likely than the average to have a history of abortion. Skjeldestad and Atrash1 excluded from both groups women who could not have been exposed to abortion or those who were less likely to have an ectopic pregnancy—primigravid women and those using effective contraception during the 4 weeks before the interview. Similarly, Atrash and coworkers4 excluded women with spontaneous abortion, primigravid women, and those with a history of ectopic pregnancy or tubal damage.
Control for Potential Confounding Factors
Regardless of the care taken in choosing an appropriate comparison group, it is impossible to ensure comparable potential exposure to prior abortion. Women with prior abortions differ from women with no prior abortions in that they are more likely to smoke10 and to have been exposed to sexually transmitted diseases (STDs). They have more sexual partners, have earlier age at first sex, have a higher risk of pre-existing pelvic inflammatory disease, and rely less on condoms for protection from STDs. Both smoking and STDs are risk factors for ectopic pregnancy. Studies that do not control for these potential confounding factors may overestimate the effect of induced abortion on ectopic pregnancy. For example, a Greek study that did not control for STDs reported an OR of 1.87 (95% CI, 0.84–4.16) for ectopic pregnancy associated with one or more induced abortions.11 All studies summarized in Table 1 controlled for both smoking and STDs, as well as other potential confounding factors, when calculating adjusted ORs. The effect of adjustment was to reduce the observed effect of induced abortion, illustrating the importance of taking these differences into account. However, no observational study can completely rule out potential confounding by unmeasured variables. Therefore, observed ORs of less than 2.0 may be challenged on the basis of unmeasured confounding.
Selective Recall
The case-control strategy recruits participants based on their health status (e.g., case subjects have an ectopic pregnancy and control subjects have a normal pregnancy) and queries them about past exposures. Accurate exposure measurement relies on accurate memory. Both case and control subjects may experience an equal likelihood of poor memory. In this instance, the OR is biased toward the null because of error in measurement. However, with a sensitive topic such as prior induced abortion, women with a health problem are more likely to report their abortion history than are women in the control group.12 This selective recall biases the OR away from the null by an estimated 20% to 50% over the true risk. Selective recall could explain the small excess risk observed in the ectopic pregnancy case-control studies summarized in Table 1. Cohort studies do not rely on recall, but rather begin with the exposure and follow women to observe which ones experience the outcome. One cohort study of Norwegian women identified those with one (N = 2662), two (N = 554), or three (N = 538) abortions.13 Followed 1 to 7 years through record linkage, women with two or more abortions experienced no excess risk for ectopic pregnancy (adjusted incidence density ratio [IDR], 1.2; 95% CI, 0.5–3.1) compared with women with one abortion. There was no excess risk if the abortions occurred in consecutive pregnancies (adjusted IDR, 0.9 for two consecutive abortions and 1.1 for three or more consecutive abortions compared with one abortion). These findings support the lack of causal association between induced abortion and ectopic pregnancy.
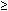 2
2