Management of Premature Rupture of the Membranes in Term Patients
Authors
INTRODUCTION
Premature rupture of the fetal membranes (PROM) is defined as rupture prior to the onset of labor. This condition occurs in 5–10% of all pregnancies.1 Preterm PROM has received considerable attention in the recent obstetric literature, and deservedly so, for it is directly responsible for approximately one-third of all preterm deliveries. Interestingly, however, at least 60% of cases of PROM occur in term patients, and even at this gestational age, clinical management can be surprisingly complicated.1
The purpose of this chapter is to provide a concise review of the treatment of term patients with PROM. The first two sections of the chapter discuss the etiology and diagnosis of PROM. The final section outlines a management algorithm for this complication, focusing in particular on the alternate approaches to patients who have a cervix that is unfavorable for induction of labor.
ETIOLOGY
The etiology of PROM is multifactorial. Conditions that overdistend the uterus, such as multiple gestation and polyhydramnios, may predispose to PROM. In a survey of data from the Collaborative Perinatal Project, Naeye2 demonstrated an association between cigarette smoking and PROM in term patients. Membranes that rupture prematurely may have different mechanical properties to those that do not rupture prematurely. For example, Artal and colleagues3 showed that prematurely ruptured membranes had definite decreases in thickness and elasticity at the site of rupture. Similarly, Vadillo-Ortega and co-workers4 measured collagen content, acid-soluble collagen, collagen degradation activity, and collagen biosynthesis in 22 normal and 20 prematurely ruptured membranes from patients at 37 weeks’ gestation or later. Collagenolytic activity and collagen solubility were higher in membranes that ruptured prematurely. Collagen synthesis was also lower in these membranes.
The role of infection in the etiology of PROM is clearly of great importance. Several lines of evidence suggest that bacterial colonization can reduce the tensile strength of membranes and, thereby, predispose to rupture. McGregor and associates5 demonstrated that, when fetal membranes were exposed to bacteria or to bacterial collagenases, the bursting load, elasticity, and work to rupture were significantly reduced. Sbarra and colleagues6 observed that growth of Escherichia coli and group B streptococci on the decidual surface of fetal membranes significantly weakened the tensile strength of the membranes compared with uninfected control membranes. In a similar model, Schoonmaker and coworkers7 demonstrated that exposure of fetal membranes to group B streptococci, Staphylococcus aureus, or activated neutrophils and neutrophil elastase resulted in significant decreases in membrane strength, elasticity, and work to rupture.
The clinical implication of these observations is that certain interventions, such as digital examination of the cervix, may increase the risk of bacterial contamination of the membranes and, simultaneously, increase the risk of PROM. In support of this hypothesis, Lenihan8 confirmed that antenatal cervical examinations were associated with an increased frequency of PROM. He conducted a prospective investigation of 349 uncomplicated term patients, 174 of whom were randomly assigned to receive weekly cervical examinations from 37 weeks to delivery. One hundred and seventy-five women were assigned to the “no examination” group. Eighteen per cent of women in the group being examined experienced PROM compared with 6% in the latter group (p = 0.001). The cesarean delivery rate was similar in the two groups.
In a follow-up to Lenihan’s investigation, McDuffie and associates9 randomly assigned 604 term patients to two groups. Half the patients received weekly cervical examinations from 37 weeks to delivery, and half had no examinations until onset of labor. These authors observed no significant differences in the frequency of PROM or prolonged rupture of the membranes (ROM). Moreover, they noted no significant differences in the frequency of cesarean delivery, oxytocin administration for induction or augmentation of labor, incidence of chorioamnionitis, or neonatal sepsis.
McDuffie and associates9 were unable to explain precisely why their results differed so markedly from those of Lenihan.8 However, in view of the multiple experiments cited previously that confirmed the effect of bacteria and bacterial proteases on membrane integrity, antenatal cervical examinations should not be performed as a matter of routine from 37 weeks until onset of labor. Rather, their use should be restricted to situations in which the results of the examination clearly will influence clinical management.
DIAGNOSIS
Patients with ruptured membranes usually note a large “gush of fluid” that wets their undergarments or bed sheets. Alternatively, they may experience a steady trickle of small amounts of clear fluid from the vagina, resulting in a constant sense of “wetness” on the perineum.
On physical examination, the most reliable method for confirmation of ruptured membranes is direct observation of amniotic fluid in the posterior vaginal vault (pooling). In some instances, gross pooling may not be evident, but amniotic fluid can be seen to leak from the cervix when the patient pushes on her fundus, coughs, or strains. Documentation of decreased or absent amniotic fluid by ultrasound is an important complement to physical examination in confirming the diagnosis of PROM.
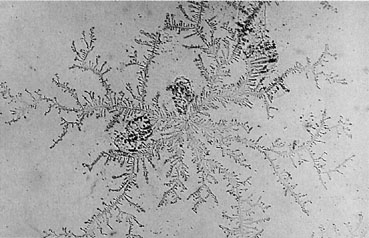
When physical examination is problematic, two simple laboratory tests may be of value. The normal pH of amniotic fluid is 7.1–7.3, whereas the pH of sterile urine and vaginal secretions is 5–6 and less than 4.5, respectively. Accordingly, if the suspect fluid has a pH of 7 or greater (nitrazine test, Fig. 1), it is most likely amniotic fluid. In addition, when amniotic fluid is allowed to dry on a glass slide, it produces a characteristic pattern of arborization, termed ferning (Fig. 2). If both the fern test and the nitrazine test are positive, the diagnosis of ruptured membranes is highly probable. However, contaminating substances in the fluid may affect the reliability of these tests. For example, blood and seminal fluid may produce a false-positive nitrazine test. Sodium chloride in the perspiration from a fingerprint or in some antiseptic solutions may produce a false-positive fern test.10, 11 In addition, if amniotic fluid is frankly bloody, the characteristic ferning pattern may be obscured. Interestingly, the presence of dilute concentrations of blood and meconium does not affect the interpretation of the fern test.12
Other laboratory tests have been proposed for confirmation of the diagnosis of ROM, such as intra-amniotic injection of indigo carmine, measurement of amniotic fluid glucose and fructose concentrations, and identification of diamine oxidase in amniotic fluid.11, 13 As a general rule, these tests are too invasive, too cumbersome, or too expensive to have routine application in clinical practice. However, an immunoassay for placental alpha microglobulin can be of great value in confirming the diagnosis in problematic cases. Cousins et al. demonstrated that the test had a sensitivity of 98.9%, specificity of 100%, positive predictive value of 100%, and a negative predictive value of 99.1%.14 In addition, detection of insulin-like growth factor binding protein 1 alone, or in combination with alphafetoprotein, appears to be of comparable value in confirming the diagnosis.15
CLINICAL MANAGEMENT
Once the diagnosis of PROM is confirmed, the next step in clinical management is to determine whether the cervix is favorable for induction of labor. In most instances, this assessment can be made by visual inspection of the cervix during a sterile speculum examination. The rationale for this recommendation is based on the twin issues of reliability and safety. With respect to reliability, Munson and colleagues16 prospectively compared the results of a digital and visual cervical examination in 133 women with spontaneous ROM. The coefficient of correlation for the two examinations was 0.74 with respect to assessment of cervical effacement and dilatation. The degree of correlation did not differ significantly depending on the level of training of the examiner.
From the perspective of safety, several authors have demonstrated an association between even a single digital examination and increased risk of neonatal infection. In an earlier investigation, Schutte and coworkers17 evaluated 189 term patients who experienced spontaneous ROM at least 24 hours before delivery. When the interval between the first vaginal examination and delivery also exceeded 24 hours, four of 17 (24%) infants developed infection. When the interval between the first examination and delivery was less than 24 hours, only one of 172 (0.6%) neonates became infected (p < 0.0001). Similarly, Wagner and associates18 also observed an increased risk of neonatal infection in women who had a single digital examination at admission and then underwent delayed induction of labor.
On visual examination, if the cervix is posterior in the vagina, thick, and closed, it should be considered “unfavorable.” If it is midposition to anterior within the vagina, moderately effaced, and approximately 2 cm dilated, it should be considered “favorable.” If the visual examination is problematic, a sterile digital examination should be performed and a Bishop score assigned. A Bishop score of 6 or more should be considered favorable for induction.
Favorable cervix
If the patient’s cervix is favorable, little is to be gained by delay. Accordingly, labor should be induced as soon as possible by administration of intravenous oxytocin. Vaginal examinations should be minimized, especially during the latent phase of labor. If the total length of ROM is expected to exceed 18 hours, or any other risk factor for group B streptococcal infection is present, prophylactic antibiotics should be administered intrapartum unless the patient has recently been tested and is known to have a negative culture for this organism. Patients who are known to have a positive genital tract culture for group B streptococci should receive intrapartum antibiotic prophylaxis. Penicillin (2.5 or 3.0 million units IV every 4 hours until delivery) or ampicillin (2 g initially, then 1 g IV every 4 hours) is the preferred agent for prophylaxis because essentially 100% of strains of group B streptococci are sensitive to these drugs. If the patient has a mild allergy to penicillin, the drug of choice is cefazolin (2 g IV every 8 h). In the event of a life-threatening allergy to penicillin, the preferred agent is vancomycin (1 g IV every 12 h) unless the organism has been confirmed to be sensitive to clindamycin (900 mg IV every 8 h).19
Unfavorable cervix
The standard textbook guidelines for management of PROM in term patients originally were based on a series of alarming reports published in the period 1950–1980. In 1952, Calkins20 demonstrated that perinatal mortality increased threefold when the fetal membranes had been ruptured for longer than 24 hours. In 1962, Russell and Anderson21 argued convincingly that this mortality could be reduced if patients with PROM were managed by prompt induction of labor. These authors observed 131 cases of intrapartum infection in 2644 women (5%) of varying gestational ages with PROM. Sixty-three per cent of infections were in term patients, and five (3.8%) of these infections resulted in maternal deaths. Four of the deaths occurred in the first phase of the study when patients were not routinely managed by prompt induction of labor. In the same period, 10 of 52 infants (19%) born to mothers with intrapartum infection died.
In 1965, Lanier and coworkers22 showed that perinatal mortality doubled in patients with prolonged ROM. Twenty-eight per cent of these women developed intrapartum or postpartum infection. In patients with intrapartum infection, the perinatal mortality was 50%. Bryans23 noted equally ominous results in a series of 192 patients with PROM. Thirty-one per cent of women with prolonged ROM developed intrapartum infection; 47% of their babies died as a result of complications of infection.
Shubeck and colleagues24 reviewed data from the Collaborative Perinatal Project and analyzed the outcome of 4868 patients with spontaneous ROM. Their investigation included patients who experienced spontaneous ROM while in labor and women with PROM. They observed that perinatal mortality increased as duration of ruptured membranes increased and cautioned that “with rupture of membranes, the clock of infection starts to tick; from this point on, isolation and protection of the fetus from external microorganisms virtually ceases.”
In 1967, Webb25 affirmed this ominous prediction with a grim survey of 54 cases of maternal death associated with PROM. Thirty-one deaths occurred in women at 36 weeks’ gestation or greater, and most were the direct result of overwhelming infection. Eighty per cent of the infants of these mothers died. Gunn and associates26 also pointed with alarm to the high frequency of infection in patients with PROM. Overall, 9% of the women in this survey with PROM developed intrapartum infection. In term patients, 24% of those with ROM of longer than 24 hours became infected. Sixteen per cent of infants delivered to mothers with intrapartum infection died. The authors concluded that, in term patients with PROM, early induction of labor was indicated to reduce the risk of ascending infection.
In a subsequent investigation, Johnson and coworkers27 evaluated 8320 women of varying gestational ages who had PROM. In women at 37 weeks’ gestation or greater with prolonged ROM, the incidence of intrapartum fever was increased significantly compared with that in the general obstetric population. When duration of membrane rupture was greater than 72 hours, there was also an increase in perinatal mortality due, primarily, to stillbirths in patients with intrapartum infection.
The reports summarized above clearly argue in favor of an aggressive approach to term patients with PROM. When interpreting these observations, however, several caveats must be observed. First, these investigations were retrospective and, largely, uncontrolled (level II and level III evidence). Therefore, treatment groups were not necessarily comparable with respect to other obstetric and medical complications that might have influenced outcome. Most of the reports were published at a time when the microbiology of intrapartum infection was not well understood and when some of the newer, broad-spectrum antibiotics were not available. Moreover, neonatal intensive care has improved dramatically in recent years, and the prognosis for infected neonates is not so unfavorable as previously reported. Many of the early investigations included both term and preterm patients, and clearly, the outcomes for these individuals are not comparable. The definitions of maternal and neonatal infection were neither uniform nor precise. The time of onset of maternal infection usually was not specified, nor was the timing of maternal antibiotic administration noted. Few of the authors employed a standardized management plan for patients with PROM. Finally, and most important, none of the authors examined maternal and neonatal outcome in the specific group of term patients who had a cervix unfavorable for immediate induction of labor.
Kappy and colleagues28 were the first group of investigators to challenge the doctrine that all term patients with PROM required immediate induction of labor, regardless of the cervical examination. In 1979, they published the results of a retrospective, uncontrolled survey of 78 term women who had PROM and whose cervix was unfavorable for induction of labor. Fifteen of these women (19%) were electively induced at the discretion of the attending physician; 63 (81%) were observed expectantly for the onset of spontaneous labor. Of the latter individuals, 85% were in labor within 24 hours of ROM. Fifteen per cent of patients had latent periods that exceeded 24 hours, and in 4%, the latent period exceeded 7 days.
Seven of the patients (47%) undergoing induction required cesarean delivery. In each instance, the indication was lack of progress in labor, although the authors did not distinguish between failed induction and true arrest of progress in the active phase of labor. Twenty-one women (33%) who initially were managed expectantly required abdominal delivery, nine (43%) for “lack of progress.” This observed difference in frequency of cesarean delivery was statistically significant. Moreover, the reduction in frequency of surgery was achieved without increasing the risk of neonatal or maternal infection.
Three years later, Kappy and associates29 provided an additional assessment of the management of PROM in term patients with a cervix unfavorable for induction of labor. In this second retrospective investigation, the authors added 72 patients to their original series of 78. Patients were included in the study if they were at term and had documented ROM, and the Bishop score by visual inspection was less than 8. During the period of this investigation, 38 patients (26%) were managed by immediate induction of labor. Most of these women were private patients of attending physicians who did not approve of conservative management. One hundred twelve women (74%) were observed expectantly for spontaneous onset of labor.
Of the 38 women managed by immediate induction of labor, 15 (39%) delivered abdominally. Twelve cesareans were performed for “lack of progress” and three for fetal distress. Of the 112 patients who were allowed to enter labor spontaneously, 21 subsequently had cesareans before labor was well established for reasons such as “breech presentation, prior cesarean, twin gestation-abnormal lies, suspicion of infection, and infertility.” Of the 91 women who were allowed to continue in labor, 11 (12%) required cesareans for either lack of progress (7) or fetal distress (4). Eighty-seven per cent of patients managed expectantly went into labor within 48 hours. Only 3.6% had a latent period that exceeded 7 days.
In this investigation, the authors defined “lack of progress” as “lack of cervical effacement and dilation despite good labor for at least 1.5 hours.” As previously noted, they did not distinguish between arrest of progress in the latent versus the active phase of labor. Despite this caveat, the observed difference in frequency of cesarean delivery in the two groups was highly significant (p <0.01). Moreover, there were no neonatal or fetal deaths and no instances of proven septicemia or meningitis in either an infant or a mother. As expected, the frequency of febrile morbidity was higher in patients who delivered by cesarean.
The two reports by Kappy and coworkers28, 29 were the impetus for a subsequent report by Duff and colleagues.1 Their investigation was the first prospective comparison of “aggressive” versus “conservative” management in term patients with PROM and an unfavorable cervix. Their study group was composed predominantly of indigent patients at 36 weeks’ or longer gestation. For the purpose of the study, the status of the cervix was assessed by a single sterile digital examination and was considered unfavorable for induction of labor if effacement was less than 80% and dilatation less than 2 cm.
Patients were assigned to treatment on the basis of their day of admission to the hospital. One group of women was managed expectantly by awaiting the spontaneous onset of labor or the development of infection. The second group underwent induction of labor within 12 hours of admission. An internal fetal heart rate electrode and uterine pressure catheter were inserted before infusion of oxytocin was begun. For women in this group, “failed induction” was defined as failure to enter the active phase of labor after 12 hours of regular uterine contractions documented by internal uterine monitoring.
Seventy-five patients were managed expectantly, and 59 underwent early induction of labor. Fifty-two per cent of women managed conservatively were in labor within 12 hours, and 75% were in labor within 24 hours. Nine women (12%) had latent periods that exceeded 48 hours. The longest latent period was 161.5 hours. Patients managed by intervention had a higher frequency of intrapartum infection (16 vs. 4%, p <0.05). They also had a higher frequency of cesarean delivery (20 vs. 8%, p <0.05). Fifty-eight per cent of the cesareans in the intervention group were for failed inductions. There were no differences in the frequency of neonatal infection or duration of maternal hospitalization.
After publication of these reports, many clinicians began to adopt a more conservative approach to the management of PROM at term, especially in patients whose cervix was unfavorable for induction of labor. However, in 1989, Wagner and associates18 injected a new note of caution into the debate concerning management of this difficult clinical problem. These authors studied a group of 182 uncomplicated, term women who received prenatal care at a large health maintenance organization (HMO) in California. The diagnosis of unfavorable cervix (<80% effacement, <2 cm dilatation) was assessed by visual inspection in 156 patients and by a single sterile digital examination in 26 women. On admission, patients were assigned to one of two treatment groups. One group underwent induction of labor within 6 hours of ROM. The second group was observed for up to 24 hours, awaiting the onset of spontaneous labor or infection. If labor did not ensue within 24 hours, oxytocin was administered.
In the group managed by early induction, 90% delivered within 24 hours of ROM. In the delayed group, only 60% delivered within 24 hours (p <0.001). Thirty-eight per cent of the latter patients required induction after a latent period of 24 hours. There were no differences between the two groups in the frequency of cesarean delivery or maternal infection. However, 48% of infants in the delayed induction group required diagnostic evaluations for sepsis compared with only 8% in the early intervention group (p <0.001). Significantly more infants in the delayed induction group received antibiotics pending the result of the evaluation for sepsis. In addition, there was an increased incidence of proven infection (p = 0.06) among the neonates delivered to mothers in the delayed induction group.
The findings of Wagner and associates18 are similar to those subsequently reported by Guise and coworkers.30 In the latter investigation, 112 uncomplicated term patients with an unfavorable cervix, defined as a Bishop score of 4 or less, were managed in a uniform manner. Patients initially were observed for approximately 24 hours for evidence of spontaneous labor or infection. Patients who did not enter labor within 24 hours were induced with oxytocin. The decision to intervene at this time was based on two factors: (1) the previous observations of other investigators that at least 75–85% of women were in spontaneous labor within 24 hours, and (2) the recognition that, in an era of increasing concern about cost containment, a more extended period of observation and hospitalization was difficult to justify to third-party payers.
Thirty-nine patients (35%) did not enter labor spontaneously and, therefore, required induction. Twenty-nine (26%) began labor spontaneously but then required oxytocin augmentation. Forty-four women (39%) began labor spontaneously and required no intervention. The frequency of maternal infection for the entire group was 24/112 (21%). Intrapartum infection was significantly more common in the women who required induction of labor. Cesarean delivery was more frequently performed in women who needed induction or augmentation compared with patients who had no intervention (p <0.05). Patients who required induction of labor had a longer hospitalization than women in the other two groups. Finally, infants born to mothers in the induction group were significantly more likely to undergo evaluations for sepsis than infants delivered to mothers who required no intervention (p <0.05).
Two other investigators reached different conclusions concerning the effect of delayed induction. Cammu and colleagues31 evaluated 105 middle-class nulliparas with uncomplicated singleton pregnancies. Once the diagnosis of PROM was confirmed, the women were observed for up to 24 hours. Patients were not stratified on the basis of their cervical examination. If spontaneous labor did not ensue within 24 hours or by the early morning following admission, oxytocin was administered. The outcome of these patients was compared with controls who had artificial ROM and spontaneous or induced labors.
Seventy-six women (72%) managed conservatively entered labor within 24 hours, and 38 of these patients (50%) required augmentation with oxytocin. Twenty-nine women (28%) were induced. Of these women, the longest interval from ROM until delivery was 43 hours. The frequency of infection in study patients and controls was very low, a fact that reflects the affluent nature of the patient population. There were no differences between study and control patients in the frequency of maternal or neonatal infection.
Grant and associates32 reported similar findings in a study of 444 term nulliparas. Patients had a single sterile speculum examination at the time of admission and then were randomized to immediate induction of labor with oxytocin (n = 219) versus an initial period of observation (n = 225). Patients initially managed expectantly received oxytocin if they were not in labor on the morning after admission. In these patients, the range in time from admission to induction was 9–33 hours. In the conservatively managed group, the cesarean delivery rate was 11.1% compared with 17.4% in the immediate induction group (p = 0.06). More spontaneous vaginal deliveries occurred in the former group, and these patients had fewer requests for analgesics during labor. There were no significant differences in the length of labor, the frequency of maternal or neonatal infection, or the requirement for therapeutic antibiotics.
Shalev and colleagues33 conducted a prospective, but nonrandomized, investigation of 566 women who were not in labor within 6 hours of ROM. Patients were assigned to induction at the end of 12 hours (n = 298) versus induction after 72 hours (n = 268). In the former group, 164 (55%) required oxytocin induction versus 47 (17.5%) in the 72-hour group (p < 0.001). There were no significant differences in the frequency of cesarean delivery or maternal and neonatal infection. The overall length of hospitalization was prolonged in the second group (5 versus 6 days). The authors concluded that an extensive delay in induction offered no measurable benefit to mother or infant and increased the expense and duration of hospitalization.
Several features unify the reports reviewed above. All authors confined their analysis to patients with an unfavorable cervix. All investigators routinely excluded from assessment patients who had any medical or obstetric complication of pregnancy that warranted immediate intervention, such as hypertension, insulin-dependent diabetes, postdates pregnancy, malpresentation, or fetal anomaly. All investigators observed patients in the hospital under a strict protocol that provided for fetal heart rate testing and surveillance for maternal infection. Cultures of the lower genital tract, assessment of the peripheral white blood cell count, and evaluation of maternal temperature were the measures most consistently employed to detect maternal infection. The authors intervened promptly by inducing labor if evidence of maternal infection developed. They also recognized that their study patients were likely to have very long latent phases and made a determined effort to minimize the number of vaginal examinations in the early portion of labor. Finally, none of the authors used laminaria, dilapan, or topical prostaglandin preparations as cervical-ripening agents.
A number of reports have appeared in the literature describing use of prostaglandin preparations in management of patients with PROM and an unfavorable cervix. Taken together, these publications have highlighted and refined a valuable new approach to patients with this troublesome complication. Granstrom and associates34 were among the first to evaluate the efficacy of prostaglandin E2 (PGE2) for cervical ripening and labor induction in patients with PROM and an unfavorable cervix. They treated 29 nulliparas and 32 multiparas with a 3-mg PGE2 vaginal suppository approximately 6 hours after ROM. The cervical examination was reassessed 5 and 24 hours later. If the cervix was favorable but no contractions were present, labor was induced with oxytocin. If the cervix was still unfavorable, a second suppository was administered. Twelve of the nulliparous women and 21 of the multiparous women were in labor within 5 hours of application of the first suppository. The mean application-to-regular contractions interval and onset of labor-to-delivery interval were 11 and 5.3 hours in the first group and 5.0 and 4.6 hours in the second group. No maternal or neonatal infections occurred.
van der Walt and Venter35 subsequently assigned 60 uncomplicated term patients with an unfavorable cervix (Bishop score 5) to three treatment groups: expectant management, immediate oxytocin induction, and intravaginal PGE2 (two 0.5-mg tablets). The dose of prostaglandin was repeated every 6 hours up to a maximum of three doses. The mean duration of labor was longest in the oxytocin group, and all six cesarean deliveries were in these patients. There was no significant difference in the frequency of maternal or neonatal infection. Women in the prostaglandin group had the shortest length of hospitalization.
Goeschen36 conducted a clinical trial comparing induction of labor with oxytocin versus PGE2. He initially enrolled 60 women who were at 36 weeks’ gestation or longer and had PROM and a Bishop score of 7 or less and assigned them to receive oxytocin (n = 25) or PGE2 gel (n = 35). The gel was applied intracervically in a dose of 0.4 mg. The interval from PROM to delivery and the duration of labor were significantly shorter in the PGE2 group. In addition, the incidence of operative delivery and the frequency of neonatal infection were lower in the prostaglandin group.
Ray and Garite37 reached similar conclusions in a prospective study of 140 women with uncomplicated pregnancies at greater than 36 weeks’ gestation and cervical dilatation less than 3 cm. Patients were randomly assigned to placebo suppository versus 3-mg PGE2 suppository vs. immediate oxytocin infusion. The dose of PGE2 was repeated in 6 hours if the patient was not in labor. Oxytocin was administered after 12 additional hours if the patient was still not in labor. Patients who received PGE2 subsequently had a decreased requirement for oxytocin compared with the placebo group. Patients in the PGE2 and oxytocin group had a significant decrease in the mean time to delivery compared with the placebo group (16.3 hours, 13.9 hours, and 21.2 hours, respectively). Significantly, the lowest incidence of chorioamnionitis and endometritis was in the women who received PGE2. There also was a trend toward decreased frequency of cesarean delivery in the prostaglandin group.
A subsequent report by Chua and coworkers38 differed significantly from the findings of van der Walt and Venter,35 Goeschen,36 and Ray and Garite.37 These authors treated 94 women admitted to the National University in Singapore with PROM at 36 weeks’ gestation or greater. Patients were assigned to treatment with oxytocin versus PGE2, administered in the form of two 3-mg vaginal pessaries, followed by oxytocin induction. The mean cervical score was 3.5 in the first group and 3.6 in the second (p = NS). The length of labor and incidence of operative delivery did not differ in the two groups.
Interestingly, however, the experience with this investigation did not deter the authors from further use of PGE2 in the same clinical setting.39 They subsequently conducted a double-blind, randomized trial comparing a 3-mg PGE2 vaginal pessary with placebo for induction of labor in 155 nulliparous women at greater than 36 weeks with PROM and a modified Bishop score of less than 6 of 10. If patients did not begin labor within 12 hours, oxytocin was administered. Compared with women in the placebo group, those who received PGE2 were less likely to require oxytocin at the end of the 12-hour observation period (37 vs. 58%, p < 0.002). In addition, they had a significantly shorter interval from admission to onset of labor, a shorter latent period from ROM to onset of labor, and shorter interval from admission to delivery. The overall incidence of maternal and neonatal infection was very low and did not differ significantly between the two groups.
Meikle and colleagues40 provided additional information concerning the safety of PGE2 preparations in term patients with PROM. They reported a retrospective survey of 148 women who were at 37 weeks’ gestation or greater and who had cervical dilatation less than 2 cm. In the first phase of the investigation, patients received PGE2 gel, 4 mg every 12 hours for two doses, and in the second phase, 3-mg PGE2 vaginal suppositories, every 4–6 hours, for a maximum of three doses. Patients not in labor after the maximum dose of prostaglandin received oxytocin. Interestingly, 55 women (37%) entered labor spontaneously following administration of PGE2. None developed chorioamnionitis or endometritis. Only three instances (2%) of hyperstimulation occurred; each of these patients subsequently delivered vaginally. The cesarean delivery rate in the study group was relatively low, 18/148 (12%). Two-thirds of the cesareans were for failure to progress.
The largest and probably best designed trial of management for term patients with PROM (level I evidence) was published by Hannah and associates.41 These authors randomly assigned over 5000 women to one of four treatment groups: immediate induction with oxytocin or PGE2 vaginal gel or expectant management followed by induction with oxytocin or prostaglandin gel if labor did not ensue in 4 days. The overall frequency of cesarean delivery was very low and did not differ among the four groups. Women in the expectant management (oxytocin) group had a significantly higher frequency of infection than women in the induction-with-oxytocin group – a finding consistent with previous reports. The rates of neonatal infection were comparably low in all groups, but neonates in the induction-with-oxytocin group were significantly less likely to receive antibiotics than those in the expectant management (oxytocin) or induction-with-prostaglandin group. Women in the study expressed a greater preference for induction of labor than expectant management. Of note, four perinatal deaths occurred, all in the expectant management groups. Two of the deaths occurred in infants of women who were treated as outpatients.
In a subsequent investigation, Gafni and coworkers42 assessed the economic impact of the four methods of management used in the aforementioned trial by Hannah and associates.41 Immediate induction with oxytocin was less expensive than immediate induction with PGE2 gel or expectant management followed by oxytocin induction after 4 days. The cost difference ranged from $30 to $49 (American dollars).
Misoprostol, an analog of PGE1, has also been shown to be effective in inducing labor in patients with an unfavorable cervix, and it offers a marked cost savings compared with commercially available preparations of PGE2.43, 44 In a prospective trial in 99 patients, Sanchez-Ramos and associates44 compared misoprostol tablets (50 μg) administered intravaginally to intravenous oxytocin for induction of labor in term patients with PROM and an unfavorable cervix. Oxytocin was used to augment labor as needed in the misoprostol group. Patients in the misoprostol group required lower total doses of oxytocin, although there were no significant differences in time interval to delivery. Fewer patients in the misoprostol group required operative vaginal delivery (23 vs. 33%, relative risk [RR] 0.76, 95% confidence interval [CI] 0.46–1.28). There were no significant differences in the frequency of cesarean delivery or maternal and neonatal complications.
In another investigation, Varaklis and colleagues45 compared intravaginal misoprostol (25 μg every 2 hours) to intracervical PGE2 gel (0.5 mg every 6 hours for two doses) for induction of labor in women with intact membranes and a Bishop score of 5 or less. Women in the misoprostol group had a significantly shorter mean time from initial administration of the drug to onset of regular uterine contractions (p = 0.007) and a corresponding decrease in the mean time to delivery (p = 0.006).
CONCLUSION
On the basis of the information presented previously, there is consistent evidence from both level I and level II clinical trials to support the following plan of management algorithm outlined in Figure 3 (grade of recommendation – A).[46][47]
Fig. 3. Algorithm for management of term patients with premature rupture of the membranes (PROM). ROM, rupture of the membranes.
REFERENCES
Duff P, Huff R, Gibbs RS: Management of premature rupture of membranes and unfavorable cervix in term pregnancy. Obstet Gynecol 63:697, 1984 |
|
Naeye RL: Factors that predispose to premature rupture of the fetal membranes. Obstet Gynecol 60:93, 1982 |
|
Artal R, Sokol RJ, Neuman M, et al: The mechanical properties of prematurely and non-prematurely ruptured membranes. Am J Obstet Gynecol 125:655, 1976 |
|
Vadillo-Ortega F, Gonzalez-Avila G, Karchmer S, et al: Collagen metabolism in premature rupture of amniotic membranes. Obstet Gynecol 75:84, 1990 |
|
McGregor JA, French JI, Lawellin D, et al: Bacterial protease-induced reduction of chorioamniotic membrane strength and elasticity. Obstet Gynecol 69:167, 1987 |
|
Sbarra AJ, Thomas GB, Cetrulo CL, et al: Effect of bacterial growth on the bursting pressure of fetal membranes in vitro. Obstet Gynecol 70:107, 1987 |
|
Schoonmaker JN, Lawellin DW, Lunt B, et al: Bacteria and inflammatory cells reduce chorioamniotic membrane integrity and tensile strength. Obstet Gynecol 74:590, 1989 |
|
Lenihan JP: Relationship of antepartum pelvic examinations to premature rupture of the membranes. Obstet Gynecol 63:33, 1984 |
|
McDuffie RS, Nelson GE, Osborn CL, et al: Effect of routine weekly cervical examinations at term on premature rupture of the membranes: A randomized controlled trial. Obstet Gynecol 79:219, 1992 |
|
Lodeiro JG, Hsieh KA, Byers JH, et al: The fingerprint, a false-positive fern test. Obstet Gynecol 73:873, 1989 |
|
Gahl WA, Kozina TJ, Fuhrmann DD, et al: Diamine oxidase in the diagnosis of ruptured fetal membranes. Obstet Gynecol 60:297, 1982 |
|
Reece EA, Chervenak FA, Moya FR, et al: Amniotic fluid arborization: Effect of blood, meconium, and pH alterations. Obstet Gynecol 64:248, 1984 |
|
Gorodeski IG, Paz M, Insler V, et al: Diagnosis of rupture of the fetal membranes by glucose and fructose measurements. Obstet Gynecol 53:611, 1979 |
|
Cousins LM, Smok DP, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of rupture of membranes. Am J Perinatol 2005; 22:1-4. |
|
Duff P. Preterm premature (prelabor) rupture of membranes. UpToDate, 2016. |
|
Munson LA, Graham A, Koos BJ, et al: Is there a need for digital examination in patients with spontaneous rupture of the membranes? Am J Obstet Gynecol 153:562, 1985 |
|
Schutte MF, Treffers PE, Kloosterman GJ, et al: Management of premature rupture of membranes: The risk of vaginal examination to the infant. Am J Obstet Gynecol 146:395, 1983 |
|
Wagner MV, Chin VP, Peters CJ, et al: A comparison of early and delayed induction of labor with spontaneous rupture of membranes at term. Obstet Gynecol 74:93, 1989 |
|
Group B streptococcal infections in pregnancy. ACOG Technical Bulletin No. 170. July 1992 |
|
Calkins LA: Premature spontaneous rupture of the membranes. Am J Obstet Gynecol 64:871, 1952 |
|
Russell KP, Anderson GV: The aggressive management of ruptured membranes. Am J Obstet Gynecol 83:930, 1962 |
|
Lanier LR, Scarbrough RW, Fillingim DW, et al: Prudence of maternal and fetal complications associated with rupture of the membranes before onset of labor. Am J Obstet Gynecol 93:398, 1965 |
|
Bryans CI: Incidence of maternal and fetal complications associated with rupture of the membranes before the onset of labor. Am J Obstet Gynecol 93:403, 1965 |
|
Shubeck F, Benson RC, Clark WW, et al: Fetal hazard after rupture of the membranes. A report from the collaborative project Obstet Gynecol 28:22, 1966 |
|
Webb GA: Maternal death associated with premature rupture of the membranes. An analysis of 54 cases Am J Obstet Gynecol 98:594, 1967 |
|
Gunn GC, Mishell DR, Morton DG: Premature rupture of the fetal membranes: A review. Am J Obstet Gynecol 106:469, 1970 |
|
Johnson JWC, Daikoku NH, Niebyl JR, et al: Premature rupture of the membranes and prolonged latency. Obstet Gynecol 57:547, 1981 |
|
Kappy KA, Cetrulo CL, Knuppel RA, et al: Premature rupture of the membranes: A conservative approach. Am J Obstet Gynecol 134:655, 1979 |
|
Kappy KA, Cetrulo CL, Knuppel RA, et al: Premature rupture of the membranes at term. A comparison of induced and spontaneous labors J Reprod Med 27:29, 1982 |
|
Guise JM, Duff P, Christian JS: Management of term patients with premature rupture of membranes and an unfavorable cervix. Am J Perinatol 9:56, 1992 |
|
Cammu H, Verlaenen H, Derde MP: Premature rupture of membranes at term in nulliparous women: A hazard? Obstet Gynecol 76:671, 1990 |
|
Grant JM, Serle E, Mahmood T, et al: Management of prelabour rupture of the membranes in term primigravidae: Report of a randomized prospective trial. Br J Obstet Gynecol 99:557, 21992 |
|
Shalev E, Peleg D, Eliyahu S, et al: Comparison of 12- and 72-hour expectant management of premature rupture of membranes in term pregnancies. Obstet Gynecol 85:766, 1995 |
|
Granstrom L, Ekman G, Ulmsten U: Cervical priming and labor induction with vaginal application of 3 mg PGE2 in suppositories in term pregnant women with premature rupture of amniotic membranes and unfavorable cervix. Acta Obstet Gynecol Scand 66:429, 1987 |
|
van der Walt D, Venter PF: Management of term pregnancy with premature rupture of the membranes and unfavourable cervix. S Afr Med J 75:54, 1989 |
|
Goeschen K: Premature rupture of membranes near term: Induction of labor with endocervical prostaglandin E2 gel or intravenous oxytocin. Am J Perinatol 6:181, 1989 |
|
Ray DA, Garite TJ: Prostaglandin E2 for induction of labor in patients with premature rupture of membranes at term. Am J Obstet Gynecol 166:836, 1992 |
|
Chua S, Arulkumaran S, Kurup A, et al: Does prostaglandin confer significant advantage over oxytocin infusion for nulliparas with pre-labor rupture of membranes at term? Obstet Gynecol 77:664, 1991 |
|
Chua S, Arulkumaran S, Yap C, et al: Premature rupture of membranes in nulliparas at term with unfavorable cervices: A double-blind randomized trial of prostaglandin and placebo. Obstet Gynecol 86:550, 1995 |
|
Meikle SF, Bissell ME, Freedman WL, et al: A retrospective review of the efficacy and safety of prostaglandin E2 with premature rupture of the membranes at term. Obstet Gynecol 80:76, 1992 |
|
Hannah ME, Ohlsson A, Farine D, et al: Induction of labor compared with expectant management for prelabor rupture of the membranes at term. N Engl J Med 334:1005, 1996 |
|
Gafni A, Goeree R, Myhr T, et al: Induction of labor versus expectant management for prelabour rupture of the membranes at term: an economic evaluation. Can Med Assoc J 157:1519, 1997 |
|
Sanchez-Ramos L, Kaunitz AM, Del Valle GO, et al: Labor induction with the prostaglandin E1 methyl analogue misoprostol versus oxytocin: A randomized trial. Obstet Gynecol 81:332, 1993 |
|
Sanchez-Ramos L, Chen A, Briones D, et al: Premature rupture of membranes at term: Induction of labor with intravaginal misoprostol tablets (PGE1 ) or intravenous oxytocin. Am J Obstet Gynecol 170:380, 1994 |
|
Varaklis K, Gumina R, Stubblefield PG: Randomized controlled trial of vaginal misoprostol and intracervical prostaglandin E2 gel for induction of labor at term. Obstet Gynecol 86:541, 1995 |
|
Duff P: Premature rupture of the membranes at term. N Engl J Med 334:1053, 1996 |
|
Duff P: Premature rupture of membranes at term: A medical and economic rationale for active management. Can Med Assoc J 157:1541, 1997 |