Physiology of Pregnancy
Authors
INTRODUCTION
Pregnancy in the human female is an unusual state in which virtually all maternal systems are dramatically altered to permit the sustenance and growth of the intrauterine conceptus. In very real ways, the maternal organism is life-adapted.
Although pregnancy is unique in many ways, it is particularly so in being limited in time. Pregnancy is a temporary state with a definite point of onset and an equally definite termination. The duration of pregnancy in humans, marked from the first day of the last menstrual period, is classically 280 days. Recent studies, however, using computerized day-counting techniques, show an average duration of 284.2 days.1 Table 1 shows the mean values and statistical dispersion of gestational duration as well as a number of other gestational milestones.
Table 1. Mean observed intervals to delivery date for last menstrual period (LMP) and obstetric milestones*
Milestone | Mean interval to | Standard |
Known LMP | 284.2 | 14.6 |
Quickening | 156.3 | 18.0 |
Fetal heart audible | 136.2 | 17.0 |
Uterus at umbilicus | 140.8 | 14.9 |
*Data from 418 patients.
(Andersen HF, Johnson TR, Flora JD, Barclay M: Gestational age assessment: II. Prediction from combined clinical observations. Am J Obstet Gynecol 140(7):770, 1981)
The changes brought about in the maternal organism by the state of pregnancy are important, because in many instances they mimic pathophysiologic responses to disease. If the constellation of changes occurring normally in pregnancy are misinterpreted as signs of disease processes, the gravid or puerperal woman may be subjected to diagnostic and therapeutic interventions that are not only unnecessary but may also be dangerous to mother and fetus.
Because so many system-specific changes occur in the course of pregnancy, it is difficult to develop a total physiologic overview. There are, however, a number of well-described adaptive physiologic states that produce changes in human systems similar to those seen in pregnancy. These adaptive states may be used as models or constructs to help integrate the diverse alterations in physiologic systems that occur during the course of normal gestation. Among the physiologic states that produce adaptive changes similar to those seen in pregnancy are the presence of a moderate-sized arteriovenous fistula, acclimation to increased environmental or internal heating, and adjustments to increasing levels of circulating progesterone.
MODELS OF PREGNANCY AS A PHYSIOLOGICALLY ADAPTED STATE
Model I: Pregnancy as an Arteriovenous Fistula
In 1938, Burwell and associates2 suggested that there are strong similarities between the physiologic alterations seen in normal pregnancy and those seen in patients with large arteriovenous fistulas. Patients on chronic renal dialysis who have peripheral shunts constructed for purposes of dialysis typically have flow rates in their shunts of approximately 600 ml/minute.3 Because uteroplacental flow rates at term (nearly 600 ml/minute4) are essentially the same as those in the artificially produced shunts, it is not surprising that there are similarities between the cardiovascular changes in the shunted patients and cardiovascular alterations in the pregnant woman, particularly as she approaches term.
In both of these circumstances there is evidence of increased peripheral circulation, decreased peripheral resistance, increased heart rate, increased cardiac output, and increased plasma volume. This particular model can be used to explain a number of other changes related not directly to the shunting mechanism but to secondary changes produced by increased peripheral circulation, such as increased renal plasma flow and the physiologic alterations associated with increased renal perfusion.
Model II: Pregnancy as a State of Heat Adaptation
Abrams and associates and others5, 6 have shown that there is a considerable temperature gradient between the mother and fetus. Aortic temperature measurements made in the pregnant ewe indicate that the fetal core temperature exceeds that of the mother by 0.5°C, which is a significant amount. Thermodynamics and the physics of heat transfer suggest that because of this temperature difference, the flow of heat from the fetus to the mother is relatively constant. As a result, the maternal organism must adjust her thermoregulatory system to permit increased heat loss to the environment. Aside from physical considerations, the need for maternal thermoregulatory adjustments is suggested by the observation that homeothermic mammals function within a very narrow range of internal temperatures. Extremes in either direction produce significant alterations in the function of fundamental systems responsible for the maintenance of life.
Responses of the homeothermic mammal to internal and environmental heating produce similar physiologic alterations. These include increased respiratory rate, increased cardiac output, increased heart rate, expansion of plasma volume, increased peripheral circulation, and a number of other changes similar to those seen in normal human gestation.
Model III: Pregnancy as a Hyperprogestational State
With the onset of normal gestation, all maternal systems are subjected to increasing levels of circulating progesterone. At first the corpus luteum of pregnancy, and later the placenta, produce large amounts of this hormone. At term, serum levels may be as high as 2.5 times those considered normal in the menstruating woman.
Increased basal body temperature and changes in the smooth muscle dynamics of the uterus, the vascular system, the urinary system, the gastrointestinal system, and the respiratory system in pregnancy have often been explained on the basis of increasing levels of serum progesterone. The mechanism proposed to explain many of these changes relates to the effect of progesterone on the electrochemical gradient at the cell membrane of individual smooth muscle fibers.7 According to this hypothesis, progesterone acts to hyperpolarize the cell membrane, depressing the resting electrical potential at the membrane to a level below that of the normal activation threshold. This effectively puts the muscle at rest, because much greater levels of stimulation are required to produce depolarization and subsequent muscle contraction. Decreased tone and overall decrease in contractile activity is seen in most of the structures that depend on smooth muscle for their action. This includes the uterus, gut, respiratory system, ureters, and peripheral vascular system.
Volume expansion of the intravascular space, decreased peripheral resistance, increased heart rate, and a number of other alterations associated with the pregnant state could theoretically be explained on the basis of progesterone's effect on smooth muscle.
Overall, it seems unlikely that a single model can be invoked to explain the varied changes that take place in the human female during the course of gestation. It is more likely that all of these mechanisms contribute, along with other factors still unidentified, to the myriad changes that constitute the physiologic alterations associated with the normal human gestation. Each model, nonetheless, helps the clinician to anticipate and integrate the changes in many of the altered systems. The constructs described permit the alterations in individual systems to be fused into a more coherent overview.
METABOLIC CHANGES
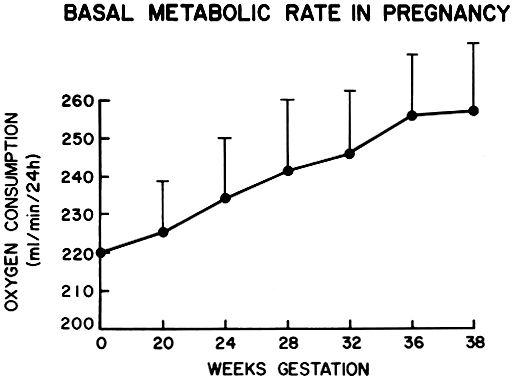
Basal metabolic studies carried out in the 1920s and 1930s by Sandiford and Wheeler8 and Rowe and Boyd9 demonstrated that pregnancy is characterized by increased metabolic activity as measured by the basal metabolic rate. In these studies, done on a small group of women throughout gestation, they found that the basal metabolic rate increased by approximately 20% as the pregnancy approached term. This trend is pictured in Figure 1. The researchers hypothesized that this increase in metabolic activity primarily represented increased fetal and placental metabolic work, with only a small fraction being attributable to increased maternal metabolic activity. At term, the products of gestation were estimated to be responsible for approximately 13% of the 20% increase in total metabolic activity. More modern studies10, 11 using oxygen consumption measures and indirect calorimetry estimate the energy output of an average-sized pregnant woman at 36 weeks' gestation to be approximately 98 W (8443 ± 243 kJ/day). This compares with an energy output of approximately 81 W (6971 ± 172 kJ/day) for a similar-sized, nonpregnant, nonlactating woman. Although the more recent studies used different methodologies, they all support the results of the earlier work.
During the course of normal pregnancy, basal body temperature is elevated. The increased metabolic activity manifested early in the gestation, along with the effects of progesterone, may be responsible for this phenomenon.
WEIGHT GAIN
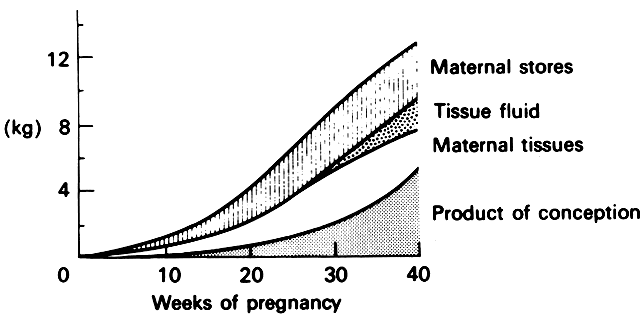
Weight gain, one of the hallmarks of pregnancy, has been extensively studied as it relates to the well-being of the fetus and infant. Optimal weight gain in pregnancy has been the subject of considerable discussion, and attitudes regarding what is appropriate have changed with time, even in recent history. Currently, a total weight gain of 10–12 kg is thought to be appropriate. The partitioning of this weight gain and its distribution are illustrated in Table 2 and Figure 2.
Table 2. Average components of weight gain in pregnancy and cumulative gain at the end of each trimester (kg)
Component | I | II | III |
Fetus | 0 | 1.0 | 3.4 |
Placenta | 0 | 0.3 | 0.6 |
Amniotic fluid | 0 | 0.4 | 1.0 |
Fetal total |
| 1.7 | 5.0 |
Uterus | 0.3 | 0.8 | 1.0 |
Breasts | 0.1 | 0.3 | 0.5 |
Blood volume | 0.3 | 1.3 | 1.5 |
Extracellular fluid | 0 | 0 | 1.5 |
Maternal total | 0.7 | 2.4 | 4.5 |
(After Pitkin R, Kaminetsky H, Newton M et al: Maternal nutrition. A selective review of clinical topics. Obstet Gynecol 40(6):773, 1972)
The total maternal weight gain indicated in Table 2 is that portion that can be explained in terms of measurable elements and is less than the 10–12-kg increase described as optimal. The difference is usually ascribed to fat storage in maternal tissues.
Weight gain in pregnancy is negligible in the first trimester, as can be seen in Table 2 and Figure 2. In the second and third trimesters, weight gain is much more appreciable and, according to the work of Hytten and Leitch,12 averages approximately 0.41–0.45 kg/week (0.9–1.0 pounds/week) in normal pregnancies in primigravidas.
NUTRITION
Studies carried out in Third World countries indicate that maternal malnutrition may interfere, at least epidemiologically, with appropriate intrauterine nutrition. Women who were marginally nourished before pregnancy deliver a higher proportion of premature and low-birth-weight infants. Feeding programs in Guatemala, Colombia, and other emerging nations have provided information that suggests that the proper nourishment of the pregnant woman may be of considerable benefit to her fetus.13
Nutritional authorities in the United States and much of the Western World have established suggested programs for appropriate nutrition in pregnancy. Basically, pregnant women require calories additional to the normal daily requirement. These recommendations, although variable from country to country, also suggest the addition of protein, iron, and other mineral and vitamin supplements to provide the necessary materials for fetal and maternal welfare during the course of gestation. Although common sense indicates that appropriate nutrition is important for maximizing the possibility of healthy offspring, Hytten and Leitch12 and others14 have pointed out that it is difficult to focus on nutrition alone as a factor in the growth and development of normal babies. Women who are appropriately nourished during the course of pregnancy are also usually better housed, better educated, and have greater access to the medical antepartum care that seems to be associated with improved pregnancy outcomes. Antepartum nutrition, however, continues to be an area of great interest because the feeding of pregnant women is a simple intervention that may have a significant impact on the outcome of reproduction. The current recommendations proposed by the Food and Nutrition Board of the US National Science Foundation are listed in Table 3.
Table 3. Recommended dietary allowances (Revised in 2005)*
Nutrient | For nonpregnant woman | For pregnant woman |
Protein | 45 g/day | + 30 g/day |
Calories | 2100 | + 300 |
Calcium | 1000 mg/day | No supplementation |
Iron | 18 mg/day | + 9 mg/day |
Folic acid | 400 μg/day | + 200 μg/day |
Ascorbic acid | 75 mg/day | + 10 mg/day |
*Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC, National Academies Press, 2005.
Caloric Requirements
Maternal mass increases by approximately 20% during the course of normal gestation. This increase in mass and the metabolic needs of the fetus require additional calories above the recommended daily allowance. In 1989, the Food and Nutrition Board of the National Academy of Sciences suggested that 300 additional calories be added to the diet of pregnant women to compensate for the additional needs of fetal growth and increased maternal metabolism.14 In pregnant adolescents, or those still in their active growth phase, additional calories beyond this recommendation are required to sustain needs related to maternal growth. The basal calorie levels for any woman can be determined from tables that take height and body habitus into consideration. A pregnant woman of average size requires approximately 2200 calories/day (Table 3).
Protein Requirements
On the basis of nitrogen balance studies, the Food and Nutrition Board has recommended that an extra 30 g of protein be supplied to pregnant women in addition to the basic requirement based on age and size.14 In the average-sized woman, this constitutes a total daily protein ration of 44–46 g/day (0.88 g/kg/day) plus the 30 gram supplement (Table 3).
Iron and Mineral Requirements
Iron supplementation is recommended in pregnancy by virtually all nutritional authorities. The requirements of the fetus and placenta and increased maternal red cell production require approximately 500 mg of elemental iron during the course of a normal pregnancy.15 This exceeds what most women have in terms of physiologic iron stores. The Food and Nutrition Board recommends 27 mg/day of supplemental iron during the course of pregnancy. This amount is usually present in prenatal vitamins. If the maternal diet is likely to have been deficient in iron before pregnancy, iron stores may be significantly less than normal, and additional iron supplementation should be considered.
Folic acid is a coenzyme essential in purine and pyrimidine metabolism and in the synthesis of DNA. Clinical evidence of folic acid deficiency is usually first evident in tissues that have rapid cell turnover, notably in hematopoiesis. Megaloblastic anemia of pregnancy as a result of inadequate intake of folic acid is not a rare cause of anemia in pregnant women in the United States. Prenatal multivitamins with folic acid often contain 1 mg of folate, which exceeds the recommended supplement.
PLASMA LEVELS OF NUTRIENTS
Changes in nutrients and related materials in the plasma of pregnant women suggest fundamental alterations in the manner in which they handle these materials. Total protein levels fall in pregnancy to levels about 1000 mg/dl less than those observed in the nonpregnant woman. Much of this decrease is in the albumin fraction, which is important in terms of its effect on the colloid osmotic pressure. Lowering of the colloid osmotic pressure allows water to flow from the plasma into cells or from vessels into the extracellular space. Globulins show a relatively small increase in serum concentration during the course of normal pregnancy. Levels of fibrinogen, an important protein in normal blood coagulation, as well as factors VII, VIII, IX, and X, are appreciably increased in the pregnant state. The increased concentration of fibrinogen (approximately 50%), is thought to be responsible for the commonly observed increase in erythrocyte sedimentation rate.
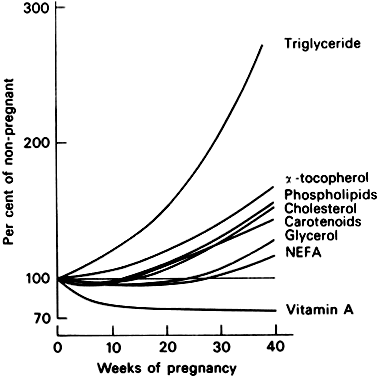
Lipid concentration in the serum is markedly increased in pregnancy (Fig. 3). Triglycerides, phospholipids, cholesterol, and free fatty acids increase markedly in amount. The increase in serum cholesterol seems to occur regardless of the dietary habits of the gravida; vegetarians show the same increase as those who eat meat.16, 17
CARBOHYDRATE METABOLISM
Carbohydrate metabolism is altered in the course of normal pregnancy, and the pregnant state has been characterized as diabetogenic. Previously undiscovered diabetes may be unmasked during the course of pregnancy, or a woman may develop diabetic levels of blood glucose as a response to pregnancy.
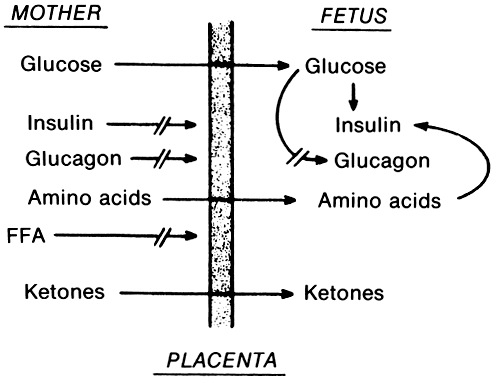
In the fasting state, glucose is transferred to the fetus from the maternal circulation, producing a decrease in maternal serum glucose levels. The major effects of pregnancy on glucose metabolism are related to the fact that the fetus withdraws glucose from the maternal circulation. In a study by Bleicher and colleagues,18 average plasma glucose values in antepartum women (75.2 mg/dl; standard error of the mean [SEM] = ±2.8 mg/dl) were significantly lower than those in postpartum women (92.5 mg/dl; SEM = ±2.7 mg/dl). This phenomenon is thought to occur because the fetus uses glucose almost exclusively as its metabolic fuel. The rate of delivery of glucose to the fetal circulation is thought to be controlled by the difference in serum concentrations between maternal and fetal serum levels. Glucose also seems to be transferred across the placenta by means of facilitated diffusion. Placental transfer of glucose and other materials related to carbohydrate handling in pregnancy is pictured in Figure 4..
Glucose utilization studies demonstrate that the fetus uses glucose at a rate of 6 mg/kg/minute at term. This rate is quite high compared with that in the normal adult, which is approximately 2.5 mg/kg/minute.In addition to glucose, amino acids are freely transported across the placenta into the fetal circulation. This transfer produces maternal hypoaminoacidemia, particularly of alanine, an important precursor of glucose in gluconeogenesis. With the fall in serum glucose levels, there is an associated decrease in plasma insulin, producing what Felig19 has referred to as an accelerated and exaggerated response to starvation. This is one explanation of the elevation in free fatty acids and triglycerides seen in the mother.
In pregnancy, feeding produces hyperglycemia, an increase in serum insulin levels, and hypertriglyceridemia. There is also a diminished response to insulin. The diminished response to insulin, which may be mediated hormonally, produces aberrations in blood sugar testing using both oral and intravenous loading with glucose. Adjusted norms are therefore necessary to diagnose diabetes in pregnancy. Bleicher and others17, 18, 19 have hypothesized that these changes are related and that the purpose of these changes in the carbohydrate metabolism of the mother is the protection of fetal tissues from fluctuations in glucose by switching the maternal tissues over to free fatty acid and triglyceride metabolism. The placental production of lactogen, a substance known to have lipolytic properties, is thought to explain these phenomena. The anti-insulin effects of placental lactogen seem to provide additional evidence for this hypothesis. The complexities of carbohydrate metabolism in pregnancy, although partially elucidated, continue to be confusing and require further investigation.
ALTERATIONS IN THE CARDIOVASCULAR SYSTEM
Regardless of the etiologic factors in the many changes that occur in the physiology of the pregnant woman, the system that undergoes some of the most significant alterations is the cardiovascular system. The changes in this system are quite profound and begin to occur almost at the time of conception. Lindhard's observations in 1915 that the cardiac output is increased in pregnancy formed the basis for much of the work subsequently done in delineating the multiple changes that occur in circulatory physiology in the pregnant woman.20
Anatomic Alterations
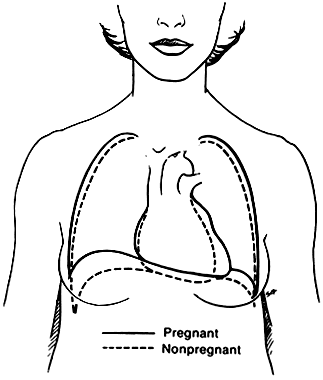
The position of the heart in the chest changes during the course of normal pregnancy. It is rotated slightly, and its apex deviates to the left. Thus, on physical examination of the chest of the pregnant woman, the point of maximum intensity of the cardiac action is often lateral to the midclavicular line and in the fourth rather than the fifth intercostal space.
Because of these changes in position and slightly increased cardiac volume (70–80 ml), the area of relative dullness over the precordium is increased, as is the cardiac shadow on x-ray examination. Lateral x-ray films of the pregnant woman's thorax may, in fact, show findings suggestive of atrial dilation, which is suggestive of stenotic mitral valvular disease. These alterations in morphology are pictured in Figure 5. The changes in heart position, increased output, and increased blood volume are probably responsible for the systolic flow-type murmur that is common in pregnancy.
Electrocardiographic Changes
These changes in cardiac position, presumably brought about by some of the functional changes in cardiodynamics, induce certain changes in the electrocardiographic (ECG) findings associated with pregnancy. As would be anticipated, ECG findings are suggestive of left axis deviation of approximately 15 degrees. There may be decreased voltage in the QRS complexes, as well as alterations in T and P waves. In a number of normal pregnant women, there may be flattening or inversion of T waves in lead III, as well as depression of the S-T segment in limb and chest leads. These findings, which are suggestive of myocardial ischemia, have been reported to occur in as many as 14% of normal pregnant women in whom none of the other demonstrable concomitants of true myocardial disease are seen. When this finding occurs in a pregnant woman, it tends to recur in subsequent pregnancies. Cardiac arrhythmias, particularly those of supraventricular origin, are also relatively common in pregnancy but are not generally productive of symptoms significant enough to require therapy.21, 22
Alterations in Cardiodynamics
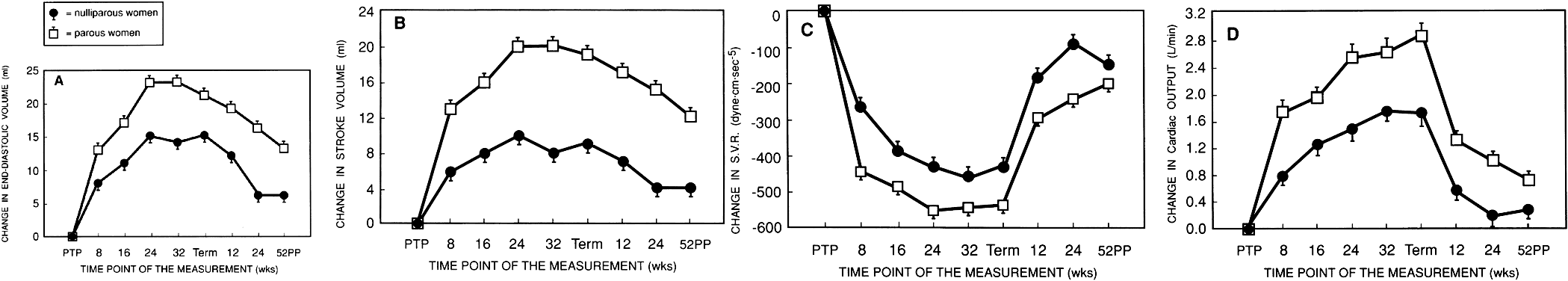
Beginning early in pregnancy, cardiac output increases significantly to maximum levels at around 20–24 weeks (Fig. 6) and maintains that level until after delivery. It is generally accepted that in the course of pregnancy the cardiac output increases to levels 30–35% in excess of that in the nonpregnant woman. Recent work by Clapp and Capeless23 has demonstrated that this increase in cardiac output may be enhanced in subsequent pregnancies. Stroke volume and end-diastolic volume are altered in a similar manner. Systemic vascular resistance is also significantly altered.
|
The factors responsible for the change in cardiac output are not completely understood but are thought to be related to one or several of the mechanisms noted in the introductory remarks in this chapter or, more likely, to some combination of all these factors. Almost all of the theories used to explain the observed changes implicate either neurohumoral factors, such as estrogen and progesterone, or the placental circulation acting as an arteriovenous fistula. Maternal heart rate is increased in pregnancy, consistent with a functional arteriovenous fistula; the increase averages approximately 15 beats/minute. Figure 7 demonstrates the increase in heart rate as pregnancy progresses.
Clinical symptoms and findings may be related to these alterations in normal cardiovascular dynamics. The pregnant woman has less measurable cardiac output, as well as decreased uterine perfusion, when in the supine position. The supine hypotensive syndrome, which is characterized by hypotension, tachycardia, diaphoresis, and discomfort, may become magnified by the effects of a conduction anesthetic that increases venous pooling and further reduces venous return to the heart. By positioning the patient to produce leftward deviation of the uterus, such postural changes in vital signs may be avoided and the need for vasopressors obviated. The case reports of O'Connor and Sevarino,24 as well as DePace and colleagues,25 suggest that an appreciation of this posture-related effect may be important in the successful cardiopulmonary resuscitation of pregnant women who have sustained a cardiac arrest.
Rapid changes in body position may produce syncope or lightheadedness because of decreased peripheral resistance in the lower extremities. These effects may be further intensified when warm environmental conditions contribute to peripheral vasodilatation. Theories relating to the effects of changes in cardiovascular dynamics on the metabolic heat production of the fetus and other products of conception are notably absent from most discussions of these phenomena, although significant fetal heat production is demonstrable in mammalian gestations.
Intravascular Volume Changes
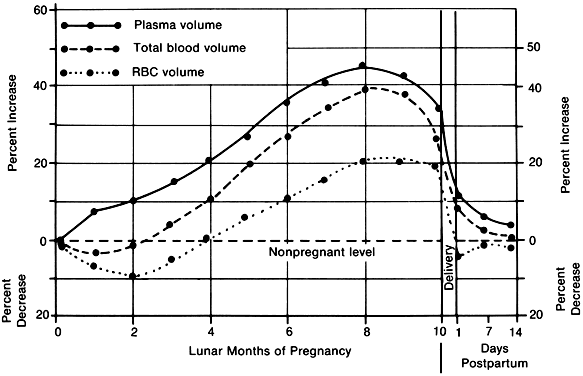
Large expansion of the intravascular volume, particularly plasma volume, is one of the hallmarks of normal pregnancy. Plasma volume increases to a significant extent early in pregnancy. Maternal blood volume expansion of 40% is not unusual in singleton pregnancy and may be even greater in multiple gestations. This expansion in blood volume is due to an increase in plasma volume of 45–55% and an increase in red cell mass of 20–30%.
The rapid increase in plasma volume, depicted in Figure 8, outstrips the manufacture of new red cells and may produce a virtual anemia in early pregnancy. The increase in plasma volume without a concomitantly rapid production of red cell mass is manifested in an apparent drop in the hemoglobin concentration, hematocrit, and red blood cell count. This plasma expansion requires that the definition of anemia in pregnancy be altered so that a pregnant woman is not considered anemic in most centers until the hemoglobin falls below 10 g/dl.26 The red cell mass increases in the course of pregnancy by 20–30%, or approximately 250–500 ml of packed red cells.
The reasons for this plasma volume expansion are probably numerous, and all suggested major causative factors, including the effects of hormonal level changes, the decrease in peripheral resistance produced by the placental and uterine shunting mechanisms, and heat-adaptive mechanisms, are likely involved to some extent. The role of increased cardiac output in the genesis of these changes is also somewhat speculative in that it is presently uncertain how plasma volume expansion is temporally related to increased cardiac output. To explain this phenomenon, Longo27 has proposed a set of mechanisms that integrate the effects of various pregnancy hormones, including estrogen and progesterone, as well as the known effects of pregnancy on the renin-angiotensin system and human placental lactogen, coupled with the changes presumably brought about by the increased uterine and placental circulation flow rates as pregnancy approaches term.
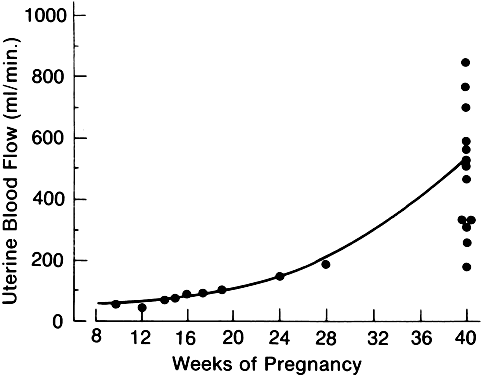
The changes in blood volume and plasma volume are associated with changes in the distribution of cardiac output. As the uterine contents increase in size and requirements for oxygen and nutrient materials grow, there is an increase in the volume of uterine artery blood flow. The studies of Romney and colleagues4 indicate that the increase in uterine perfusion is associated with the increasing size of the products of conception. The flow rates in the uterine and placental circulation approximate roughly 10 ml/minute per 100 g of fetal tissue. The association between the mass of the conceptus and uterine blood flow in terms of gestational age is displayed in Figure 9.
Alterations in Formed Elements of the Blood
Normal pregnancy is characterized by leukocytosis. This increase in white cell count is mostly due to increased numbers of polymorphonuclear leukocytes, which increase in number from the time of fertilization until the time of labor. A study by Griffin and Beck26 indicates, as does older literature,28 that leukocyte counts increase significantly from their previously elevated level in pregnancy at around 35 weeks of gestation and continue to rise until the time of parturition. At the time of delivery in this study, the leukocyte counts were in excess of 14,000 × 103 cells/ml (standard deviation = ±1.62 × 103 cells/ml). Perhaps because of the rapid production of new cells, a small number of myelocytes or metamyelocytes are normally present in the pregnant woman's peripheral circulation.
Lymphocyte and monocyte numbers are not altered significantly during the course of pregnancy, but eosinophil levels are reported to decrease sharply during labor and delivery. Basophil levels may be reduced in pregnancy.
Platelets are an important element in the coagulation process; their levels decrease slightly during the course of pregnancy. This decrease may be a function of the increase in plasma volume and is not reported to be associated with any significant change in platelet function.
Alterations in Venous Pressure
The state of pregnancy, either through the effects of progesterone on the veins of the periphery or via the shunting mechanisms of placental circulation, increases the venous capacitance. This is more marked in the lower extremities, where dependent edema is seen in as many as 30% of pregnant women at some time during the gestational period. Venous pressure above the level of the umbilicus is generally normal, whereas that below the level of the umbilicus is elevated. These changes are portrayed in Figure 10.
|
The clinical effects of this elevation in venous pressure are thought to be responsible for the pedal and pretibial edema seen in many pregnancies, particularly approaching parturition. The increased severity of varicosities of the lower extremities and of the vulva and vagina, as well as hemorrhoids, may be at least partially explained by this effect. Obstruction of the venous return to the right heart as a result of uterine compression of the vena cava producing supine hypotensive syndrome has been mentioned as a cause of hypotension following the administration of conduction anesthesia; spinal, epidural, and caudal anesthesia produce sympathetic blockade, which increases the venous capacitance to even greater levels than that already produced by the effects of progesterone on the smooth muscle of the vessels.
The increased venous pressure below the level of the umbilicus also suggests the use of alternative paths for venous return to the heart, including the azygos and hemiazygos systems and the paravertebral and epidural veins. Such pathways, particularly the paravertebral and epidural pathways, may be responsible for the decrease in volume of the subarachnoid space. Because of this change, smaller amounts of anesthetic drugs are required for the induction of spinal anesthesia.29, 30 This is an important point, because excessively high levels of spinal anesthesia may be obtained with what would be a usual dosage in a nonpregnant woman.
Respiratory Changes
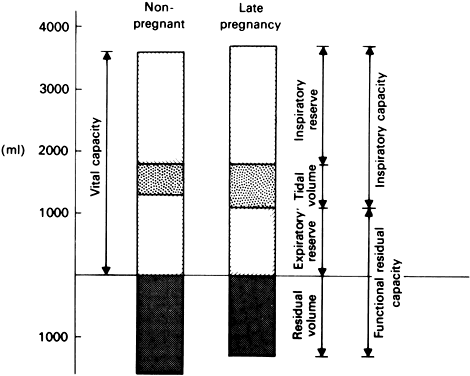
Along with the changes in the cardiovascular system, numerous changes occur in the pulmonary physiology that reflect changes in lung perfusion and the mechanisms of lung action. As would be expected from the increase in fetal requirements for oxygen as its mass increases, the changes in pulmonary physiology reflect the process of fetal growth. The changes that occur as a result of that growth include changes in the profile of the maternal chest and, obviously, in the level and movement of the diaphragms, which are mechanically interfered with by the presence of the gravid uterus and the displacement of the peritoneal contents. The circumference of the chest in pregnancy increases as the subcostal marginal angle is increased from approximately 70 degrees to more than 100 degrees near term, and the transverse diameter of the chest increases by nearly 2 cm. Although the respiratory rate in pregnancy is not appreciably increased, the pregnant woman experiences a relative hyperventilation during the course of pregnancy. The tidal volume, normally 450 ml/minute, is increased to 650 ml/minute, producing a greater gaseous exchange despite the same frequency of inspiration and expiration. These alterations in functional parameters are depicted in Figure 11.
|
The etiologic factors in the changes in the functional elements of respiration are obscure because the changes seen may be mediated by any number of stimuli present in pregnancy. Increased demand for oxygen for the growing fetus, the need for increasing heat transfer to the external environment, and the effects of progesterone may all play some role in the genesis of the ventilatory changes that take place in the course of normal gestation. The progesterone-related argument is especially cogent because it has recently been demonstrated in animal models that progesterone administered chronically induces hyperventilation in a dosage-related fashion. Factors related to hyperventilation, such as increased arterial pH and decreased PCO2, are also altered in the progesterone-treated animals in a manner similar to that seen in the pregnant human woman.31
Renal Changes
Increased cardiac output results in an increased volume of blood flow to the kidneys. Because of this increase in blood flow, the kidneys are perfused with larger amounts of blood, and therefore with larger amounts of solute and water volume, than usual; thus the kidneys do more filtering of the blood. This extra kidney filtering action reduces the values of some common laboratory blood tests; blood urea nitrogen levels are decreased markedly, as are creatinine levels.
Morphologic changes occur in the kidneys and collecting systems. Marked hydronephrosis and hydroureter often are present in normal pregnant women. In Fried's31 series of studies of 109 normal pregnant women, the incidence of hydronephrosis and hydroureter by ultrasonic measurements was 93.6%. In this study, the right side was more profoundly affected than the left. These new data support earlier work using radiographic techniques. These changes are thought to be due to the effects of progesterone or the mechanical obstruction of the ureters and renal pelves by the gravid uterus or markedly distended ovarian veins. The right-sided ureteral and renal dilatation may be produced by pressure on the right ureter at the level of the pelvic rim. On the left side, the ureter is protected and padded by the presence of the sigmoid colon. The increased volume of the collecting system and ureters is thought to predispose to upper urinary tract infection by increasing the urinary dead space and possibly the amount of reflux from the bladder to the ureters.
As the pregnancy progresses, the glomerular filtration rate increases, and it does so more significantly than can be explained by increased cardiac output alone. Atherton and Green32 have suggested that the mechanisms producing this disparate increase may be related to increased prolactin or dopamine secretion in the pregnant woman. In most studies using rats, progesterone has not had this effect. With cardiac output increasing by 30–35% and renal plasma flow increasing by more than 50%, the tubular reabsorptive capacity for several substances is exceeded. This produces conditions such as glycosuria and aminoaciduria.11 Although values vary, about 12–15% of gravid women have glycosuria at some time during their pregnancy, often shortly after the woman eats foods high in simple sugars. Lactose and other sugars may also appear in the urine in the course of the pregnancy.
SALT AND WATER
Despite the increase in sodium and water presented to the glomerulus in pregnancy, more reabsorption of both occurs than in the nongravid situation. This implies an increase in total body water of approximately 6–8 L, as well as an increase in sodium content of nearly 950 mEq/L. Among the hormones that are increased in the serum during the course of pregnancy, several have been noted to produce sodium loss. Among these are progesterone (a partial inhibitor of aldosterone), prostaglandins, and dopamine. Atherton and Green32 have suggested that several other circulating hormones may act as antinatriuretics. Among these, cortisol, deoxycortisone, estrogen, human placental lactogen, and prolactin may all have some role in opposing the salt-losing properties of progesterone. In addition, the decrease in serum protein concentration, the decrease in arterial pressure, and the effects of postural changes in blood flow in pregnancy may act together in determining the final amount of sodium reabsorption in the pregnant state. The changes in renal physiology in pregnancy, although manifold and presumably related to other changes in maternal physiology, remain somewhat obscure in terms of the underlying mechanisms in pregnancy and where these mechanisms act in the glomerulus and tubule.
THE GASTROINTESTINAL SYSTEM
Among the other profound changes that occur in the course of normal gestation are many alterations in the physiology of the gastrointestinal system. The most marked of these are probably related to the global effects of progesterone on the smooth muscle, which makes up the largest portion of the gut. Kumar,33 and more recently Gill and colleagues,34 have described the effects of progesterone on extrauterine smooth muscle. Progesterone has the effect of producing a dose-dependent and reversible inhibition of the electrical and mechanical events associated with the contraction of smooth muscle fibers. Lawson and colleagues35 demonstrated prolongation of gastrointestinal transit time in the second and third trimesters but showed no statistical differences from the nonpregnant state in the first trimester or postpartum.
Depression of muscular tone and action may explain the numerous alterations commonly seen in the pregnant woman; these alterations may have clinical implications. The increased occurrence of regurgitation and often troublesome pyrosis of pregnancy may be explained by decreased tone at the cardioesophageal junction, as well as by increased intraabdominal pressure. In animal models, Fisher and associates36 demonstrated decreased activity of the circular smooth muscle in the lower esophagus in response to both estrogen and progesterone. Decreased muscle action also explains the increased emptying time of the stomach and duodenum, as well as the delay in gallbladder emptying. Constipation may be related to this phenomenon as well, in that decreased colonic motility may provide the opportunity for increased water removal from the stool. This mechanism may aggravate hemorrhoids produced by the increased venous pressure below the level of the gravid uterus.
The major clinical implications of these processes in the gastrointestinal system are most relevant to the problems produced in the administration of general anesthesia. However they are mediated, the effects of pregnancy on the motility of the entire gastrointestinal system make virtually every pregnant woman who requires a general anesthetic during labor a high-risk patient for aspiration pneumonia. Aspiration of gastric contents during or after the administration of general anesthesia continues to be an important cause of maternal mortality.37 When general anesthesia is indicated, often under emergent conditions, endotracheal intubation should be performed
SKIN CHANGES
Pregnancy produces many changes in the skin that, although physiologic, may cause some concern in the pregnant woman. Among these changes is hyperpigmentation, which often occurs in the areolae, the perineal skin, the anal region, the inner thighs, and the linea nigra, which appears on the abdominal wall. Melasma or chloasma is a blotchy, sharply marginated hyperpigmentation that occurs on the face of dark-haired and dark-complexioned women. It is most often centrally distributed on the face.
Vascular “spiders” occur in about 67% of white patients and 11% of black patients by the time pregnancy has reached the third trimester. These lesions occur on the neck, throat, face, and arms. Most of these fade after the seventh postpartum week. Circulating estrogens in high levels are thought to be responsible for the appearance of these lesions.
Palmar erythema, which is also common in liver disease, hyperthyroidism, and collagen vascular diseases, appears commonly in pregnancy. Approximately 67% of white women and 33% of black women experience palmar erythema during the course of gestation. Striae are common among white women in late pregnancy but are less common in black and Asian women. There seems to be a familial tendency in the occurrence of these lesions. When they occur, they first appear during the sixth and seventh months of gestation on the abdominal skin; they then occur on the breasts, upper arms, lower back, buttocks, and thighs. The cause of these lesions is unclear, but they have been related to a combination of stretching of the skin and increased levels of corticosteroids and estrogen in pregnancy.38
SUMMARY
The pregnant state is characterized by a myriad of alterations in the normal physiology of the gravid woman. An understanding of some of the major mechanisms that produce these changes is helpful in the analysis of symptoms and problems that arise during the course of a normal gestation. When associated disease is present, understanding of these alterations becomes more important in that they must be distinguished from pathophysiologic changes wrought by the disease process. The interaction between disease and gestational physiology may make the appropriate diagnosis and management of the pregnant woman difficult. When a pregnant woman requires medical or surgical therapy, the consultative services of an obstetrician or clinician trained in the complexities of maternal physiology is absolutely critical to the proper management of clinical problems.
REFERENCES
Andersen HF, Johnson TR, Flora JD, Barclay ML: Gestational age assessment: II. Prediction from combined clinical observations. Am J Obstet Gynecol 140 (7): 770, 1981. |
|
Burwell CS, Strayhorn WD, Flinkinger D et al: Circulation during pregnancy. Arch Intern Med 62: 979, 1938. |
|
Johnson G, Blythe WB: Hemodynamic effects of arteriovenous shunts used for hemodialysis. Ann Surg 171 (5): 715, 1970. |
|
Romney SL, Reid DE, Metcalfe J, Burwell CS: Oxygen utilization by the human fetus in utero. Am J Obstet Gynecol 70: 791, 1955. |
|
Abrams R, Caton D, Curet LB et al: Fetal brain-maternal aorta temperature differences in sheep. Am J Physiol 217 (6): 1619, 1969. |
|
Macaulay JH, Randall NR, Bond K, Steer PJ: Continuous monitoring of fetal temperature by noninvasive probe and its relationship to maternal temperature, fetal heart rate and cord arterial oxygen and pH. Obstet Gynecol 79 (3): 469– 474, 1992. |
|
Csapo AI: The molecular basis of myometrial function and its disorders. In La Prophylaxie en Gynecologie et Obstetrique, p 693. Geneva, Georg, 1954. |
|
Sandiford I, Wheeler T: The basal metabolism before, during, and after pregnancy. J Biol Chem 62: 329, 1924. |
|
Rowe AC, Boyd WC: The metabolism in pregnancy: IX. The fetal influence on the basal rates. J Nutr 5: 551, 1932. |
|
Emerson K, Saxena BN, Poindexter EL et al: Caloric cost of normal pregnancy. Obstet Gynecol 40(6):786 1972. |
|
Heini A, Shutz Y, Jequier E: Twenty-four-hour energy expenditure in pregnant and nonpregnant women, measured in a whole-body indirect calorimeter, Am J Clin Nutr 55(6):1078–1085, 1992. |
|
Hytten FE, Leitch I: The Physiology of Human Pregnancy, 2nd ed. Oxford, Blackwell Scientific Publications, 1971. |
|
Rush D, Stein S, Susser M (eds): Prenatal nutritional, quasi, and natural experiments in the past decade: an overview. In: Diet in Pregnancy: A Randomized Controlled Trial of Nutritional Supplements, Chap 5, March of Dimes Birth Defects Foundation. Birth Defects: Original Article Series, XVI(3). New York, Alan R Liss, 1980. |
|
Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences: Nutrition During Pregnancy: Part I: Weight Gain, Part II: Nutrient Supplements. Washington, DC, National Academy Press, 1990. |
|
Pritchard JA, Scott DE: Iron demands in pregnancy. In Hallbert L, Harwerth HG, Vanotti A (eds): Iron Deficiency Pathogenesis. Clinical Aspects, Therapy. New York, Academic Press, 1970. |
|
Mullick S, Bagga OP, DuMullick V: Serum lipid studies in pregnancy. Am J Obstet Gynecol 89: 766, 1964. |
|
Reece EA, Homko C, Wixnitzer A: Metabolic changes in diabetic and nondiabetic subjects during pregnancy. Obstet Gynecol Surv 49 (1): 64– 71, 1994. |
|
Bleicher SJ, O'Sullivan JB, Freinkel N: Carbohydrate metabolism in pregnancy: V. The interrelations of glucose, insulin, and free fatty acids in late pregnancy and postpartum. N Engl J Med 271 (17): 866, 1964. |
|
Felig P: Body fuel metabolism and diabetes mellitus in pregnancy. Med Clin North Am 61 (1): 43, 1977. |
|
Lindhard J: Uber das minutevolum des herzens bei ruhe und bei meskelarbeit. Arch Ges Physiol 161: 223, 1915. |
|
Mathew JP, Fleisher LA, Rhinehouse JA et al: ST segment depression during labor and delivery. Anesthesiology 77 (4): 635-641, 1992. |
|
Veille JC, Kitzman DW, Bacevice AE: Effects of pregnancy on the electrocardiogram in healthy subjects during strenuous exercise. Am J Obstet Gynecol 175: 1360–1364, 1996 |
|
Clapp JF III, Capeless E: Cardiovascular function before, during and after the first and subsequent pregnancies. Am J Cardiol 80 (11): 1469–1473, 1997. |
|
O'Connor RL, Severino FB: Cardiopulmonary arrest in the pregnant patient: a report of a successful resuscitation. J Clin Anesth 6 (1): 66–68, 1994. |
|
DePace NL, Betesh JS, Kotler MN: Postmortem cesarean section with recovery of both mother and offspring. JAMA 248: 971–973, 1982. |
|
Griffin JFT, Beck I: A longitudinal study of leukocyte numbers and mitogenesis during the last ten weeks of human pregnancy. J Reprod Immunol 5: 239, 1983. |
|
Longo LD: Maternal blood volume and cardiac output during pregnancy: A hypothesis of endocrinologic control. Am J Physiol 245: R720, 1983. |
|
Assali MS, Prystowsky H: Studies on autonomic blockade. I. Comparison between the effects of tetraethylammonium chloride (TEAC) and high selective spinal anesthesia on the blood pressure of normal and toxemic pregnancy. J Clin Invest 29: 1367, 1950. |
|
Batson OV: The function of the vertebral veins and their role in the spread of metastasis. Ann Surg 112: 138, 1940. |
|
Hytten F, Chamberlain G: Clinical Physiology in Obstetrics, p 85. Boston, Blackwell Scientific, 1980. |
|
Fried AM: Hydronephrosis of pregnancy: Ultrasonographic study and classification of asymptomatic women. Am J Obstet Gynecol 135 (8): 1066, 1979. |
|
Altherton JC, Green R: Renal function in pregnancy. Clin Sci 65: 449, 1983. |
|
Kumar D: In vitro inhibitory effect of progesterone on extrauterine smooth muscle. Am J Obstet Gynecol 84 (10): 1300, 1962. |
|
Gill RC, Bowes KL, Kingma YJ: Effect of progesterone on canine colonic smooth muscle. Gastroenterology 88 (6): 1941, 1985. |
|
Lawson M, Kern F, Everson GT: Gastrointestinal transit time in human pregnancy, prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology 89: 996–999, 1985. |
|
Fisher RS, Roberts GS, Grabowski CJ, Cohen S: Inhibition of lower esophageal sphincter circular muscle by female sex hormones. Am J Physiol 243 (3): E243, 1978. |
|
Crawford JS: Maternal mortality associated with anesthesia. Lancet 2: 918, 1972. |
|
Wong RC, Ellis CN: Physiologic skin changes in pregnancy. J Am Acad Dermatol 10 (6): 929, 1984. |