Anatomy of the Pelvis
Authors
INTRODUCTION
This chapter will focus on those aspects of pelvic anatomy that have special importance to the practice of obstetrics. By providing a broad framework, it will help to put into perspective some of the more detailed aspects of anatomy that are contained in other chapters within this volume that deal with a specific subject in which anatomy plays an important role.
THE PELVIC WALLS AND ASSOCIATED STRUCTURES
The Bony Pelvis
The pelvis is made of a bony girdle that has a central canal. Its structure must be strong enough to transfer the weight of the body from the spine to the femurs, and yet it must have a large enough opening to allow for a term fetus to be delivered through it.
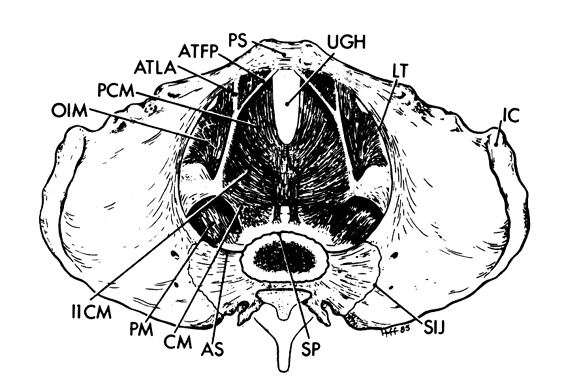
There are three separate parts to the pelvis: the single midline sacrum and paired innominate bones (os coxae). The line of division between the sacrum and the innominate bones is the sacroiliac joint, while the two innominate bones are separated from one another by the pubic symphysis (Fig.1 and Fig. 2).
Each innominate bone is formed from three bones: the ilium, the ischium, and the pubis. These bones have fused into a single unit before reproductive age is reached. Their individual names persist, however, in terms such as the iliac crest, ischial tuberosity, and pubic ramus.
These bony parts are assembled to form a pelvis, which has traditionally been divided into an upper false pelvis and a lower true pelvis, separated from one another by the linea terminalis. The false pelvis forms the lower part of the abdominal cavity. It is bounded laterally by the iliac bones, posteriorly by the lumbar spine, and anteriorly by the abdominal wall. It has little obstetrical significance.
The canal of the true pelvis is bent forward in its lower portion (see Fig. 2) in the curve of Carras. The change in direction of this space is partly due to the curve of the sacrum but is also caused by the muscles of the pelvic floor.
The rim that surrounds the upper opening of the true pelvis is called the inlet and is formed by the promontory and alae of the sacrum posteriorly, by the inner surface of the superior pubic rami anteriorly, and by the linea terminalis laterally (see Fig.1 and Fig. 2). The shape of the inlet as well as the other planes of the pelvis are important to the mechanism of labor and are covered in a later chapter in this volume.
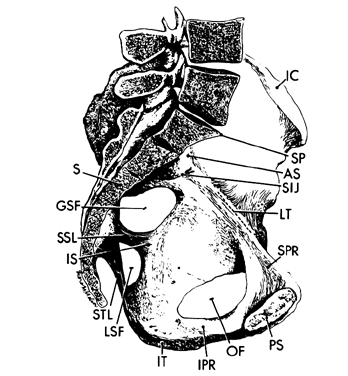
Within the true pelvis the lateral pelvic walls slant toward one another at approximately a 15° angle so that lines drawn down these walls would intersect at the level of the knees. Because of the inward inclination of the walls and protrusion of the ischial spines into the pelvic cavity (without concomitant shortening of the anterior posterior dimension), the middle portion of the pelvic cavity becomes longer in its anterposterior diameter than in the transverse. The level of the midplane is marked on each wall by the ischial spine. It passes through the lower border of the pubic symphysis and the junction of the fourth and fifth sacral vertebrae (see Fig. 2).
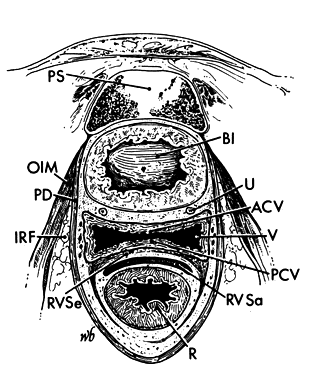
The pelvic outlet (Fig. 3) is formed by two triangles fitted base to base to form a diamond. The anterior (urogenital) triangle has its corners at the lower end of the pubic symphysis and the inner aspects of the ischial tuberosities. It is bounded laterally by the inferior ischiopubic rami. The posterior (anal) triangle has its apex at the tip of the sacrum and shares its base with the anterior triangle. The lateral borders of the posterior triangle are the sacrotuberous ligaments.
The pelvis has three joints: two sacroiliac joints and the pubic symphysis. The sacroiliac joints are true synovial joints, but the symphysis is a synchrondrosis, without a synovial space. Although immobile during most of life, these joints do display some movement during pregnancy. 1,2
Each hemipelvis contains three lateral openings in its wall. These are the obturator foramen and the greater and lesser sciatic foramina (see Fig. 2). The sacrospinous ligament separates the two sciatic foramina and has a broad attachment to the lateral surfaces of the lower sacrum and coccyx and an apical insertion into the ischial spine. It is covered on its pelvic surface by the coccygeus muscle (Fig.2 and Fig. 3). At the bottom of the lesser sciatic foramen is the sacrotuberous ligament. It arises from the posterior iliac spines and the back and side of the lower sacrum and coccyx and ends at the ischial tuberosity. These ligaments help to stabilize the pelvis and probably undergo the same softening that the ligaments around the pelvic articulations experience.
Muscles of the Pelvis
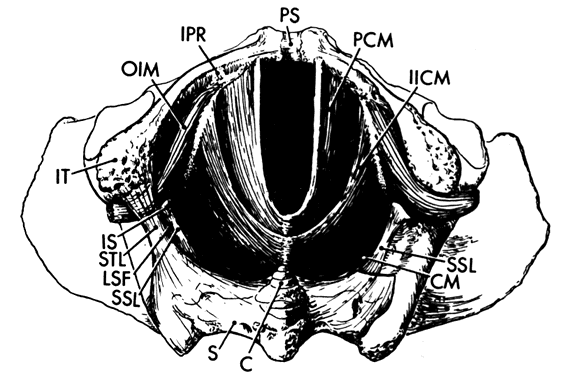
When a bony pelvis and model of a fetal head are used to demonstrate forceps delivery or mechanisms of labor there exists an obvious space between the fetal head and the osseous structures that is so large that the head can easily fall through the pelvic canal. This space is filled by the soft tissues of the pelvis. There are two components to these tissues. The first includes the muscles of the pelvic wall (Fig. 4), a series of muscles that cover the inner surfaces of the bony pelvis, following its shape and exerting their action on the hip. The second component is the pelvic floor, which supports the abdominal and pelvic viscera and whose resistance must be overcome by the presenting part during delivery. The pelvic floor consists of the pelvic diaphragm, which stretches across the entire pelvic cavity at the level of the midplane, and the urogenital diaphragm and external genital muscles, which span the anterior portion of the pelvic outlet.
MUSCLES OF THE PELVIC WALL.
Arising from the inner surface of the obturator foramen and membrane, the obturator internus muscle leaves the pelvis through the lesser sciatic foramen to insert into the medial surface of the greater trochanter. The piriformis muscle arises from the anterior aspect of the sacrum. It passes through the greater sciatic foramen and inserts into the upper border of the greater trochanter. The obturator internus muscle is innervated by the obturator nerve and the piriformis muscle is innervated by a branch from the sacral plexus. Both muscles are lateral rotators and abductors of the thigh.
MUSCLES OF THE PELVIC DIAPHRAGM.
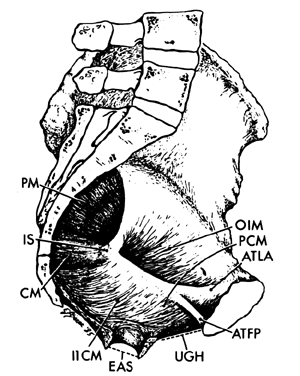
The muscles of the pelvic wall do little to close off the opening in the pelvis. A fetal head would still fall through the pelvic opening were it not closed off by the pelvic diaphragm. There are three muscles in the pelvic diaphragm: the pubococcygeus, the iliococcygeus, and the coccygeus. The pubococcygeus muscle arises from the inner surface of each pubic bone to pass posteriorly on either side of the urethra, vagina, and rectum. Behind the rectum some fibers bend back anteriorly to join the muscle on the other side, thereby forming a sling. Other fibers insert into the sacrum and coccyx. Some of the most medial fibers within this group attach to the walls of the vagina and rectum.
The iliococcygeus muscle arises from a series of tendon-like fibers that attach to the fascia of the obturator internus muscle (the arcus tendineus levator ani). From these broad origins the fibers of the iliococcygeus insert into the anococcygeal raphe and the coccyx. The coccygeus arises from the ischial spine and the sacrospinous ligament to insert into the borders of the coccyx and the lowest segment of the sacrum.
These muscles are covered on their superior and inferior surfaces by the superior and inferior fasciae of the pelvic diaphragm. These fasciae arise from the obturator internus fascia and also in certain areas from the pubic bones. They fuse at the urogenital hiatus with the urogenital diaphragm and vagina.
The iliococcygeus and coccygeus muscles receive their innervation from an anterior branch of the ventral rami of the third and fourth sacral nerves while the pubococcygeus muscle is supplied by the pudendal nerve.
The muscle fibers of the pelvic diaphragm form a “U” with the open end directed anteriorly. The open area within the “U” through which the urethra, vagina, and rectum pass is called the urogenital hiatus. The normal tone of these muscles keep the base of the “U” pressed against the back of the pubic bones, squeezing the urethra, vagina, and rectum closed. During labor, this sling pulls the presenting part anteriorly once it has passed the midplane to assist in rotation.
Blood Vessels of the Pelvic Wall
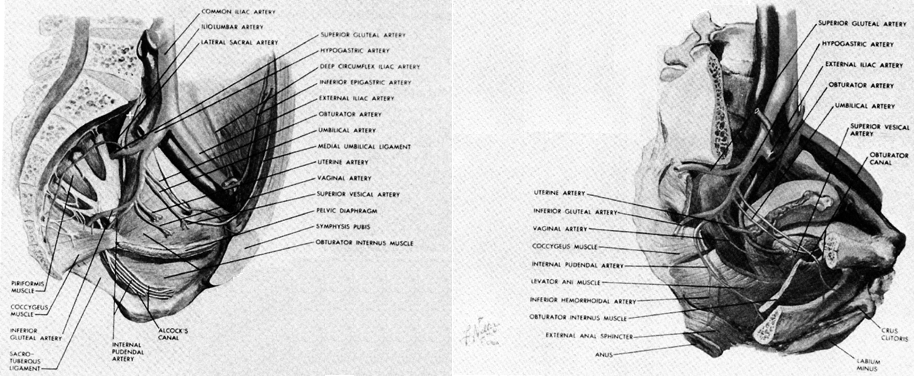
The aorta lies on the lumbar spine slightly to the left of the vena cava, which it overlies (Fig. 5). Both the aorta and vena cava are subject to compression by the uterus in this location when a patient is supine. At the level of the fourth lumbar vertebra (on a line between the tops of the iliac crests) the aorta bifurcates into the left and right common iliac arteries. After about 5 cm the common iliac arteries give off the internal lilac artery from their medial side and continue toward the inguinal ligament as the external iliac artery. This division occurs in the area of the sacroiliac joint.
The branching pattern of the internal iliac arteries and veins is extremely variable.3 A description of a common variant will be included here. The internal iliac artery supplies the viscera 9f the pelvis as well as many of the muscles of the pelvic wall and gluteal region. It usually divides into an anterior and posterior division 3 cm to 4 cm after leaving the common iliac artery. The vessels of the posterior division (the iliolumbar, lateral sacral, and superior gluteal) leave the internal iliac artery from its lateral surface to provide some of the blood supply to the pelvic wall and gluteal muscles. Trauma to these hidden vessels should be avoided during internal iliac artery ligation.
The anterior division has both parietal and visceral branches. The obturator, internal pudendal, and inferior gluteal vessels primarily supply muscles, while the uterine, superior vesical, vaginal (inferior vesical), and middle rectal vessels supply the pelvic organs. The internal iliac veins begin lateral and posterior to the arteries and have a much more plexiform arrangement.
Although the external iliac vein lies above the pelvic brim, the internal iliac vein crosses the linea terminalis. During obstructed labor, the internal iliac vein may be compressed for long periods of time, giving rise to the vulvar and pelvic soft tissue swelling seen in this situation. The legs, however, remain relatively uninvolved because of the external lilac vein's position above the true pelvis.
Ligation of the internal iliac artery has proven to be helpful in the management of postpartum hemorrhage. Burchell's arteriographic studies have demonstrated that physiologically active anastomoses were immediately patent following ligation of the internal iliac artery and connected the following arteries of the internal iliac system with systemic blood vessels4:
Internal iliac--Systemic
Iliolumbar  Lumbar
Lumbar
Lateral Sacral  Middle sacral
Middle sacral
Middle hemorrhoidal  Superior hemorrhoidal
Superior hemorrhoidal
(These were quite different than the anastomoses that had previously been hypothesized on purely anatomical grounds.) The lumbar arteries arise directly from the aorta; the middle sacral, again from the aorta; and the superior hemorrhoidal, from the inferior mesenteric.
Further detailed anatomy of the internal iliac artery and its surgical exposure are covered in the first volume of this series (see Volume 1, Chapter 73, Surgical Management of Intractable Pelvic Hemorrhage).
Nerves of the Pelvic Wall
There are instances in which paralysis and numbness of the lower extremity occur following difficult vaginal delivery, which in some cases may be explained by nerve compression in the pelvis.
As the lumbar and sacral nerves exit from the intervertebral foramina, they form the lumbar and sacral plexuses (see Fig. 5). The lumbar nerves and plexus lie deep within the psoas muscle on either side of the spine. The sacral plexus lies on the piriformis muscle, and its major branch, the sciatic nerve, leaves the pelvis through the lower part of the greater sciatic foramen. The sacral plexus supplies nerves to the muscles of the hip, pelvic diaphragm, and perineum, as well as to the lower leg (through the sciatic nerve). The femoral nerve from the lumbar plexus is primarily involved in supplying the muscles of the thigh.
In the interval between these two plexuses lies the lumbosacral trunk, which is composed of fibers from the fourth and fifth lumbar nerves. It curves in an exposed location over the brim of the pelvis at the sacroiliac articulation to join in the formation of the sacral plexus. In this position it lies directly on the bone of the pelvic inlet. Its fibers are destined to join in the formation of the sciatic nerve and eventually to form the common peroneal nerve and the tibial nerve.
After difficult labor and/or delivery, weakness and numbness in the distribution of the peroneal nerve develops in a manner suggesting compression of the lumbosacral trunk against the ala of the sacrum.5 In this way, dorsiflexion of the foot is impaired, along with sensation of the skin on the dorsum of the foot and lateral aspect of the lower leg. In some cases these signs may be secondary to herniation of an intervertebral disk.6
Posterior Abdominal Wall
After the viscera of the abdomen and pelvis have been removed from a cadaver the general shape and contour of the posterior abdominal wall may be appreciated. There is a prominent midline ridge formed by the lumbar spine. The psoas muscles produce ridges that extend downward from the spine on either side of the pelvic inlet.
The prominence of the lumbar spine forces the pregnant uterus to choose either the left or right gutter in which to lie. Because of the bulk of the sigmoid colon on the left side of the abdomen and pelvis, the uterus is found more frequently in the right gutter in a “dextrorotated” position.
The psoas ridges become important during external cephalic version. As the fetal head is moved downward toward the pelvis, some resistance is often encountered as attempts are made to push the head over the inlet and past the psoas ridge. Gentle upward pressure on the head through the flank can be used at this point to lift the head over this ridge and position it over the pelvic inlet.
PERINEUM
The perineum (Fig. 6) is the second of the two components of the pelvic floor (the other being the pelvic diaphragm). The structures of the perineum lie below the pelvic diaphragm and are most easily viewed from below. The more superficial features of this region will be described first.
External Genitalia
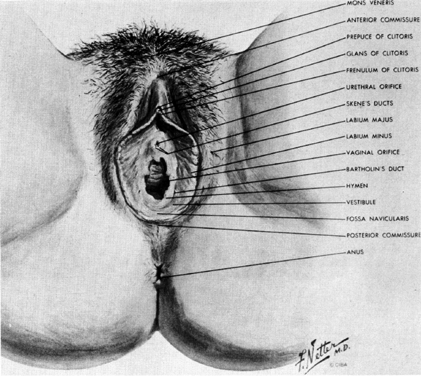
The external genitalia of the female consist of the mons, labia, clitoris, and vestibule (Fig. 7). Anteriorly, the mons overlies the pubic bones and is composed of hair-bearing skin over a cushion of adipose tissue. Extending posteriorly from the mons, the labia majora are composed of similar hair-bearing skin and adipose tissue, which contain the termination of the round ligaments of the uterus and the obliterated processus vaginalis.
Between the two labia majora the labia majora vestibule and glans clitoris can be seen. The labia minora are hairless skin folds. Anteriorly, each labium minus splits to run over, and under the glans of the clitoris. The more anterior folds unite to form the hood shaped prepuce of the clitoris, while the posterior folds insert into the under side of the glans as the frenulum.
The area between the labia minora and surrounding the vagina is called the vestibule. Its moist squamous epithelium overlies the vestibular bulbs. In the posterior lateral aspect of the vestibule, the duct of the major vestibular gland can be seen 3 mm to 4 mm outside the hymenal ring. The minor vestibular glands can be found in a line parallel and just exterior to the hymen. The urethra protrudes slightly through the vestibular skin anterior to the vagina and posterior to the clitoris.
The subcutaneous tissue underneath the skin, and superficial to the external genital muscles, consists of lobules of fat interlaced with connective tissue (see Fig. 6). The superficial layer of this tissue, where fat predominates, has been called Camper's fascia. In the deeper layers, there is less fat and the fibrous connective tissue strata between the lobules of fat are more evident. This fibrous layer is called Colle's fascia.7 Its fibrous strata are not actually a single layer as is often depicted, but they attach laterally to the ischiopubic rami and fuse posteriorly with the posterior edge of the urogenital diaphragm. These fibrous connections, which limit the spread of hematomas or infection in this deep compartment, gave rise to considering Colle's fascia as a separate entity from the superficial Camper's fascia, which lacks these connections. There is no attachment of Colle's fascia to the pubic bones anteriorly and fluid collections in this space may extend into the abdominal wall.
Erectile Bodies and Associated Structures
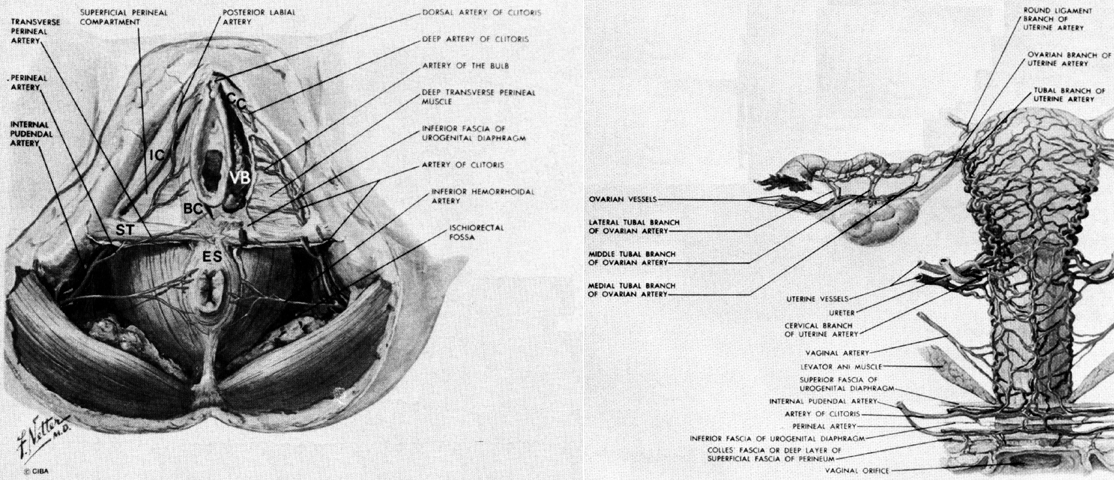
The erectile bodies and their associated muscles lie deep to the subcutaneous tissues of the vulva and are applied to the surface of the urogenital diaphragm (Fig. 6 and Fig. 8). The clitoris is composed of a midline shaft (body) capped with the glans. This shaft is suspended from the pubic bones by a subcutaneous suspensory ligament. The paired crura of the clitoris bend downward from the shaft and are firmly attached to the inferior aspects of the pubic rami. The ischiocavernosus muscles originate at the ischial tuberosities and the free surfaces of the crura, to insert on the upper crura and body of the clitoris. Contraction of these muscles pulls the shaft downward. There are a few muscle fibers that originate in common with the ischiocavernosus muscle and that run medially to the perineal body. These are called the superficial transverse perineus muscles.
The paired vestibular bulbs are composed of erectile tissue and are applied to the undersurface of the vestibular skin and vaginal wall. They are covered by the bulbocavernosus muscles that originate in the perineal body and lie over their lateral surfaces. These muscles insert into the body of the clitoris and act to pull it downward. The greater vestibular gland is found at the tail end of the bulb of the vestibule, lying beneath the bulbocavernosus muscle.
Urogenital Diaphragm
There is a triangular sheet of dense musculofascial tissue that spans the anterior half of the pelvic outlet. Rather than forming an uninterrupted sheet in the female, as it does in the male, the urogenital diaphragm attaches the vagina and perineal body to the anterior bony outlet (see Fig. 6). This layer arises from the inner aspect of the inferior ischiopubic rami above the ischiocavernosus muscles and the crura of the clitoris. The medial attachments of the urogenital diaphragm are to the walls of the vagina and to the perineal body. Within the substance of the urogenital diaphragm, there is skeletal muscle, called the deep transversus perineus. It is never as well developed as many illustrations would imply.8
The primary function of the urogenital diaphragm in relation to obstetrics has to do with its attachment to the vagina and perineal body. By attaching these structures to the pelvic bones, their descent is limited during the great downward pressure that is exerted on the vagina and perineum during the second stage of labor. If these attachments remain intact after parturition, they will contribute to the normal support of the vaginal outlet. If torn or detached from the vagina and perineal body, then the pelvic floor sags and the introitus gapes.
Ischiorectal Fossa
There is an ischiorectal fossa that lies on either side of the rectum and vagina (see Fig. 6 and Fig. 8). It is a wedge-shaped space whose base is formed by the perineal skin. The apex of the wedge is formed by the junction of the pelvic diaphragm and the obturator internus muscle. Anteriorly, there is a recess of the ischiorectal fossa above the urogenital diaphragm. Posteriorly, it extends as far as the fat that lies under the gluteus maximus muscle.
In the area above the urogenital diaphragm, the fat of the ischiorectal fossa is separated from the vaginal wall by the pelvic diaphragm. Therefore, when the fat of the anterior recess of the ischiorectal fossa is seen in a vaginal laceration or deep mediolateral episiotomy, the pelvic diaphragm has been transected and must be repaired. Because of the pelvic diaphragm's attachment to the obturator internus muscle at the apex of this space, abscesses here usually do not extend upward into the abdomen.
Pudendal Nerve and Vessels
The pudendal nerve is the sensory and motor nerve of the perineum (see Fig. 5 and Fig. 8). It arises from the anterior branches of the second, third, and fourth sacral nerves to innervate the skin, erectile tissues, and muscles of the perineum. (Its course and distribution in the perineum parallels the pudendal artery and may be followed in the illustrations of arterial anatomy.)
The pudendal nerve and vessels arise from the sacral plexus and the internal lilac artery, respectively. They leave the pelvis through the greater sciatic foramen by hooking around the ischial spine and sacrospinous ligament to enter the pudendal (Alcock's) canal through the lesser sciatic foramen.
As the nerve enters the lesser sciatic foramen, it does so through the triangle bounded by the sacrotuberous ligament, sacrospinous ligament, and the medial edge of the obturator internus muscle. Here it is in a relatively fixed position just medial and inferior to the junction of the sacrospinous ligament and the spine. The proper site for needle insertion during a pudendal nerve block, therefore, is just posterior and inferior to the junction of the spine and ligament. The nerve lies medial to the pudendal vessels at this point, so that if blood is aspirated during preparation for injection, the needle should be withdrawn and placed slightly medial to the previous injection site.
In passing around the sacrospinous ligament, the nerve and vessels enter the pudendal canal on the inner aspect of the obturator internus muscle in the ischiorectal fossa. As the vessels reach the posterior border of the urogenital diaphragm, they bend forward to supply the structures of the perineum.
Radiographic studies with radiopaque anesthetic agents show that the injected fluid extends along the sacral nerves toward the sacrum rather than into the pudendal canal.9 Infection from pudendal nerve block follows the tissue plane of the obturator internus muscle out of the pelvis, forming an abscess in the hip rather than in the pelvis or ischiorectal fossa.10
There are three branches of the pudendal nerve: the dorsal nerve of the clitoris, the perineal nerve, and the inferior hemorrhoidal nerve. The clitoral branch insinuates itself into the urogenital diaphragm along its path to innervate the clitoris. The perineal branch of the pudendal nerve (the largest of the three branches), enters the subcutaneous tissues of the vulva behind the urogenital diaphragm. Here it supplies the bulbocavernosus, ischiocavernosus, and transverse perineus muscles. It also supples the skin of the inner portions of the labia majora, the labia minora, and the vestibule.
The inferior hemorrhoidal nerve usually arises from the main trunk of the pudendal nerve, but it sometimes follows a separate parallel course to supply the external anal sphincter and skin around the anus.11 This aberrant path is responsible for the occasional lack of anesthesia in the anal sphincter sometimes seen despite an otherwise effective block.
Blockade of the pudendal nerve abolishes the sensation to pain over an area including the labia majora, labia minora, clitoris, and vestibule as far as the level of the hymenal ring. The cutaneous sensory innervation of the pudendal nerve extends as far posteriorly as a line that runs from the ischial tuberosity to the posterior aspect of the perianal skin.
Motor effects from this anesthetic produce relaxation of the bulbocavernosus and ischiocavernosus muscles as well as the muscle associated with the urogenital diaphragm. In addition, the pubococcygeus muscle will be affected, although other parts of the pelvic diaphragm will not.
Perineal Body
Within the area bounded anteriorly by the lower vagina, inferiorly by the perineal skin, and posteriorly by the anus is a mass of connective tissue called the perineal body (see Fig. 8). The term central tendon (or point) of the perineum has also been applied to the perineal body and is quite descriptive because it represents a central point into which a number of muscles insert.
The perineal body is attached to the inferior pubic rami and ischial tuberosities through the urogenital diaphragm and transverse perineus muscles. Anterolaterally, it receives the insertion of the bulbocavernosus muscles. On its lateral margins the upper portion of the perineal body are connected with some of the fibers of the pelvic diaphragm. Posteriorly the superficial portion of the external anal sphincter is connected to the coccyx and provides posterior traction on the perineal body. All of these connections anchor the perineal body and its surrounding structures to the bony pelvis and help to keep it in place.
The downward force on the perineal body during the second stage of labor occurs because the vaginal outlet is smaller than the presenting part. If this force is excessive, then the perineal body or its attachments will be torn, thereby weakening the support of the pelvic floor.
The anterior portion of the external anal sphincter lies within the perineal body. The deep external sphincter is circularly disposed and encompasses the anal canal, while the superficial portion of this muscle is fusiform and runs from the coccyx to the perineal body. The external anal sphincter is surrounded by a connective tissue capsule that aids in its reapproximation after it has been severed or torn.
The internal anal sphincter is a thickening in the circular muscle of the anal wall. It can be identified just beneath the anal submucosa in a fourth-degree laceration of the perineum and is usually reapproximated along with the wall of the bowel.
VAGINA
The vagina is a fibromuscular tube lined by a noncornified squamous epithelium (Fig. 6 and Fig. 9). Its lower third is vertical in the standing woman with the upper two thirds lying at a 120° angle from the lower portion.12 The cervix is situated in the anterior wall so that the distance from the introitus to the anterior fornix is shorter by the diameter of the cervix than the distance from the introitus to the posterior fornix.
Underneath the epithelium of the vagina is a dense layer of connective tissue that forms the submucosa. Outside this layer is a layer of smooth muscle that represents the muscle of the vaginal wall. This muscle does not have well-defined circular and longitudinal layers such as are found in the bowel wall but has a somewhat more complex spiral arrangement.13
The innervation of the vagina comes from the uterovaginal plexus (Frankenhauser's ganglion). This nerve supply modulates the tone of the smooth muscle of the vaginal wall and the vaginal vascular tone. There are only occasional free nerve endings in the vaginal wall.14 This, and a lack of specialized receptor units, explains the relative insensitivity of the vagina to touch, pressure, and pain.
The blood supply to the vagina comes from several different sources, with the largest branches lying on the lateral wall. A downward extension of the uterine artery, the vaginal branch of the internal iliac artery, and the pudendal artery all contribute.
The major branches of these vessels lie outside the muscular coat of the vagina within the loose adventitial layer that surrounds it. Superficial lacerations that extend only as far as the submucosa rarely cause significant hemorrhage, but lacerations that traverse the muscularis may injure some of these large vessels. When such deep lacerations occur and significant hemorrhage is encountered, surgical repair should be undertaken so as to include the deep vessels that may have retracted within the loose adventitial layer just outside the vaginal wall muscularis.
Lacerations that involve the vaginal wall above the outlet may occasionally involve deeper structures, and an appreciation of the adjacent anatomy will help suggest the nature of these lesions, thereby facilitating their recognition and repair (see Fig. 9). The anterior wall lies adjacent to the urethra, bladder, and ureters. The posterior wall is next to the perineal body, rectum, and peritoneal cavity (at the pouch of Douglas), while the two lateral walls lie against the pelvic diaphragm and major vaginal vessels.
Anatomical landmarks within the vagina can be used to locate the position of such structures as the ureter and urethra and warn of their possible involvement in a vaginal laceration. Anteriorly, a narrow ridge (the urethral carina) can be seen in the lower third of the vagina where the urethra bulges into the vaginal canal. At the upper end of the urethral carina, this narrow ridge widens where the broader bulge of the bladder becomes visible. The combination of these two anterior ridges is called the anterior column of the vagina. In the upper third of the vagina, the ureters lie between the vaginal wall and the bladder in the anterior and lateral fornices.15
In the middle of the posterior wall, the rectum causes a longitudinal midline bulge (posterior column of the vagina). This bulge is lost in the lower third of the vagina, where the rectum is separated from the vagina by the perineal body. In its upper third, the vagina is adjacent to the pouch of Douglas.
Awareness of these anatomical relationships will indicate possible occult damage to other visceral or muscular structures and avoid missing a chance to repair this damage primarily.
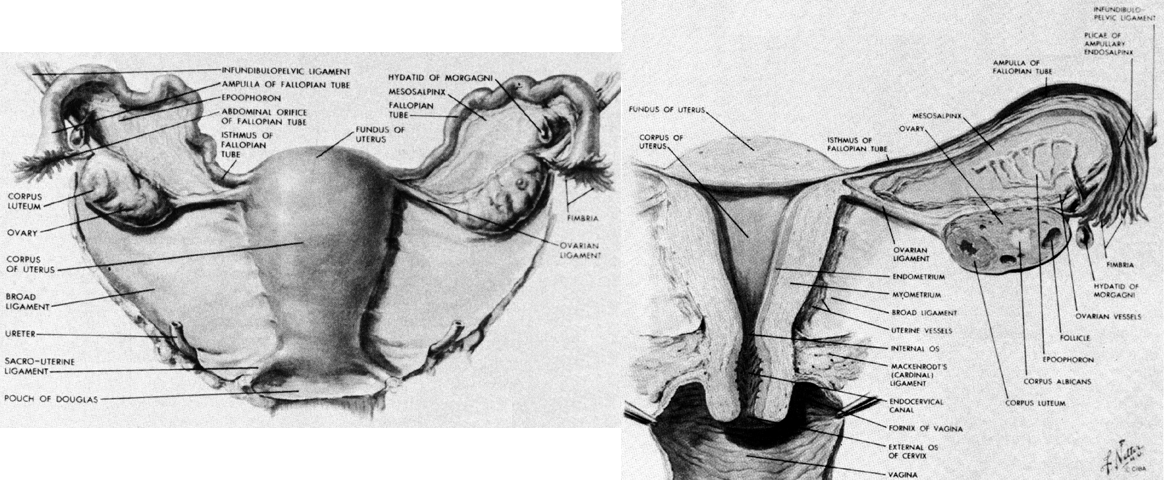
THE UTERUS AND ADNEXA
The uterus is a muscular organ whose endometrial lining provides the implantation site of the developing embryo (Fig. 10). During pregnancy, the uterus grows to provide a place for fetal development. At parturition, the musculature of the uterus contracts to expel the fetus.
The uterus is divided into two portions. The upper part is the uterine corpus and consists primarily of uterine smooth muscle. The lower part, the uterine cervix, is composed largely of fibrous tissue.
Uterine Corpus
Within the corpus there is a small, triangularly shaped endometrial cavity surrounded by a thick muscular wall. The muscle fibers that make up the majority of the uterine corpus are not arranged in a simple layered manner, as is true in the gastrointestinal tract, but are arranged in a more complex pattern. 16
The clinical aspects of uterine fiber direction can be summarized as follows. On the anterior uterine wall, the fibers from each side crisscross diagonally with those of the opposite side but run in a predominantly transverse direction. This can be appreciated from the gaping that occurs in a classic uterine incision as well as the predilection of a uterus which contains a scar from a previous classical cesarean section to rupture during and before labor. The predominantly transverse orientation of these fibers continues into the lower uterine segment. Blunt separation of fibers during a low segment cesarean section results in a transverse laceration. Inspection of the lateral edges of this wound reveal an overlapping of fibers in this area that belies the fact that they are not completely parallel. Most obstetricians have also noted that there is a grossly recognizable band of muscle fibers that runs in an anterior and posterior direction over the fundus of the uterus. Its significance is not entirely clear.
Uterine Cervix
The cervix is divided into two portions: the portio vaginalis, which is that part that protrudes into the vagina, and the portio supravaginalis, which lies above the vagina and below the corpus (see Fig. 10).
The portio vaginalis is covered by nonkeratinizing squamous epithelium. Its canal is lined by a columnar mucus-secreting epithelium which is thrown into a series of folds, the palmate folds or plicae palmatae, which form crypts that are often called the cervical glands. The upper border of the cervical canal is marked by the internal os where the narrow cervical canal widens out into the endometrial cavity. Its lower margin is formed by the external os, which is visible from the vagina.
The substance of the cervical wall is made up of dense fibrous connective tissue with only a small (approximately 10%)17 amount of smooth muscle. What smooth muscle there is lies on the periphery of the cervix, connecting the myometrium with the muscle of the vaginal wall.18
The fibrous tissue of the cervix is composed predominantly of collagen, which makes up more than three fourths of the cervical wall.19 In the nonpregnant state, the collagen fibers are dense and highly cross-linked, making them difficult to dilate. Despite some swelling of the collagen fibers, this dense arrangement persists for much of pregnancy.
Near term the cervix becomes softer and thinner and begins to dilate in a process known as ripening. These changes are necessary for the cervix to open during labor and are associated with a “loosening” of the collagen network seen microscopically as a finer looser fibrous mesh. This is associated with a decline in the collagen cross-linking, making it more loosely dispersed and, therefore, less able to resist stretching.20 This process also involves a decrease in the amount of collagen present and an increase in quantity and change in composition in the ground substance.21
Growth of the Uterus
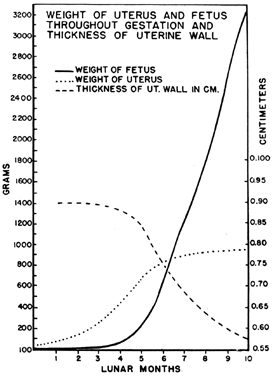
It is obvious that the uterus must undergo significant enlargement to accommodate the growing fetus (Fig. 11). Its weight increases from approximately 60 g to 1 kg. The volume of its cavity grows from 10 ml to 6 to 10 liters, and its external dimensions from 8 × 2.5 × 4 cm to 32 × 24 × 22 cm at term.22
|
The way in which these changes occur, however, is not readily apparent. The growth that occurs in the substance of the uterus itself occurs during the first half of gestation. During the first 5 months of pregnancy the uterus grows faster than the conceptus so that it is only during the middle of pregnancy that the conceptus actually catches up with the growth of the uterus to fill the uterine cavity, as will be seen when the development of the isthmus of the cervix is considered.
There are three phases of uterine growth23: hyperplasia, which occurs before nidation; hypertrophy, which happens during the first half of pregnancy; and dilation, which occurs during the second half of pregnancy.
During the first and early second trimesters of pregnancy, the mass of the uterus increases in a fairly linear fashion to the full weight that it will be at term. This means that although the external dimensions of the uterus will continue to enlarge during the second half of pregnancy, the uterus will not gain additional tissue. The wall of the uterus, therefore, thickens or remains a constant thickness in the first half of pregnancy but becomes thinner as it must stretch to surround a growing fetus later on. In contrast to the uterus, which has achieved its full weight by the middle of the second trimester, at this same time the fetus has only undergone one sixth of the total growth that it will achieve by term.
Lower Uterine Segment
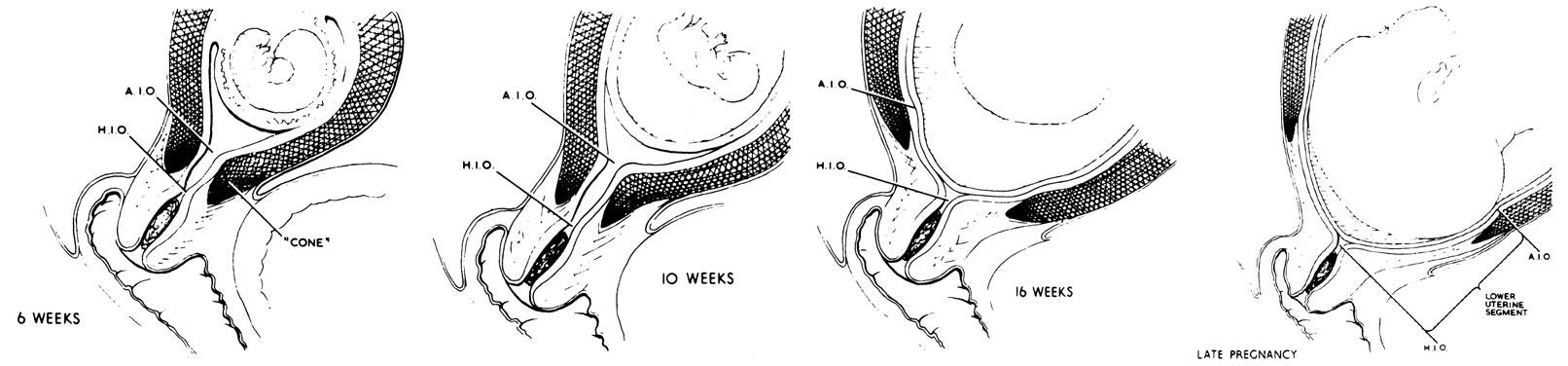
The lower uterine segment is that portion of the myometrium that must dilate during the process of delivery in order to allow the presenting part to deliver (Fig. 12). The tissue that will make up the lower uterine segment begins as a part of the cervix, and as pregnancy progresses, it comes to lie in the lower portion of the corpus. It goes through several stages of development.
The division between the muscular uterine wall and the fibrous cervix is not always at the internal os of the cervix. In the nonpregnant and early pregnant uterus the line of demarcation between the fibrous and muscular parts of the uterus actually occurs below the anatomical internal os of the uterus (see Fig. 12, “6 weeks”). Early in pregnancy the relatively small conceptus occupies a portion of a large uterus. At about the 16th week, fetal growth catches up with uterine growth so that the products of conception fill the entire uterine cavity. The continued fetal growth past the time when uterine hypertrophy has ceased stretches the uterine wall,24 as evidenced by the thinning of the muscular wall of the corpus (see Fig. 11). As this stretching increases, the muscular portion of the cervix is placed under tension and, having little collagenous tissue to resist this force, opens as far as the musculofibrous junction.
As pregnancy progresses the lower uterine segment begins to develop as a clinically distinctive entity at about 34 weeks' gestation, roughly the same time that Braxton-Hicks contractions become clinically evident.25 At this time the increasing tension of this tissue causes it to widen and therefore becomes proportionately thinner.
This widening of the lower uterine segment is responsible for two clinical phenomena. First, it explains the apparent upward migration of a low lying placenta during the latter phases of pregnancy as the lower uterine segment between the placenta and cervix widens. Second, with a placenta that is implanted in the lower uterine segment, stretching of this area may cause shearing between the unyielding placenta and the placental bed, which changes as the lower uterine segment develops. This phenomenon explains the fact that patients with placenta previa begin to bleed at about 34 weeks' gestation when the lower uterine segment begins to develop.
The lower uterine segment is also that portion of the corpus that must dilate during parturition, thinning as the muscle of the corpus shortens and thickens.26 This implies that the muscle in this area is less able to contract than the muscle of the upper part of the uterus. Because it is thin and avascular, this part of the uterus makes it a good location for cesarean section incisions. The dilation of the cervix enhances the primarily transverse orientation of the fibers in this area, thereby creating little tension on the closure line.
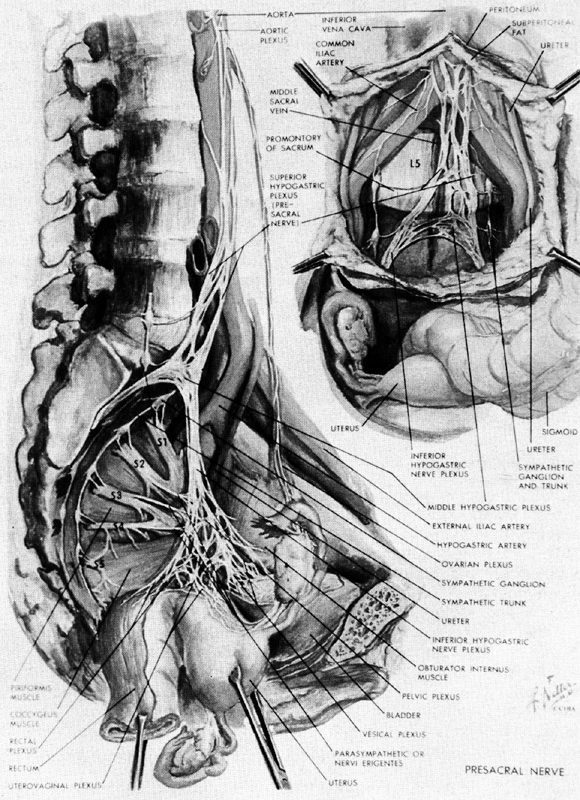
Innervation of the Uterus and Adnexa
The uterus receives its nerve supply from the uterovaginal plexus (Frankenhauser's ganglion), which lies in the connective tissue of the cardinal ligament (Fig. 13).27 This local condensation of nerve fibers is a part of a larger plexus of nerves--the pelvic (inferior hypogastric) plexus, which also supplies the bladder and rectum. The adnexal structures receive their innervation from nerve fibers coursing along the ovarian blood vessels. These latter fibers are derived primarily from the tenth thoracic segment.
The uterovaginal plexus contains fibers that are derived from two sources. It receives sympathetic and sensory fibers from the tenth thoracic through the first lumbar spinal cord segments. These nerves travel through the superior hypogastric plexus along the inferior hypogastric nerve to reach the pelvic (inferior hypogastric) plexus. The second input comes from the second, third, and fourth sacral segments and consists primarily of parasympathetic nerves, which reach the pelvic plexus through the nervi erigentes. Clinically there appear to be no significant afferent fibers from the uterus and cervix in these sacral nerves.28
These anatomical facts explain several clinical observations. Injection of anesthetic agents into the paracervical tissues, transection of the superior hypogastric plexus (presacral neurectomy), and segmental blockade of the tenth thoracic through first lumbar spinal nerves all are effective in alleviating the pain of uterine contraction and cervical dilation, while low caudal or saddle anesthesia that blocks the sacral segments is not.
The autonomic nervous system modulates the smooth muscle contractions of most viscera, and its action on the uterus has been clinically useful in the inhibition of uterine activity. There are unmyelinated nerve fibers visible within the wall of the uterus, and although most end in the smooth muscle of the uterine blood vessels, some seem to terminate on smooth muscle cells of the myometrium.27 Because there is no clearly established pacemaker or conduction system as there is in the heart, the influence of the autonomic nervous system is probably exerted directly on the muscle cells themselves. There has been one report of a band of specialized muscle cells that run from the cervix to the cornu of the uterus, which, on morphologic grounds, was thought to be a conduction system, but the function of the tissue has not been studied.29 Conduction of electrical impulses in the myometrium seems to occur through direct cell-to-cell contact through gap junctions and to proceed without any anatomical conduction system.30
The sympathetic nervous system is of some importance to the clinician in that β-adrenergic medications are helpful in inhibiting uterine contractions in preterm labor. Histochemical techniques show presumptive adrenergic nerves within the myometrium, separate from the blood vessels of the uterine wall, which are numerous near the cervix and sparse in the corpus? There is general agreement that the parasympathetic nervous system has little effect on the activity of the myometrium. The parasympathetic fibers that do go to the uterus primarily supply the smooth muscle of vascular walls.32
Blood Vessels of the Uterus and Adnexa
The uterus, tubes, and vagina develop from paired müllerian ducts. Each of these tubes is connected to the lateral pelvic wall by a mesentery destined to become the broad and cardinal ligaments. Vessels that run within this mesentery become the ovarian, uterine, and vaginal vessels and are interconnected by an anastomotic arcade that runs through the adnexus and along the lateral margin of the uterus and vagina (see Fig. 8).
THE UTERINE VESSELS.
The uterine artery originates from the internal iliac artery. It usually arises independently from this source but may have a common origin with either the internal pudendal or vaginal artery.33 It joins the uterus at approximately the junction of the corpus and cervix, but this varies considerably both with the individual and also with the amount of upward or downward traction placed on the uterus. The diameter of the uterine arteries increases from an average of about 2 mm at the beginning of pregnancy to between 3 mm and 4 mm at term.33 Accompanying each uterine artery are several large uterine veins that drain the corpus and cervix.
On arriving at the lateral border of the uterus (after passing over the ureter and giving off a small branch to this structure), the uterine artery flows into the side of the marginal artery, which runs along the side of the uterus. Through this connection it sends blood both upward toward the corpus and downward to the cervix. As the marginal artery continues along the lateral aspect of the cervix it eventually crosses over the cervicovaginal junction and lies on the side of the vagina. It receives the vaginal arterial branch from the internal iliac artery and also, sometimes, a separate cervical branch. The further details of the vagina's blood supply will be described under that heading.
A number of short arteries run from the marginal arteries perpendicularly into the wall of the uterus. They split into anterior and posterior arcuate arteries that continue around the circumference of the uterine wall. These vessels form anastomotic connections freely across the midline.34,35 The arcuate arteries, in turn, send off radial arteries inward toward the endometrial cavity. The course of these vessels through the myometrium places them in positions where contraction of the muscle may impede blood flow during contraction of the uterine musculature. The venous pattern parallels the flow of the arterial system but has a greater degree of intercommunication among venous channels.
THE OVARIAN VESSELS.
The blood supply of the upper genital tract comes from the ovarian arteries that drain into the anterior surface of the aorta just below the level of the renal arteries. The accompanying plexus of veins arise from the vena cava on the right and the renal vein on the left. The arteries and veins follow a long retroperitoneal course before reaching the cephalic end of the ovary. They pass along the mesenteric surface of the ovary to connect with the upper end of the marginal artery of the uterus. As the ovarian artery runs along the hilum of the ovary, in addition to supplying the gonad, it sends a number of small vessels through the mesosalpinx to supply the fallopian tube.
Adnexal Structures
Although the fallopian tube and ovary play an extremely important role in conception and the establishment of pregnancy, their role during the rest of gestation is somewhat secondary. Their anatomy has been covered in the first volume of this series. (See Volume 1, Chapter 2,) Clinical Anatomy of the Uterus, Fallopian Tubes, and Ovaries.)
LIGAMENTS OF THE GENITAL TRACT
The term ligament is most familiar when it describes a dense connective tissue band that links two bones, but it also describes structures that support various viscera. There is great diversity among the ligaments of the uterus and its adnexal structures (see Fig. 10). Although they share a common designation, they are composed of many types of tissue and have different functions.
The broad ligaments are primarily peritoneal folds that extend laterally from the uterus to end on the pelvic wall. They have several specialized areas. The main sheet of tissue extending on either side of the uterus where there is anterior peritoneum on posterior peritoneum is called the mesometrium. Below it are the cardinal ligaments and at its upper border are the mesovarium and mesosalpinx.
At the lateral end of the ovary and extending upward the ovarian vessels raise a ridge of peritoneum from the lateral pelvic wall. This ridge and its contained vessels are called the suspensory ligament of the ovary or the infundibulopelvic ligament. At the other end of the ovary, connecting it to the uterus is the ovarian ligament, which is a fibromuscular structure separate from the vascular pedicle.
At the lower end of the uterus, somewhat above the external os, two fibromuscular bands called the uterosacral ligaments run from the posterolateral aspects of the cervix to the presacral connective tissue over the second, third, and fourth sacral vertebrae. They lie on either side of the pouch of Douglas and are composed of smooth muscle, nerves, and connective tissue.36 These ligaments hold the cervix posteriorly in the pelvis over the pelvic diaphragm. They do not undergo as much hypertrophy in pregnancy as the round ligaments do and probably have no significant role in labor.
The round ligaments are extensions of the uterine musculature. They begin as broad bands that arise on the lateral aspect of the anterior corpus. They assume a more rounded shape before they enter the retroperitoneal tissue where they pass lateral to the deep inferior epigastric vessels and enter the internal inguinal ring. After traversing the inguinal canal, they exit the external ring and distribute to the subcutaneous tissue of the labia majora. These ligaments undergo significant hypertrophy during pregnancy and have sufficient bulk to make the contention that they help pull the uterus forward during contractions plausible.
The cardinal ligaments lie at the lower edge of the broad ligaments, between their peritoneal leaves. They run from the lateral pelvic walls to the lateral edges of the cervix and the upper third of the vagina. Although when placed under tension they feel like ligamentous bands, they are composed simply of the vascular and neural elements that supply the uterus and vagina.37 These structures have considerable strength, and the lack of a separate “ligamentous band” in this area does not detract from their supportive role. They not only provide support to the cervix and uterus but also support the upper portion of the vagina to keep these structures positioned over the pelvic diaphragm away from the urogenital hiatus.
When a parturient pushes before the cervix is completely dilated, the descent of the uterus causes the blood vessels, nerves, and connective tissue of the cardinal ligament as well as the fibromuscular tissue of the uterosacral ligament to become taut so that they retard the downward movement of the cervix. Some damage to these structures may occur as a result of this set of circumstances, and if the pelvic floor is also damaged, there appears to be an increased chance in later life that genital prolapse will develop.
THE LOWER URINARY TRACT
The bladder and urethra are intimately connected with the female genital tract, and they undergo significant changes in their positions during labor. The ureters undergo some dilation due to the hormonal changes of pregnancy, but they are not specifically altered in their position during gestation.
The changes that occur in the positions of the bladder and urethra were defined by Malpas and co-workers.38 At the beginning of labor, the bladder base and urethra lie in their normal positions with the bladder base and urethrovesical junctions on the line from the inferior border of the pubic symphysis to the sacrococcygeal joint. Little change occurs due to cervical dilation, but as the presenting part descends into the pelvis, the urethra and vesical neck are pushed anteriorly toward the pubic bone. The extent to which this occurs depends on the relative sizes of the fetal head and pelvic cavity. When there is ample room in the pelvis for the head to pass there is little displacement of these structures. When there is relative disproportion the bladder becomes closely applied to the symphysis and is also pulled upward to the level of the top of the pubic bones. Because these displacements move the vesical neck upward in most cases, they would not put the supportive tissues that attach the vesical neck to the pelvic wall on stretch. All obstetricians, however, remember cases in which there is great descent of the urethra in front of the presenting part. In these cases there is likely to be considerable stretch in the supportive tissues of the bladder base and vesical neck, which may become manifest later in life as the tissues of the pelvis undergo the atrophy that accompanies advancing age and the menopause.
The course of the ureter is unchanged during pregnancy. Ureters do, however, undergo significant dilation above the pelvic brim beginning at about 20 weeks' gestation. This is much more frequent on the right side than the left and occurs to a greater extent there,39 and it resolves rapidly post partum.
REFERENCES
Borell U, Fernstrom I: Movements at the sacroiliac joints and their importance to changes in pelvic dimensions. Acta Obstet Gynecol Scand 36: 42, 1957 |
|
Abramson D, Roberts SM, Wilson PD: Relaxation of the pelvic joints in pregnancy. Surg Gynecol Obstet 58: 595, 1934 |
|
Roberts WH, Krishingner GL: Comparative study of human internal iliac artery based on Adachi classification. Anat Rec 158: 191, 1967 |
|
Burchell RC: Arterial physiology of the human female pelvis. Obstet Gynecol 31: 855, 1968 |
|
Cole JT: Maternal obstetric paralysis. Am J Obstet Gynecol 40: 372, 1946 |
|
O'Connell JEA: Maternal obstetrical paralysis. Surg Gynecol Obstet 79: 374, 1944 |
|
Tobin CE, Benjamin JA: Anatomic and clinical re-evaluation of Camper's, Scarpa's, and Colle's fasciae. Surg Gynecol Obstet 88: 545, 1949 |
|
Oelrich TM: The striated urogenital sphincter muscle in the female. Anat Rec 205: 223, 1983 |
|
Kobak AJ, Sadove MS, Mazeros WT: Anatomic studies of transvaginal regional anesthesia: Roentgenographic visualization of neural pathways. Obstet Gynecol 19: 302, 1962 |
|
Wenger DR, Gitchell RG: Severe infections following pudendal block anesthesia: Need for orthopaedic awareness. Am J Bone Joint Surg 55: 202, 1973 |
|
Klink RE: Perineal nerve block: An anatomic and clinical study in the female. Obstet Gynecol 1: 137, 1953 |
|
Funt MI, Thompson JD, Birch H: Normal vaginal axis. South Med J 71: 1534, 1978 |
|
Schreiber H: Konstruktionsmorphologische Betrachtungen uber den Wandungsbau der menschlichen Vagina. Arch Gynaek 174: 222, 1942– 1943 |
|
Krantz KE: Innervation of the human vulva and vagina: A microscopic study. Obstet Gynecol 12: 382, 1958 |
|
Brash JC: The relation of the ureters to the vagina: With a note on the asymmetrical position of the uterus. Br Med J 2: 790, 1922 |
|
Goerttler K: Die Architektur der Muskelwand des menschlichen Uterus und ihre funktionelle Bedeutung. Morph Jarb 65: 45, 1930 |
|
Schwalm H, Dubrausky V: The structure of the musculature of the human uterus. Am J Obstet Gynecol 94: 392, 1966 |
|
Hughesdon PE: The fibromuscular structure of the cervix and its changes during pregnancy and labour. J Obstet Gynaecol Br Commonw 59: 763, 1952 |
|
Buckingham JC, Buethe RA, Danforth DN: Collagen-muscle ratio in clinically normal and clinically incompetent cervices. Am J Obstet Gynecol 91: 232, 1965 |
|
Danforth DN, Buckingham JC, Roddick JW: Connective tissue changes incident to cervical effacement. Am J Obstet Gynecol 80: 939, 1960 |
|
Danforth DN, Veis A, Breen M, et al: The effect of pregnancy and labor on the human cervix: Changes in collagen, glycoproteins, and glycosaminoglycans. Am J Obstet Gynecol 120: 641, 1974 |
|
Miller NF, Evans TN, Haas RL: Human Parturition: Normal and Abnormal Labor, p 25. Baltimore, Williams & Wilkins, 1958 |
|
Gillespie EC: Principles of uterine growth in pregnancy. Am J Obstet Gynecol 59: 949, 1950 |
|
Danforth DN: The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and non-gravid uteri. Am J Obstet Gynecol 53: 541, 1947 |
|
Huszar G, Naftolin F: The myometrium and uterine cervix in normal and preterm labor. N Engl J Med 311: 571, 1984 |
|
Danforth DN, Ivy AC: The lower uterine segment: Its derivation and physiologic behavior. Am J Obstet Gynecol 57: 831, 1949 |
|
Krantz KE: lnnervation of the human uterus. Ann NY Acad Sci 75: 770, 1959 |
|
Bonica JJ: Obstetric Analgesia and Anesthesia, 2nd ed, p 45. Amsterdam, World Federation of Societies of Anaesthesiologists, 1980 |
|
Toth S, Toth A: Undescribed muscle bundle of the human uterus: Fasciculus cervicoangularis. Am J Obstet Gynecol 118: 979, 1974 |
|
Garfield RE: Control of myometrial function in preterm versus term labor. Clin Obstet Gynecol 27: 572, 1984 |
|
Nakanishi H, McLean J, Wood C, Burnstock G: The role of sympathetic nerves in control of the nonpregnant and pregnant human uterus. J Reprod Med 11: 20, 1969 |
|
Bell C: Autonomic nervous control of reproduction: Circulatory and other factors. Pharmacol Rev. 24: 657, 1972 |
|
Fernstrom I: Arteriography of the uterine artery. Acta Radiol [Suppl] 122: 21, 1955 |
|
Farrer-Brown G, Beilby JOH, Tarbit MH: The blood supply of the uterus: I. Arterial vasculature. J Obstet Gynaecol Br Commonw 77: 673, 1970 |
|
Itskovitz J, Lindenbaum ES, Brandes JM: Arterial anastomosis in the pregnant human uterus. Obstet Gynecol 55: 67, 1980 |
|
Campbell RM: The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol 59: 1, 1950 |
|
Range RL, Woodburne RT: The gross and microscopic anatomy of the transverse cervical ligaments. Am J Obstet Gynecol 90: 460, 1964 |
|
Malpas P, Jeffcoate TNA, Lister UM: The displacement of the bladder and urethra during labour. J Obstet Gynaecol Br Emp 56: 949, 1949 |
|
Shulman A, Herlinger H: Urinary tract dilatation in pregnancy. Br J Radiol 48: 638, 1975 |