Preterm Premature Rupture of the Membranes
Authors
INTRODUCTION
Rupture of the fetal membranes is an integral part of the normal and abnormal parturition process. Rupture of the membranes before the onset of contractions (premature rupture of the membranes: PROM) complicates 10% of pregnancies, with 3% of pregnant women having PROM before 37 weeks' gestation. Preterm PROM (pPROM) is more likely to occur in populations of lower socioeconomic status and complicates one-quarter to one-third of preterm births.1, 2 As such, PROM and pPROM complicate more than 400,000 and 120,000 pregnancies, respectively, in the United States each year.
Unfortunately, pPROM is generally followed by delivery soon after membrane rupture. Additionally, pPROM is associated with an increased risk of maternal and infant infection. Because of this, the major impacts of pPROM on pregnancy are those associated with preterm birth and infectious complications. Respiratory distress syndrome (RDS) is the most common complication after preterm PROM at any gestation. Serious perinatal morbidities that may lead to long-term sequelae or death are common when PROM leads to preterm birth remote from term. Acute neonatal morbidities such as necrotizing enterocolitis, intraventricular hemorrhage (IVH), and sepsis commonly complicate early preterm birth but are relatively uncommon near term. It has been established that preterm birth is a significant risk factor for long-term sequelae such as chronic lung disease, neurosensory impairment, cerebral palsy and developmental delay. Perinatal infection has also been linked to neurologic complications. Cerebral palsy and periventricular leukomalacia have been linked to amnionitis, which is commonly seen after pPROM.3 Elevated amniotic fluid cytokines and fetal systemic inflammation have also been associated with pPROM and with periventricular leukomalacia.4, 5, 6 However, despite these known associations, it has not been shown that delivery immediately after pPROM will prevent these adverse outcomes.
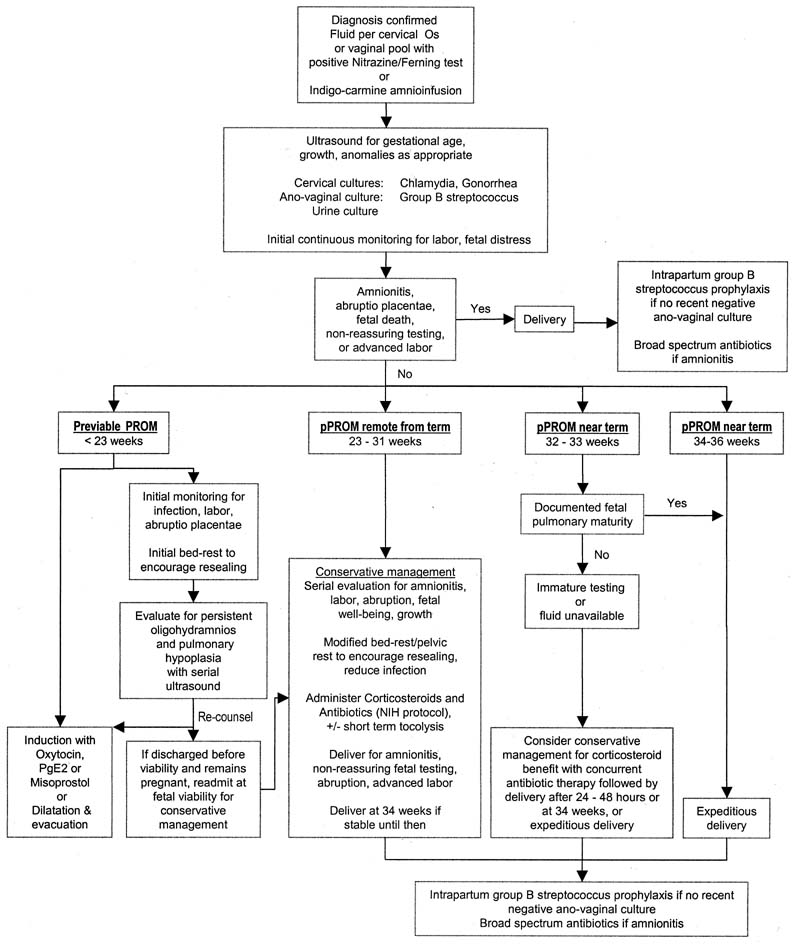
Although the diagnosis of membrane rupture can usually be made unequivocally, the management of women with pPROM is sometimes controversial. This chapter addresses the causes, diagnosis, and clinical course of preterm PROM, as well as the risks associated with expectant and active management of pPROM. A potential algorithm for assessment and treatment of women presenting with suspected membrane rupture preterm is offered (Fig. 1). In addition, available adjunctive therapies (e.g., antibiotics, corticosteroids, tocolysis) are reviewed, and those circumstances in which the risks and management of previable PROM may be specifically altered (previable PROM, twin pregnancy, cervical cerclage, herpes infections) are be discussed.
DEFINITIONS
PROM is defined as spontaneous rupture of the membranes before the onset of labor; pPROM includes those women presenting with PROM before 37 weeks 0 days' gestation. Midtrimester PROM applies to those with premature membrane rupture at 14–26 weeks' gestation. Although the term midtrimester PROM has been consistent with previable PROM in the past,7 this is no longer the case. Currently, it is more clinically relevant to differentiate previable PROM, which occurs before the limit of viability (<23 weeks' gestation),8preterm PROM remote from term (from viability to approximately 31 weeks' gestation), and preterm PROM near term (approximately 32–36 weeks' gestation). When previable PROM occurs, immediate delivery will lead to neonatal death. Immediate delivery after pPROM remote from term is associated with a significant risk of perinatal morbidity and mortality and this risk decreases with advancing gestational age at birth. When preterm PROM occurs near term, expeditious delivery will likely lead to survival; short-term morbidity is unlikely if fetal pulmonary maturity has occurred, and long-term morbidity is uncommon. Conservative (expectant) management of pPROM includes therapy directed toward extending the pregnancy to improve neonatal outcome and should not be confused with nonintervention.
ETIOLOGY OF pPROM
The fetal (amniochorionic) membrane results from fusion of the amnion and chorion concomitant with obliteration of the chorionic cavity in the first trimester. This complex is fused to the decidua capsularis, which is juxtaposed to the decidua parietalis through the remainder of pregnancy. Membrane rupture usually occurs near the internal cervical os, but it may occur remote from that site.9, 10
A number of mechanisms have been proposed for PROM.11, 12, 13 These include intrinsic membrane weakness, mechanical stress, and ascending infection among others. Factors that could cause weakening of the fetal membranes and have been associated with PROM include local inflammation and infection, poor maternal nutrition, maternal smoking, and collagen deficiency syndromes. Choriodecidual inflammation plays an important role in pPROM, particularly when membrane rupture occurs remote from term.14 The lower genital tract is a potential reservoir for bacteria that may ascend through the cervical canal and cause localized inflammation. Bacteria and maternal neutrophils are able to produce a number of proteolytic enzymes (e.g., collagenase, elastase, gelatinase) that can cause local weakening of the membranes. Subsequent prostaglandin production resulting from localized inflammation can lead to occult contractions and increased shearing stress at the internal cervical os. Factors associated with mechanical distention of the membrane near the internal os include polyhydramnios, twin gestation, and incompetent cervix. Trauma may be associated with pPROM through an acute increase in intra-amniotic pressure or through the production of occult contractions. In many cases, the cause of premature membrane rupture remains unknown.
PREDICTION OF pPROM
Because the clinical course of pPROM is often unalterable once membrane rupture has occurred, it would be beneficial to identify women at risk and prevent membrane rupture from occurring. Women with a previous early preterm birth (23–27 weeks) have a 25% risk for preterm birth in a subsequent gestation.15 Those with a previous history of pPROM have a 3.3-fold increased risk of preterm birth caused by pPROM (13.5 vs. 4.1%, p < 0.01) and a 14-fold higher risk of pPROM before 28 weeks (1.8 vs. 0.13%, p < 0.01) in a subsequent pregnancy. While a number of socioeconomic, demographic, and clinical risk factors for pPROM have been identified, these tend to have low predictive values and identify a large number of women who would ultimately deliver at term despite their increased risk. Though bacterial vaginosis has been established as being associated with preterm birth and pPROM, it is unclear if this is a cause–effect relationship or if bacterial vaginosis only identifies those at risk for infection and inflammation.16, 17 A short cervix (<25 mm) by transvaginal ultrasonography has been associated with pPROM in both nulliparas and multiparas, and a positive fetal fibronectin screen has also been associated with pPROM in multiparas.18 Nulliparas with a positive cervicovaginal fetal fibronectin and a short cervix have been found to have a one-in-six risk (16.7%) of preterm birth caused by pPROM, whereas multiparas with a previous history, a short cervix, and a positive fetal fibronectin have a 31-fold higher risk of PROM with delivery before 35 weeks than those without risk factors (25 vs. 0.8%, p = 0.001). Despite our increasing ability to identify those at increased risk for pPROM, such expensive and inconvenient testing will only identify a small fraction of women ultimately delivering preterm.
Progesterone therapy, given to women with a prior spontaneous preterm birth due to preterm labor or PROM, has been shown to reduce the risk of recurrent preterm birth.19, 20 A recent study provided some evidence to suggest that vitamin C supplementation might reduce the risk of subsequent PROM (7.6 vs. 24.5%, p = 0.02).21 However, review of studies in which vitamin C was given alone or in combination with other supplements to women without a prior preterm birth suggests no reduction, and a possible trend towards increased preterm birth with treatment (relative risk 1.38, 95% confidence interval: 1.04–1.82).22 Because of this, vitamin C supplementation to prevent preterm birth due to PROM is not recommended until there is solid evidence to suggest benefit.
DIAGNOSIS OF PPROM
More than 90% of patients presenting with pPROM will provide a clinical history and findings of fluid leakage from the vagina to allow the unequivocal diagnosis of membrane rupture. Some will have preceding symptoms, including a change in vaginal discharge and pelvic pressure, or even a prior history of preterm labor.23 Occasionally, patients will present with an equivocal history of perineal dampness, intermittent leakage, or an isolated loss of fluid. The differential diagnosis of pPROM includes increased physiologic secretions of pregnancy, pathologic discharge associated with vaginitis or cervicitis, urinary incontinence that may be associated with lower urinary tract infection, vesicovaginal or rectovaginal fistula in women with a history of previous delivery, urogenital tract trauma or surgery, and Crohn's disease. Occasionally, excess fluid may result from exogenous sources, such as semen and vaginal douches.
Initial evaluation of the patient presenting with pPROM includes determination of the duration, amount, and persistence of fluid leakage. In the absence of a classic history, leakage associated with symptoms that increase abdominal pressure (e.g., coughing, straining) would suggest urinary incontinence but can also be associated with pPROM. If the clinical history is not clear, patients should be questioned regarding recent vaginal or cervical infection, recent sexual activity, douching, previous pelvic surgery, and inflammatory disease.
The diagnosis of membrane rupture is typically made through visual examination of perineum with detection of the typical pungent odor, or by sterile speculum examination of the vagina and cervix. At speculum examination, the diagnosis is generally confirmed by visualization of fluid passing from the endocervical canal into the vagina, or by the presence of alkaline fluid (pH > 7.0 confirmed by a phenaphthazine "Nitrazine" paper) and a ferning pattern (multiple branches of crystalized dried amniotic fluid on microscopic examination) in a sample obtained from the vaginal sidewall with attention to avoidance of cervical mucous which can cause false-positive results.
Blood or semen contamination, alkaline antiseptics, and bacterial vaginosis can increase the vaginal pH, leading to a false-positive Nitrazine test. Women with chronic membrane rupture or severe oligohydramnios may not demonstrate the classic physical findings. In the absence of a visible pool or positive Nitrazine paper and ferning tests, the patient can be retested after prolonged recumbency. If amniotic fluid leakage cannot be confirmed on speculum examination, membrane rupture can be confirmed by collection of fluid using a perineal pad or a modified vaginal pouch24 or by ultrasound-guided amniocentesis with injection of indigo carmine dye and observation for vaginal leakage. Oligohydramnios demonstrated by ultrasound may be helpful but can be confounded by other causes, including renal agenesis, renal dysplasia, and intrauterine growth retardation. Other adjunctive tests, including Nile blue sulfate staining of vaginal smears, and vaginal alpha-fetoprotein screening have been suggested as adjuncts to assist in the diagnosis of membrane rupture, but these have either not proved clinically practical or have not been studied for their predictive value when the diagnosis remains unclear after traditional testing. While a positive cervicovaginal screen for fetal fibronectin may be a marker for pPROM, the impact of prolonged membrane rupture on the fibronectin result has not been determined, and a positive test may reflect disruption of the decidua rather than membrane rupture.
CLINICAL COURSE AFTER PPROM
The natural history of pPROM is one of short latency followed by labor and delivery. While median latency does increase with earlier membrane rupture, this is partially artifactual. Those who present with PROM remote from term have the potential to continue their pregnancies for up to 16–26 weeks if conservative management is pursued, whereas those with membrane rupture near term can not anticipate such an extended latency as labor will be induced within weeks if it does not occur spontaneously. Overall, approximately 50% of women presenting with pPROM will deliver within 48 hours of membrane rupture and approximately 80% will deliver within 1 week in the absence of adjunctive treatments to prolong pregnancy. The incidence of chorioamnionitis is approximately 15–20% overall, and 40–60% if membrane rupture occurs in the near the limit of viability.25, 26 Chorioamnionitis subsequent to membrane rupture tends to occur early in the course of PROM, with those having chorioamnionitis also having a shorter latency, on average.27, 28, 29 Abruptio placentae may lead to PROM or occur secondarily. Overall, women with pPROM have a 4–12% risk of abruptio placentae.30, 31, 32, 33 Fetal demise is anticipated in 1–2% of conservatively managed pregnancies after PROM. Amniotic fluid leakage will cease in 2.6–11% of cases (9.7–21% after midtrimester PROM).27, 34, 35 In 1989, Gold and associates reported on seven patients with amniotic fluid leakage subsequent to genetic amniocentesis (1.2% incidence).36 Leakage resolved in all seven patients within 1 week of PROM (100%) and was associated with uncomplicated term deliveries in six of seven (85.7%). In a similar study, fluid leakage stopped in nearly all cases though it sometimes took significant time for a normal fluid volume to reaccmulate (8–51 days).37
INITIAL EVALUATION AFTER PPROM
When the diagnosis of membrane rupture is considered at speculum examination, visual assessment of the cervix should be performed to evaluate dilatation and effacement.38 Until such time, as the diagnosis of membrane rupture is excluded, it is prudent to avoid digital cervical examination as this may decrease latency and increase infectious morbidity without adding substantial information to that obtained by careful visualization.39, 40 Cervical cultures for Neisseria gonorrhoeae and Chlamydia trachomatis can also be obtained from the endocervix at the time of speculum examination if these have not been recently obtained or there is a clinical suspicion of infection. Anovaginal cultures for group B streptococcus should be obtained if these have not been recently performed. Because the vagina is colonized by a large variety of aerobic and anaerobic bacteria and contains numerous maternal leukocytes, vaginally collected amniotic fluid cultures are not representative of the intrauterine environment and should not be used to predict intrauterine infection. The initial speculum examination provides the best opportunity for collection of amniotic fluid for assessment of fetal pulmonary maturity, if desired. With increasing latency, prolonged leakage will often lead to oligohydramnios and an inadequate vaginal pool fluid for laboratory testing.
When a woman presents with pPROM, it is important to establish gestational age as accurately as possible. Gestational age should be determined based on menstrual dating if more secure criteria are not available (e.g., in vitro fertilization, artificial insemination, or ovulation induction), and the earliest available ultrasound examination. Although ultrasound is more difficult in the presence of oligohydramnios, evidence suggests that estimation of fetal weight and gestational age is not affected adversely by oligohydramnios.41 Because dolichocephaly (narrow biparietal diameter compared with occipitofrontal diameter) is more commonly seen with oligohydramnios and breech presentation, it is prudent to use the head circumference rather than biparietal diameter to evaluate gestational age and estimate fetal weight in this circumstance. Fetal abnormalities that may be associated with oligohydramnios or polyhydramnios (e.g., hydrops fetalis, gastrointestinal obstruction, oropharyngeal and neck masses, severe hydrocephalus, renal agenesis or dysplasia, urinary tract obstruction) should be considered and fetal presentation assessed. If fetal malpresentation coexists with significant cervical dilatation, there is an increased risk of umbilical cord prolapse, which may increase the risk of fetal loss. A low residual amniotic fluid volume has been associated with shorter latency, but this finding is inadequately discriminative to alter management.42, 43
Fetal heart rate monitoring should be performed to establish fetal well-being. Fetal tachycardia may reflect fetal compromise or intrauterine infection. Variable-type fetal heart rate decelerations caused by umbilical cord compression are identified in up to 75% of pregnancies complicated by pPROM.44 Maternal uterine activity should be monitored to identify early labor.
Because pPROM is highly associated with infection, maternal and fetal evaluation should be performed with this in mind. A history should be elicited, noting fever, recent infection, uterine tenderness, abnormal vaginal discharge, dysuria, change in urinary patterns, and flank pain. At the time of physical examination, attention should be paid to maternal tachycardia, fever, uterine tenderness, and abnormal cervical discharge. Urinary tract infection should be excluded. Because of the high number of leukocytes and the presence of numerous microorganisms capable of causing urinary tract infection in the vagina, a catheterized urine specimen should be considered for women with membrane rupture. Maternal white blood cell count determination is not always helpful in the evaluation of infection because of the tendency for increased levels in normal pregnancy. However, demonstration of a increasing blood leukocyte count as compared with a baseline level on admission, or a single value equal to or greater than 18,000, could be considered supportive evidence of infection if the clinical picture is unclear. For this reason it is reasonable to obtain a maternal blood leukocyte count on admission for future comparison.
MANAGEMENT of PPROM
General Considerations
Because the frequency of perinatal complications decreases with increasing gestational age at membrane rupture and at delivery, a gestational age-based approach to the management of pPROM is useful. While there is a potential to reduce infant morbidity when conservative management of pPROM is undertaken for the immature fetus, this benefit can only occur through a reduction of gestational age dependent morbidity with extended pregnancy prolongation and/or antenatal corticosteroid administration, or through prevention of perinatal infection. In general, the risk of neonatal infection increases if maternal chorioamnionitis occurs during conservative management. Treatment should be based on individual and ongoing assessment of the predicted maternal, fetal, and neonatal risks for complications with either conservative management or expeditious delivery. If conservative management of pPROM is attempted, the patient should be admitted to a facility capable of providing emergent care for placental abruption, fetal malpresentation in labor, fetal distress caused by umbilical cord compression, and/or in utero infection. The facility should also be capable of providing 24-hour neonatal resuscitation and intensive care, because conservative management should generally be performed only in pregnancies at significant risk for neonatal morbidity and mortality. When appropriate, patient transfer should be undertaken early in the course of management to avoid emergent transfer once complications arise.
pPROM Near Term (at 32–36 Weeks' Gestation)
While infants born near term are more likely to suffer complications than term babies,45 conservatively managed pPROM at this advanced gestational age is not without risks, and there is a low risk of severe acute morbidity and mortality with expeditious delivery at 34–36 weeks' gestation. Antenatal corticosteroids are generally not administered to these women at this gestation to accelerate fetal pulmonary maturity. Conservative management at 34–36 weeks' gestation is associated with an eight-fold increase in amnionitis (16 vs. 2%, p = 0.001) and prolonged maternal hospitalization (5.2 vs. 2.6 days, p = 0.006) without a significant reduction in perinatal morbidity related to prematurity.46 Thus, the woman with pPROM at 34–36 weeks is generally best served by expeditious delivery. When pPROM occurs at 32–33 weeks, fetal pulmonary maturity assessment from a vaginal pool specimen should be obtained if feasible (see later). Amniocentesis by a skilled clinician should be considered if there is inadequate vaginal fluid for pulmonary maturity testing. The infant with documented lung maturity at 32–36 weeks' gestation is at low risk for major morbidities related to preterm birth.47 Alternatively, conservative management of pPROM near term will increase latency only briefly (36 vs. 14 hours, p < 0.001), increases the risk of amnionitis (27.7 vs. 10.9%, p = 0.06), and places the fetus at risk for occult cord compression with prolonged oligohydramnios. Because there is little neonatal benefit to be gained by brief pregnancy prolongation when fetal pulmonary maturity is evident with pPROM after 32 weeks, and the risk of amnionitis increases with conservative management, expeditious delivery is recommended.
When fetal pulmonary maturity assessment is unavailable or if fetal pulmonary immaturity is suspected through testing after pPROM at 32 or 33 weeks' gestation, there may be a significant risk of complications related to pulmonary immaturity and other gestational age-dependent morbidity. Unfortunately, there are no studies involving only this specific population on which to base management. Cox and coinvestigators evaluated 129 women undergoing immediate delivery or conservative management after pPROM at 30–33 weeks and 6 days' gestation,48 and found only a brief increase in latency (59 vs. 100% delivered at 48 hours, p < 0.001), a significant increase in amnionitis (15 vs. 2%, p = 0.009), and no reduction in infant morbidity with conservative management. This population had a significant risk of RDS (35%), one stillbirth caused by suspected cord compression with conservative management and three neonatal deaths (2 sepsis, 1 pulmonary hypoplasia) with immediate delivery. However, antenatal steroids were not administered to reduce the risk of RDS, and this study was performed before antibiotics for pregnancy prolongation and group B streptococcus prophylaxis were routinely administered. While this study suggested that immediate delivery might reduce fetal exposure to intrauterine infection and avoid loss caused by cord compression, it confirms the risk for neonatal morbidity in the infant delivered at 30–33 weeks. If amniotic fluid is unavailable for testing or immature at 32 or 33 weeks, conservative treatment with close fetal monitoring, adjunctive antibiotic therapy, and induction of fetal pulmonary maturation should be considered. Alternatively, if these measures to reduce gestational age-dependent and infectious morbidity are not attempted, such patients may be better served by expeditious delivery.
It is important to reiterate that all three prospective studies evaluating pPROM near term have found significant increases in perinatal infection and only brief pregnancy prolongation with conservative management. As such, expeditious delivery should be considered unless the fetus is considered to be at significant risk for gestational age-dependent morbidity and efforts to suppress infection and enhance fetal maturity are undertaken.
pPROM Remote from Term (at 23–31 Weeks' Gestation)
Delivery before 32 weeks' gestation is associated with a significant risk of neonatal morbidity and death. Because of this, attempts to prolong pregnancy and reduce gestational age-dependent morbidity are generally appropriate if there is no maternal indication for immediate delivery. In the presence of active labor, vaginal bleeding, intrauterine infection, or evidence of fetal compromise, delivery is required. The decision as to whether expectant management should be pursued is based on the potential benefit of delaying delivery versus the concurrent maternal and fetal risks of such therapy, particularly the increased risk of intrauterine infection with conservative management.
Generally, hospitalization for bed rest and pelvic rest is indicated. Recognizing that latency is frequently brief, that intrauterine infection and fetal infection may occur and that the fetus is at risk for umbilical cord compression, ongoing surveillance of both mother and fetus is necessary. Attention should be paid to findings that would require delivery for either fetal or maternal indication. Evaluation of the mother should include observation for infection, abruptio placentae, and labor. Symptoms including uterine contractions, pelvic pressure, lower abdominal or back pain, and bleeding, as well as the mother's impression of fetal activity, should be elicited. Periodic uterine contraction monitoring of symptomatic and asymptomatic women will allow early identification of labor so that fetal evaluation and appropriate interventions be initiated. Evaluation of the fetus should include serial determination of fetal well-being based on maternal recollection of fetal activity, and fetal evaluation by fetal heart rate monitoring and/or biophysical testing. In addition, if delivery is adequately delayed, evaluation of continued fetal growth should be considered.
The optimal treatment for those reaching an advanced gestational age after conservative management of pPROM remote from term is not known. It is reasonable to consider labor induction when the pregnancy reaches 34 weeks' gestation. It may also be reasonable to assess fetal pulmonary maturity at any time from 32 to 34 weeks and consider delivery once maturity has been documented. However, this approach has not been studied.
ADJUNCTS TO MANAGEMENT OF PPROM
Assessment of Fetal Pulmonary Maturity From Vaginal Pool Specimens
Fetal pulmonary maturity testing is helpful when pPROM occurs near term. Clinical studies suggest a useful role for vaginally collected amniotic fluid specimens for assessment of fetal pulmonary maturity.49 Neither lecithin nor sphingomyelin have been isolated in significant amounts from vaginal lavage fluid when the membranes are intact.50 This group found the L/S ratio to be unaffected by vaginal cervical saline washes, and others have found no differences in the L/S ratio or lecithin between vaginal and amniocentesis specimens.51 Golde found the L/S ratio, PG, and PI levels to be similar when collected directly from the vagina or collected over a period of hours from perineal pads.52 Shaver and coworkers found a close correlation between L/S ratios obtained from vaginal pool and amniocentesis and an 89% concordance regarding fetal pulmonary maturity.53 The mean L/S ratio from vaginal pool was not significantly higher than from amniocentesis (2.56 vs. 2.3:1, p = 0.06). In that study, all patients with a positive amniocentesis result also had PG present in the vaginal pool. In an evaluation of 447 women with PROM, PG determinations from vaginal fluid collected via perineal pads were found to be highly predictive of fetal pulmonary maturity (0.8%) and similarly predictive of pulmonary immaturity (34%) to specimens collected by amniocentesis.54 No cases of RDS occurred after a mature L/S ratio, PG, or TDx FLM result greater than 50 mg/g from vaginal fluid collection.55 A study of 153 women with pPROM at 30–36 weeks found a mature S/A ratio (55 mg/g or more) to carry a predictive value of 98%, whereas 24% of infants with an immature result less than 40 mg/g had RDS.56
Nonpulmonary phospholipids in blood may alter the results of nonspecific fetal pulmonary maturity tests for pulmonary phospholipids. Because maternal serum has an intrinsic L/S ratio of 1.3 to 1.5:1, significant blood contamination could falsely elevate an immature result and lower a mature result;57 however, a mature L/S ratio result should be reassuring, as blood contamination would not be expected to increase to an immature result to a mature level. Because red blood cell phospholipids may interfere with the TDx FLM result, some elect not to perform a TDx FLM II assay if there is blood in the specimen.58 Meconium contamination increases the L/S ratio by 0.1 to 0.2:1 in preterm infants and by as much as 0.5:1 after 35 weeks.59 The PG test is not affected by blood or meconium contamination. Practically, if significant blood or meconium is present in a vaginal pool specimen, serious consideration should be given to expeditious delivery for fetal indication, rather than conservative management.
Amniocentesis
Amniocentesis may be particularly helpful in the setting of pPROM, assisting in the diagnosis of membrane rupture, evaluation of fetal pulmonary maturity, and assessment of intrauterine infection, when appropriate. Amniocentesis and amnioinfusion should be performed under ultrasound guidance. Particular attention should be paid to avoid the umbilical cord, which may be confused with an amniotic fluid pocket in the case of severe oligohydramnios.
Amnioinfusion of indigo carmine dye will effectively confirm or rule out the diagnosis of membrane rupture if noninvasive testing is equivocal. One technique is to infuse 10 mL of sterile saline mixed with 0.5–1 mL of indigo carmine dye into the amniotic fluid using a 22-gauge spinal needle under direct ultrasound visualization. The absence of blue discoloration on a perineal pad worn for several hours effectively rules out the diagnosis of membrane rupture. Methylene blue should not be injected for this purpose because it can exacerbate methemoglobinemia and anemia in individuals with glucose-6-phosphotase deficiency, among other potential side-effects.
Amniocentesis may be performed to obtain an adequate fluid volume for fetal pulmonary maturity testing if there is inadequate vaginal fluid for collection. There is no reason to believe that amniocentesis fluid after pPROM will yield less predictive results than that obtained at amniocentesis with intact membranes.
The diagnosis of intrauterine infection is not always clear. The combination of fever (temperature exceeding 38°C or 100.4°F) with uterine tenderness and/or maternal or fetal tachycardia are suggestive of chorioamnionitis in the absence of another source of infection. However, maternal fever is not always present early in the course of infection. While a rising maternal blood leukocyte count may be useful in the presence of suspicious clinical findings, the count may be artificially elevated if antenatal corticosteroids have been administered within 5–7 days. Should maternal symptoms, physical findings, and/or hematological findings be equivocal, amniocentesis may be useful in evaluating for intra-amniotic infection. An amniotic fluid glucose concentration less than 16–20 mg/dl (sensitivity and specificity 80–90% for positive culture) , a positive Gram stain (sensitivity 36–80%, specificity 80–97% for positive culture), and elevated interleukin levels (not generally available for clinical care) have been associated with increased early delivery and perinatal infectious morbidity.60, 61, 62, 63 The presence of leukocytes in the amniotic fluid after PROM is not well correlated with intrauterine infection. While a positive amniotic fluid culture is associated with brief latency and amnionitis, the results of this testing are not generally available in time to assist in management decisions. A positive preliminary amniotic fluid result should be correlated to clinical findings before action is pursued. Definitive intervention should not be delayed for a test result if the clinical diagnosis of infection is clear. A recent study has suggested that a low vaginal fluid glucose level below 5 mg/dl is 74.2% accurate in predicting amniotic fluid culture results.64 If additional studies confirm this preliminary finding, this simple and non-invasive test could be an appealing adjunct to clinical care.
Assessment of Fetal Well-Being
MATERNAL PERCEPTION OF FETAL ACTIVITY
Fetal testing may be useful in the identification of fetal compromise, umbilical cord compression, fetal infection, or labor. Fetal activity does not decrease significantly after membrane rupture.65 The simplest form of fetal testing is maternal observation of fetal activity. A maternal perception of decreasing fetal activity is helpful in identifying patients who require more intensive or prolonged fetal evaluation.
FETAL HEART RATE TESTING
Fetal heart rate monitoring, including the nonstress test, is particularly well-suited for the evaluation of pregnancies complicated by pPROM. Such testing may identify umbilical cord compression by the presence of variable-type heart rate decelerations, fetal compromise by the presence of a nonreactive nonstress test, fetal tachycardia and/or recurrent late-onset decelerations, and infection by the presence of fetal tachycardia and/or a lack of fetal heart rate reactivity.60, 66, 67 Such testing also offers the opportunity to evaluate for the presence of uterine contractions by external tocodynamometry. Occasionally, recurrent variable decelerations will be identified before the identification of symptomatic uterine contractions.44, 67, 68
BIOPHYSICAL PROFILE TESTING
Fetal biophysical activity, not only is affected by fetal compromise, but also is affected by intrauterine infection. A number of studies have demonstrated decreasing fetal breathing activity, movement, and tone before maternal and neonatal infection.69, 70, 71, 72 Other studies have found the relationship between fetal biophysical activity and intrauterine infection to be weak.73, 74 Because fetal nonstress testing has a high false-positive rate, biophysical profile testing is a useful adjunct that may allow continued expectant management in the face of a nonreactive nonstress test. Such testing is particularly useful remote from term, when fetal heart rate accelerations are less likely to be seen.
Antibiotic Therapy
GROUP B STREPTOCOCCUS PROPHYLAXIS
Intrapartum antibiotic prophylaxis against group B streptococcus is indicated for women with pPROM who are known group B streptococcus carriers or whose carrier status is unknown.75 Intrapartum group B streptococcus prophylaxis with intravenous penicillin as a 5-million unit initial bolus followed by 2.5 million units every 4 hours or ampicillin 2 g followed by 1 gram intravenously every 4 hours (erythromycin, 500 mg intravenous every 6 hours or clindamycin, 900 mg intravenous every 8 hours, if penicillin-allergic) should be administered during labor or before cesarean section after pPROM unless there is a negative anovaginal culture obtained within 5 weeks of delivery. Because of increasing resistance of group B streptococcus to erythromycin and clindamycin, ACOG and the Centers for Disease Control have recommended intravenous treatment based on culture sensitivities, cefazolin or vancomycin therapy for those with documented penicillin allergy. Vancomycin should be considered for those with a significant likelihood of anaphylaxis or compromise with penicillin exposure, unless sensitivity to erythromycin or clindamycin has been demonstrated.76, 77, 78, 79 Because latency is generally short and delivery may occur rapidly in the face of nonreassuring fetal testing, it may be prudent to initiate group B streptococcus therapy pending the receipt of a negative culture. Group B streptococcus carriers who are treated and have prolonged latency may have persistent vaginal colonization despite treatment or may become recolonized from the rectum. Therefore, women with group B streptococcus colonization on admission should be administered prophylaxis again in labor.
THERAPEUTIC ANTIBIOTICS
Antibiotic therapy should be administered during conservative management of pPROM remote from term. Such treatment has been shown to prolong pregnancy and offer the opportunity for reduced neonatal infectious and gestational age-dependent morbidity. More than two dozen randomized trials in this regard are addressed in detail elsewhere.80, 81, 82, 83, 84 These studies are generally small and use varied approaches to antibiotic, corticosteroid, and tocolytic administration, making direct comparison difficult. Two large multicenter clinical trials have adequate power to evaluate the usefulness of adjunctive antibiotics after pPROM in reducing infant morbidity and approached this issue differently.85, 86 The combination of these studies, and specific details from individual trials, offer valuable insights.
The National Institutes of Child Health and Human Development Maternal Fetal Medicine Units (NICHD-MFMU) research network studied women with pPROM from 24 to 32 weeks and 0 days' gestation.85 This study was designed to determine if antibiotic therapy would reduce the number of infants who were acutely ill after birth. These investigators gave initial aggressive intravenous therapy (48 hours) with ampicillin (2 g intravenous every 6 hours) and erythromycin (250 mg intravenous every 6 hours), followed by limited duration oral therapy (5 days) with amoxicillin (250 mg orally every 8 hours) and enteric-coated erythromycin base (333 mg orally every 8 hours). These agents offered the benefits of broad-spectrum antimicrobial coverage and demonstrated safety in pregnancy. The duration of therapy was limited to minimize selection of resistant bacteria that could be more difficult to treat should neonatal infection occur. All group B streptococcus carriers were treated with ampicillin for 1 week and then again in labor, regardless of whether they were assigned to antibiotic or placebo therapy. Antibiotic treatment improved neonatal health by reducing the number of babies with one or more major infant morbidity (composite morbidity: death, RDS, early sepsis, severe IVH, severe necrotizing enterocolitis) from 53 to 44% (p < 0.05). Antibiotic therapy also led to significant reductions in individual infant morbidities including RDS (40.5 vs. 48.7%), stage 3 or 4 necrotizing enterocolitis (2.3 vs. 5.8%), patent ductus arteriosus (11.7 vs. 20.2%), and bronchopulmonary dysplasia (13.0 vs. 20.5%, p = 0.05 or less) for each. Regarding infectious morbidity, the antibiotic study group had a lower incidence of neonatal group B streptococcus sepsis (0 vs. 1.5%, p = 0.03), amnionitis (23 vs. 32.5%, p = 0.01), neonatal sepsis (8.4 vs. 15.6%, p = 0.009), and pneumonia (2.9 vs. 7.0%, p = 0.04) among those who were not group B streptococcus carriers. Similar to other studies, antibiotic treatment increased the likelihood that patients would remain undelivered after 7 days of treatment two-fold. Despite discontinuation of antibiotics at 7 days, treated women were more likely to remain pregnant for up to 3 weeks after randomization, suggesting that antibiotic therapy actually successfully treated subclinical infection rather than just suppressing it. Mantel–Haensel chi square analysis of randomized trials with initial broad-spectrum parenteral therapy for women with pPROM at less than 34 weeks82, 83, 84, 85, 87, 88, 89, 90, 91, 92, 93 reveals that antibiotics increase the likelihood of women remaining pregnant more than 1 week (odds ratio [OR] and 95% confidence interval [CI]: 2.52 and 1.92–3.30), reduce amnionitis (OR: 0.60; 95% CI: 0.47–0.78), reduce neonatal sepsis (OR: 0.47; 95% CI: 0.33–0.67), reduce RDS (OR: 0.76; 95% CI: 0.60–0.96), reduce IVH (OR: 0.70; 95% CI: 0.52–0.95), without an increasing the risk of necrotizing enterocolitis (OR: 1.28; 95% CI: 0.84–1.98).
Kenyon and associates performed a large multi-arm, multicenter placebo-controlled trial of oral antibiotics for pPROM at less than 37 weeks.86 Treatment included oral erythromycin, oral amoxicillin-clavulanic acid, both, or placebo for up to 10 days. Oral erythromycin led to brief pregnancy prolongation (not significant at 7 days), reduced need for supplemental oxygen (31.1 vs. 35.6%, p = 0.02), and reduced positive blood cultures (5.7 vs. 8.2%, p = 0.02), but not other morbidities. In singleton gestations, erythromycin treatment reduced oxygen dependence at 28 days (6.9 vs. 8.9%, p = 0.03), positive blood cultures (5.3 vs. 7.4%, p = 0.04), abnormal cerebral ultrasonography (3.0 vs. 4.6%, p = 0.04), and composite morbidity (11.2 vs. 14.4%, p = 0.02). Oral amoxicillin-clavulanic acid prolonged pregnancy (43.3 vs. 36.7% undelivered after 7 days, p = 0.005) and reduced the need for supplemental oxygen (30.1 vs. 35.6%, p = 0.05), but increased the risk of necrotizing enterocolitis (1.9 vs. .5%, p = 0.001) without reducing other neonatal morbidities. Similar findings were seen with the combination of oral amoxicillin-clavulanic acid and erythromycin. The authors concluded that oral erythromycin had significant effects on major neonatal disease and might have a substantial health benefit, but raised concern regarding the increased incidence of necrotizing enterocolitis with oral amoxicillin-clavulanic acid. The latter finding is at odds with the NICHD-MFMU trial finding of reduced stage 2 or 3 necrotizing enterocolitis with aggressive antibiotic therapy in a higher-risk population.
In summary, aggressive antibiotic therapy with erythromycin and amoxicillin/ampicillin during conservative management of pPROM remote from term appears to offer benefit in reduction of infant infectious and gestational age-dependent morbidity. Two recent studies evaluated the impact of shorter duration antibiotic therapy after pPROM, but neither is of inadequate size and power to evaluate effectiveness in preventing infant morbidities and brief duration therapy is not recommended for this indication.94, 95 If aggressive therapy is not administered, oral erythromycin may offer some potential benefit. The combination of oral erythromycin and extended-spectrum ampicillin-clavulanic acid in a lower-risk population near term does not appear to be beneficial and may be harmful. This latter regimen is not recommended.
Antibiotic therapy of positive amniotic fluid cultures in the asymptomatic patient remote from term is attractive, but controversial. One case report has suggested the efficacy of such therapeutic antibiotic intervention in a patient with a positive amniotic fluid culture for Bacteroides bivius, Veillonella parvula, and Peptococcus at 29 weeks' gestation.96 However, there are no properly conducted trials to address the safety and efficacy of therapeutic antibiotics in this setting.
Induction of Fetal Pulmonary Maturity
The current NIH consensus conference recommendations regarding corticosteroid administration in the setting of pPROM are that a single course of betamethasone (12 mg intramuscular every 24 hours for 2 doses) or dexamethasone (6 mg intramuscular every 12 hours for 4 doses) be administered during conservative management of pPROM before 30 to 32 weeks' gestation because of the potential for reduction of IVH.97 The efficacy of corticosteroids in the setting of pPROM has been questioned. While it has been suggested that women with pPROM will deliver too quickly to accrue the potential benefits of antenatal steroids, that pPROM itself might induce fetal pulmonary maturation, thereby making corticosteroids unnecessary, and that antenatal corticosteroids might increase the risk of perinatal infection, data in this regard are not convincing. Approximately 80% of women with pPROM remote from term will remain pregnant for at least 24 hours, if antibiotic therapy is administered. RDS remains the most common morbidity after preterm birth caused by pPROM remote from (41% in the NICHD-MFMU trial).85 Studies of antenatal steroid administration performed before the era of antibiotic treatment during conservative management of pPROM revealed conflicting results regarding neonatal infection. However, two recent trials have assessed antenatal corticosteroid administration concurrent to adjunctive antibiotic administration and found no increase in infectious morbidity with antenatal corticosteroid administration. Lewis and associates found antenatal corticosteroids to reduce the incidence of RDS (18.4 vs. 43.6%, p = 0.03) without increasing perinatal infection (3 vs. 5%, p = not significant [NS]), when antibiotics were administered concurrently.98 In a second trial, women with pPROM at 28–34 weeks' gestation received amoxicillin and metronidazole for 5 days and were randomized to antenatal dexamethasone or placebo.99 No increase in maternal or neonatal infectious morbidity was evident with corticosteroids. Among those remaining pregnant at least 24 hours, the corticosteroid group had less perinatal death (1.3 vs. 8.3%, p = 0.05) The most recent meta-analysis in this regard found corticosteroid administration after pPROM to reduce the risks of RDS (20 vs. 35.4%), IVH (7.5 vs. 15.9%), and necrotizing enterocolitis (0.8 vs. 4.6%; p < 0.05 for each), without increasing the risks of maternal (9.2 vs. 5.1%) or neonatal infection (7.0 vs. 6.6%; p > 0.05 for both).100
Based on current information, a single course of antenatal corticosteroids with concurrent adjunctive antibiotic therapy is recommended during conservative management of pPROM before 32 weeks. Similar treatment could also be considered if conservative management is pursued for suspected fetal pulmonary immaturity at 32–34 weeks' gestation. Absent adequate data regarding safety and efficacy, repeated administration of antenatal corticosteroids is not recommended.
Tocolysis
Both prophylactic and therapeutic tocolysis have been used to prolong the latency interval after preterm PROM.101, 102, 103 Some clinicians consider labor to be a presumptive sign of intrauterine infection and therefore a contraindication to tocolytic therapy. In 1980, Christensen administered Ritodrine intravenously to patients with pPROM in labor and compared this with a placebo for a 24-hour course.101 None of the patients delivered while administered the intravenous infusion. The difference between the two groups was statistically significant at 24 hours. Levy and Warsof randomized 42 women to a prophylactic oral ritodrine group versus a control group and found a significant pregnancy prolongation at 48 hours (OR: 0.19; 95% CI: 0.05–0.72) but not at 10 days.104 They did not demonstrate any benefit in terms of respiratory distress, perinatal mortality, or neonatal infection. Another study by Weiner and colleagues indicated a significant delay in delivery only among pPROM patients undergoing tocolysis before 28 weeks' gestation.105 Alternatively, Garite and associates, in a study randomizing women on admission to conservative management or to an intent to treat with tocolytics should labor ensue, found no improvement in pregnancy prolongation.102 Similarly, improved neonatal outcome has not been conferred with a practice of tocolysis and serial assessment of fetal pulmonary maturity followed by delivery.106Most recently, in a retrospective comparison of aggressive tocolysis versus treatment for contractions as needed during the first 48 hours, latency was not increased with aggressive therapy (3.8 vs. 4.5 days, p = 0.16).107 A secondary analysis from the Collaborative Study on Antenatal Steroids found increased neonatal respiratory distress syndrome after tocolytic therapy in the setting of pPROM, however it is unclear how this association is biologically plausible.108,
While no study has found tocolytic therapy to improve infant outcomes, such studies have not evaluated tocolysis when corticosteroids and antibiotics are administered concurrently. It is plausible that prophylactic tocolysis could increase the time for corticosteroid action on the fetus and allow more time for antibiotics to act against subclinical decidual infection. Given the above, while it may be reasonable to initiate tocolysis concurrent to antibiotic and antenatal corticosteroid therapies directed to improve neonatal outcomes, this approach should not be considered an expected practice. Further research is needed to clarify this issue.
SPECIAL CIRCUMSTANCES AFFECTING THE MANAGEMENT OF PPROM
Previable pPROM (at less than 23 Weeks' Gestation)
There are limited data available regarding acute and long term outcomes after previable PROM before 23 weeks' gestation.109 Additional information must be extrapolated from older studies regarding midtrimester PROM and currently available information for gestational age appropriate outcomes.49
Approximately one half of women with midtrimester PROM will deliver within 1 week of membrane rupture and up to 22% will remain undelivered for at least 1 month. Maternal morbidities after conservative management of midtrimester PROM include chorioamnionitis (39%), endometritis (14%), abruptio placentae (3%), and retained placenta with postpartum hemorrhage necessitating dilatation and curettage (12%). Maternal sepsis is a rare but serious complication (0.8%).110 Stillbirth subsequent to midtrimester PROM (15%) is more common than that seen later in pregnancy (approximately 1%) and may reflect fetal susceptibility to umbilical cord compression and intrauterine infection and/or nonintervention for fetal distress in the periviable fetus. Periviable delivery after pPROM may be associated with more frequent morbidity and higher mortality than that seen with delivery because of other causes because of the associations between this complication, perinatal infection, and pulmonary hypoplasia. Estimates of long-term morbidity after midtrimester PROM from the 1980s are no longer appropriate for the current limit of viability or current practice. Counseling in this regard is limited to data available for periviable delivery in general.111, 112, 113, 114
Because of the high risk of maternal and neonatal adverse outcomes after previable PROM, some women will request expeditious delivery. This can be accomplished by dilatation and evacuation, labor induction with high-dose oxytocin, or by vaginal prostaglandin therapy, depending on caregiver and concurrent factors. If conservative management is elected, initial observation may include strict bed and pelvic rest to enhance the opportunity for resealing, and for early identification of infection and abruption. Initial ultrasound should exclude fetal anomalies to the extent possible. Follow-up ultrasound should be considered, initially to evaluate for re-accumulation of amniotic fluid and subsequently to evaluate interval pulmonary growth. Some will elect not to continue the pregnancy if interval ultrasound suggests a high likelihood of lethal pulmonary hypoplasia based on the presence of persistent severe oligohydramnios and/or lagging pulmonary growth. Some clinicians will discharge women with pPROM, prescribe bed rest at home after initial observation, and readmit them to hospital once the pregnancy reaches 23 or 24 weeks. There are no published data evaluating the risks and benefits of this strategy of continued hospitalization
Pulmonary Consequences of Prolonged Oligohydramnios
Pulmonary hypoplasia results from a variety of conditions that are associated with fetal lung compression and/or oligohydramnios. Pathologically, there is a failure of proliferation of alveoli with a corresponding decrease in lung size. The incidence of pulmonary hypoplasia is difficult to determine, ranging from 9% to 46%, as reported in various studies.115, 116, 117, 118, 119, 120 Women with early previable PROM before 20 weeks, and those with persistent oligohydramnios, are at particular risk for pulmonary hypoplasia. Lethal pulmonary hypoplasia rarely occurs with membrane rupture subsequent to 24 weeks' gestation, presumably because alveolar growth adequate to support postnatal development has occurred.117, 118, 121, 122
The etiology of hypoplastic lung growth subsequent to pPROM is not clear. Although absent fetal breathing has been associated with pulmonary hypoplasia in fetuses at risk for myotonic dystrophy,123 the association between fetal breathing activity and pulmonary hypoplasia after PROM is controversial.115, 124, 125, 126, 127, 128, 129, 130 It has been speculated that membrane rupture results in a relative increase in the pressure gradient from the lung to the amniotic cavity, leading to increased loss of fluid produced by the lung. This might be associated with the loss of an intrinsic pulmonary growth factor or collapse of the tracheobronchial tree with failure of subsequent alveolar development. This theory is supported by evidence suggesting that amniotic cavity pressure is decreased subsequent to membrane rupture.131 Moreover, prolonged tracheal drainage leads to pulmonary hypoplasia, whereas ligation of the trachea prevents pulmonary hypoplasia subsequent to prolonged oligohydramnios.124
The antenatal prediction of lethal pulmonary hypoplasia has been the subject of significant study, as summarized by Laudy et al. in 2002.132 In 1985, Callan and colleagues demonstrated a progressive lag of ultrasonographic thoracic growth in a fetus with lethal pulmonary hypoplasia.133 This group subsequently demonstrated a correlation between the thoracic circumference-to-abdominal circumference ratio (TC-to-AC ratio) and pulmonary hypoplasia (Pearson correlation coefficient: 0.75).116 Since then, a variety of authors have studied the indirect ultrasonographic estimation of fetal lung size through evaluation of chest circumference and cardiothoracic ratio.115, 117, 120, 125, 134, 135, 136, 137, 138 Correction for fetal size has been also attempted by evaluating a ratio of chest circumference-to-body size.115, 120, 125, 134 van Eyck and associates demonstrated a loss of the normal reduction in peak ductus arteriosus blood velocity with breathing movements in fetuses destined to demonstrate pulmonary hypoplasia.122
Sealing of the membranes after previable PROM (e.g., amnioinfusion, fibrin/platelet/cryoprecipitate, or gel-foam instillation) has been preliminarily investigated for prevention of pulmonary hypoplasia and is described in a recent review.139, 140, 141, 142, 143 Imanaka and colleagues suggested that the use of an indwelling transcervical catheter (PROM fence) with antibiotic infusion might reduce the incidence of infection, prolong pregnancy, and reduce the incidence of adverse effects related to oligohydramnios.144, 145, 146 This technique requires the insertion of a cervical cerclage to maintain catheter position. The benefits of this intervention have not been subsequently confirmed. The maternal risks and fetal benefits of these aggressive interventions are not yet known. At present, each should be considered experimental. Nonpulmonary Consequences of Prolonged Oligohydramnios
The pattern of fetal restriction deformities occurring as a result of prolonged intrauterine crowding is similar to those seen with Potter's syndrome. The nonpulmonary features include abnormal facies and limb-positioning abnormalities.147 The newborn may have low-set ears and epicanthal folds. The extremities, hands, and feet may be flattened and malpositioned. Fortunately, these abnormalities can usually be treated with splinting and/or physical therapy, and surgical intervention is generally not required. Intrauterine growth retardation has been suggested to occur more frequently with pPROM; however, there are few data in support of this conclusion. Amnion nodosum consists of elevated, eosinophilic, amorphous plaques of fetal epithelial cells found on the fetal surface of the chorioamnion occurring after prolonged membrane rupture. This finding is also nonspecific to the etiology of oligohydramnios but may be helpful in the determination of chronicity if an adequate history cannot be obtained.148
Herpes Simplex Virus and PROM
Approximately 10–20% of women of childbearing age from higher socioeconomic groups and 50–60% from lower socioeconomic groups have detectable antibodies to herpes simplex virus 1 (HSV-1) or herpes simplex virus 2 (HSV-2).149 Eighty percent of previously infected women will have two to four symptomatic recurrences in pregnancy, with cervical shedding of virus present in 15% of women with clinical recurrences.150, 151, 152 Approximately 14% of women with recurrent HSV will have genital lesions at delivery,153 with 20–33% of symptomatic lesions actively shedding virus.153, 154 The estimated incidence of neonatal infection ranges from 1 in 2000 to 1 in 30,000 deliveries.155, 156, 157
Fetal/neonatal infection is presumed to result from one of several mechanisms, including direct contact during passage through the birth canal, the hematogenous/transplacental spread, ascending of the infection after membrane rupture, and direct contact with parents and other caregivers postnatally. Approximately 90% of cases of neonatal infection result from direct maternal transmission.158 In 1989, Baldwin suggested that approximately 5% of cases of neonatal infection occurred in utero.159 Infants of women with primary HSV infections are at increased risk because of the lack of protective maternal antibodies, as well as the longer duration (2–3 weeks) and higher volume of viral shedding.160 This is further supported by a higher incidence of cervical shedding of virus during primary HSV infection.161, 162 Infants delivered during a primary infection have a vertical transmission rate of 34–80%162 as opposed to a risk of approximately 1–5% after delivery during a secondary infection.156 Of infants with neonatal HSV infection, 85% are delivered of mothers who are asymptomatic at the time of delivery, 20–30% of infected neonates are delivered by cesarean section, and 8% of infected infants are delivered by cesarean section with membranes intact at delivery.163, 164 In the face of clinically evident herpetic lesions, cesarean section has long been suggested to reduce the risk of intrapartum direct maternal-to-fetal transmission of HSV. In 1971, Amstey suggested that cesarean section not be performed if the membranes have been ruptured for more than 6 hours.165 The 4-hour rule was subsequently introduced later that year by Nahmias and colleagues.166 Other reports, however, have demonstrated a lack of neonatal infection with delivery from 24 hours to several weeks after membrane rupture with genital lesions present at the time of membrane rupture.167, 168, 169, 170
In 1985, Ray and colleagues described the cases of three women who were expectantly managed in the face of recurrent vulvar herpes and pPROM.168 In 1987, Utley and associates described the case of a patient admitted with primary herpes vulvovaginitis and PROM at 25.5 weeks' gestation.169 The patient was managed expectantly with parenteral acyclovir (500 mg every 8 hours) until labor ensued 6 days later. She was delivered abdominally of a 700-gram infant who demonstrated no signs of infection. More recently, Major and colleagues presented a review of 18 women with culture-proven recurrent genital herpes complicated by PROM remote from term.170 Expectant management was associated with latencies of 12 hours to 5 weeks. Three women had more than one recurrence subsequent to membrane rupture, and eight women were delivered by cesarean section because of the presence of lesions at the time of delivery. None of the 18 infants demonstrated evidence of neonatal herpes infection.
In general, immediate cesarean section in the face of active genital herpes and PROM is prudent. However, the risks of preterm birth may exceed those of conservative management when membrane rupture occurs near the limit of viability. It should be reinforced that most cases studied to date involved patients with recurrent herpes virus infections and PROM. These patients would be anticipated to have a lower incidence, duration, and volume of cervical shedding. In addition, although the existing data are encouraging, they do not compare HSV subjects against a control group and may reflect a publication bias.
Multifetal Gestation and PROM
RUPTURE OF THE PRESENTING SAC
Twin gestation is associated with a high incidence of spontaneous preterm birth with resultant infant morbidity and mortality.171, 172, 173 Despite extensive literature describing the clinical course of singleton pregnancy complicated by pPROM, there are few comparable data available concerning pPROM in twin gestation. One published study has reviewed the clinical outcome of 99 twin pregnancies complicated by pPROM.174 Another study, reported in abstract form, revealed similar results.175
Latency in conservatively managed twin pPROM is similar to that in singleton gestations, with 64% of pregnancies delivering within 48 hours of membrane rupture and 89% delivering within 1 week.174 Twin PROM occurring before 30 weeks' gestation have longer latency period than that occurring after 30 weeks (median 1.6 vs. 0.9 days; p < 0.03). Other than an increased incidence of cesarean delivery in twin pregnancies (42 vs. 27%; p < 0.02), maternal morbidity is remarkably similar between singleton and twin gestations. Complications of twin gestations were chorioamnionitis (19%), postpartum fever (15%), and placental abruption (6%).
Infant morbidity after pPROM in a twin gestation is common. As in singleton pregnancies complicated by pPROM, RDS is the most common sequela after pPROM in the twin gestation. Montgomery and colleagues demonstrated similar infant morbidity and mortality in singleton and twin gestations (respiratory distress, 35%; infection, 19%; survival, 89%).175 Leveno and associates demonstrated pulmonary maturity testing to be synchronous in twins.21 In contrast, Arnold and colleagues suggested that the nonpresenting infant is at higher risk for respiratory compromise.22 Mercer and colleagues supported these findings, showing an increased incidence of hyaline membrane disease (21 vs. 7.1%; p < 0.01) and oxygen requirement (44 vs. 23%; p < 0.003) in the nonpresenting infant.174
Generally, management of twin pregnancies with pPROM is similar to that for singleton gestations. When faced with extreme prematurity, it is important to counsel parents about the potential for demise of one twin caused by umbilical cord compression and the possibility of further conservative measures to enhance survival of the fetus with intact membranes.
PREMATURE RUPTURE OF THE NONPRESENTING SAC
The incidence of membrane rupture of the nonpresenting sac is not known. One report described a case involving intra-amniotic injection of Evans blue dye into the upper sac to confirm the diagnosis of PROM at 15 weeks' gestation.176 The patient was treated conservatively with oral cephalothin and intravaginal povidone–iodine. Leakage resolved spontaneously at 19 weeks, and the patient went on to deliver two healthy infants at 32 weeks after recurrent pPROM.
Cervical Cerclage and pPROM
pPROM complicates approximately 1 in 4 pregnancies with a cerclage in place; it complicates 1 in 2 pregnancies after emergent cerclage.177, 178 There are no prospective data available regarding the optimal treatment of pPROM with a cervical cerclage in situ. Retrospective studies have suggested that when cerclage is removed after pPROM, the risk of adverse perinatal outcomes is similar to that seen after pPROM without cerclage.179, 180 Studies comparing pregnancies with retained or removed cerclage after pPROM have been small and conflicting.181, 182, 183 Each has shown a statistically insignificant increased risk of maternal infection with retained cerclage. One study found increased infant mortality and death from sepsis with retained cerclage.181 In that study, the only benefit of cerclage retention was a decreased likelihood of delivery within 24 hours. Another study, which evaluated differing practices at two institutions, found significant pregnancy prolongation with cerclage retention.182 Cerclage retention after pPROM before 28 weeks was associated with higher birthweight (942 vs. 758 g, p = 0.04). No study has found a reduction in infant morbidity with cerclage retention after pPROM.
Given the potential risk without evident neonatal benefit when the stitch is left in place after pPROM, the general approach should include early cerclage removal after membrane rupture occurs. The role for short-term cerclage retention while attempting to enhance fetal maturation with antenatal corticosteroids in the periviable gestation has not been determined.
REFERENCES
Meis JP, Ernest M, Moore ML: Causes of low birth weight births in public and private patients. Am J Obstet Gynecol 156:1165, 1987 |
|
Savitz DA, Blackmore CA, Thorp JM: Epidemiologic characteristics of preterm delivery: Etiologic heterogeneity. Am J Obstet GynecoI 164:467, 1991 |
|
Wu YW, Colford JM Jr: Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA 1417:284, 2000 |
|
Yoon BH, Jun JK, Romero R et al: Amniotic fluid inflammatory cytokines (interleukin-6, interleukin 1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 19:177, 1997 |
|
Yoon BH, Romero R, Kim CJ et al: High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol 406:177, 1997 |
|
Yoon BH, Romero R, Yang SH et al: Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1433:174, 1996 |
|
Taylor J, Garite T: Premature rupture of the membranes before fetal viability. Obstet Gynecol 64:615, 1984 |
|
Mercer BM: Premature Rupture of the membranes. An expert's view. Obstet Gynecol 178:101, 2003 |
|
McParland, P.C., Taylor, D.J., Bell, S.C. Mapping of zones of altered morphology and choriodeciduaic connective tissue cellular phenotype in human fetal membranes (amnion and deciduas) overlying the lower uterine pole and cervix before labor at term. Am J Obstet Gynecol 2003;189:1481-1488. |
|
Moore RM, Mansour JM; Redline RW; Mercer BM; Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties.(Review) Placenta. 2006;27:1037-51. |
|
Lonky NM, Hayashi RH: A proposed mechanism for pre mature rupture of the membranes. Obstet Gynecol Surv 43:22, 1988 |
|
Naeye RL: Factors that predispose to premature rupture of the fetal membranes. Obstet Gynecol 60:93, 1982 |
|
Gibbs RS, Blanco JD: Premature rupture of the membranes. Obstet Gynecol 60:671, 1982 |
|
Bendon RW, Faye-Petersen O, Pavlova Z et al: Fetal membrane histology in preterm premature rupture of membranes: comparison to controls, and between antibiotic and placebo treatment. Pediatr Dev Pathol 552:2, 1999 |
|
Mercer BM, Goldenberg RL, Moawad AH et al: The preterm prediction study: Effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol 1216:181, 1999 |
|
Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol. 2004;190:1509-19. |
|
Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG 2nd, Rao AV, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737-42. |
|
Mercer BM, Goldenberg RL, Meis PJ et al: The preterm prediction study: Prediction of preterm premature rupture of the membranes using clinical findings and ancillary testing. Am J Obstet Gynecol 738:183, 2000 |
|
Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O'Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379-85. |
|
da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419-24. |
|
Leveno KJ, Quirk JG, Whalley PJ et al: Fetal lung maturation in twin gestation. Am J Obstet Gynecol 148:405, 1984 |
|
Arnold C, McLean FH, Kramer MS et al: Respiratory distress syndrome in second-born versus first-born twins: A matched case-control analysis. N Engl J Med 317:1121, 1987 |
|
Iams JD, Stilson R, Johnson FF et al: Symptoms that precede preterm labor and preterm premature rupture of the membranes. Am J Obstet Gynecol 162:486, 1990 |
|
O'Brien JM, Mercer BM, Sibai BM: The use of a modified vaginal pouch for the diagnosis and management of premature rupture of the membranes. Am J Obstet Gynecol 172:1565, 1995 |
|
Garite TJ, Freeman RK: Chorioamnionitis in the preterm gestation. Obstet Gynecol 59:539, 1982 |
|
Simpson GF, Harbert GM Jr: Use of betamethasone in management of preterm gestation with premature rupture of membranes. Obstet Gynecol 66:168, 1985 |
|
Beydoun S, Yasin S: Premature rupture of the membranes before 28 weeks: Conservative management. Am J Obstet Gynecol 1155:471, 1986 |
|
Major CA, Kitzmiller JL: Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol 163:838, 1990 |
|
Moretti M, Sibai B: Maternal and perinatal outcomes of expectant management of premature rupture of the membranes in the midtrimester. Am J Obstet Gynecol 159:390, 1988 |
|
Gonen R, Hannah ME, Milligan JE: Does prolonged pre term premature rupture of the membranes predispose to abruptio placentae. ? Obstet Gynecol 74:347, 1989 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: Preterm premature rupture of the membranes: A risk factor for the development of abruptio placentae. Am J Obstet Gynecol 156:1235, 1987 |
|
Nelson DM, Stempel LE, Zuspan FP: Association of prolonged, preterm premature rupture of the membranes and abruptio placentae. J Reprod Med 31:249, 1986 |
|
Mercer BM, Moretti ML, Prevost RR et al: Erythromycin therapy in preterm premature rupture of the membranes: A prospective, randomized trial of 220 patients. Am J Obstet GynecoI 166:794, 1992 |
|
Johnson JWC, Egerman RS, Moorhead J: Cases with ruptured membranes that “reseal”. Am J Obstet Gynecol 163:1024, 1990 |
|
Mercer BM: Management of premature rupture of membranes before 26 weeks' gestation. Obstet Gynecol Clin North Am 19:339, 1992 |
|
Gold RB, Goyer GL, Schwartz et al: Conservative management of second trimester post-amniocentesis fluid leak age. Obstet Gynecol 74:745, 1989 |
|
Borgida AF, Mills AA, Feldman DM, Rodis JF, Egan JFX. Outcome of pregnancies complicated by ruptured membranes after genetic amniocentesis. Am J Obstet Gynecol 2000;183:937-9. |
|
Munson LA, Graham A, Koos BJ et al: Is there a need for digital examination in patients with spontaneous rupture of the membranes. ? Am J Obstet Gynecol 153:562, 1985 |
|
Lewis DF, Major CA, Towers CV et al: Effects of digital vaginal examinations on latency period in preterm premature rupture of membranes. Obstet Gynecol. 630:80, 1992 |
|
Alexander JM, Mercer BM, Miodovnik M et al: The impact of digital cervical examination on expectantly managed preterm rupture of membranes. Am J Obstet Gynecol 1003:183, 2000 |
|
Bottoms SF, Welch RA, Zador IE et al: Clinical interpretation of ultrasound measurements in preterm pregnancies with premature rupture of the membranes. Obstet Gynecol 69:358, 1987 |
|
Silver RK, MacGregor SN, Hobart ED: Impact of residual amniotic fluid volume in patients receiving parenteral tocolysis after premature rupture of the membranes. Am J Obstet GynecoI 161:784, 1989 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: Qualitative amniotic fluid volume versus amniocentesis in predicting infection in preterm premature rupture of the membranes. Obstet Gynecol 67:579, 1986 |
|
Moberg U, Garite TJ, Freeman RK: Fetal heart rate patterns and fetal distress in patients with preterm premature rupture of membranes. Obstet Gynecol 64:60, 1984 |
|
Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30:28-33. |
|
Naef RW 3rd, Allbert JR, Ross EL et al: Premature rupture of membranes at 34 to 37 weeks' gestation: Aggressive versus conservative management. Am J Obstet Gynecol 126:178, 1998 |
|
Mercer BM, Crocker LG, Boe NM et al: Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: A randomized trial. Am J Obstet GynecoI 169:775, 1993 |
|
Cox SM, Leveno KJ: Intentional delivery versus expectant management with preterm ruptured membranes at 30–34 weeks' gestation. Obstet Gynecol 875:86, 1995 |
|
American College of Obstetricians and Gynecologists. ACOG Committee Opinion: number 279, December 2002. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2002;100:1405-12 |
|
Sbarra AJ, Blake G, Cetrulo CL et al: The effect of cervical/vaginal secretions on measurements of lecithin/sphingomyelin ratio and optical density at 650 nm. Am J Obstet Gynecol 139:214, 1981 |
|
Phillippe M, Acker D, Torday J et al: The effects of vaginal contamination on two pulmonary phospholipid assays. J Reprod Med 27:283, 1982 |
|
Golde SH, Petrucha R, Meade KW et al: Fetal lung maturity: the adjunctive use of ultrasound. Am J Obstet Gynecol 142:445, 1982 |
|
Shaver DC, Spinnato JA, Whybrew D et al: Comparison of phospholipids in vaginal and amniocentesis specimens of patients with premature rupture of membranes. Am J Obstet Gynecol 156:454, 1987 |
|
Estol PC, Poseiro JJ, Schwarcz R: Phosphatidylglycerol determination in the amniotic fluid from a PAD placed over the vulva a method for diagnosis of fetal lung maturity in cases of premature ruptured membranes. J Perinat Med 20:65, 1992 |
|
Russell JC, Cooper CM, Ketchum CH et al: Multicenter evaluation of TDx test for assessing fetal lung maturity. Clin Chem 35:1005, 1989 |
|
Edwards RK, Duff P, Ross KC: Amniotic fluid indices of fetal pulmonary maturity with preterm premature rupture of membranes. Obstet Gynecol 96:102, 2000 |
|
Buhi WC, Spellacy WN: Effects of blood or meconium on the determination of the amniotic fluid lecithin/sphingomyelin ratio. Am J Obstet Gynecol 121:321, 1975 |
|
Apple FS, Bilodeau L, Preese LM et al: Clinical implementation of a rapid, automated assay for assessing fetal lung maturity. J Reprod Med 39:883, 1994 |
|
Tabsh KM, Brinkman CR 3rd, Bashore R: Effect of meconium contamination on amniotic fluid lecithin: Sphingomyelin ratio. Obstet Gynecol 58:605, 1981 |
|
Asrat T, Nageotte MP, Garite TJ et al: Gram stain results from amniocentesis in patients with preterm premature rupture of membranes: Comparison of maternal and fetal characteristics. Am J Obstet Gynecol 163:887, 1990 |
|
Romero R, Mazor M, Morrotti R et al: Infection and labor: VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term Am J Obstet Gynecol 166:29, 1992 |
|
Santhanam U, Avila C, Romero R et al: Cytokines in normal and abnormal parturition: Elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine 3:155, 1991 |
|
Romero R, Yoon BH, Mazor M et al: A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin 6, and Gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 169:839, 1993 |
|
Buhimschi CS, Sfakianaki AK, Hamar BG, Pettker CM, Bahtiyar MO, Funai E, Norwitz ER, Copel JA, Lockwood CJ, Buhimschi IA. A low vaginal "pool" amniotic fluid glucose measurement is a predictive but not a sensitive marker for infection in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2006;194:309-16. |
|
Ohel G, Sadovsky E, Aboulafia Y et al: Fetal activity in premature rupture of membranes. Am J Perinatol 3:337, 1986 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol 155:149, 1986 |
|
Zeevi D, Sadovsky E, Younis J et al: Antepartum fetal heart rate characteristics in cases of premature rupture of membranes. Am J Perinatol 5:260, 1988 |
|
Schwarcz R, Althabe O, Belitzky R et al: Fetal heart rate patterns in labors with intact and with ruptured membranes. J Perinat Med 1:153, 1973 |
|
Vintzileos AM, Feinstein SJ, Lodeiro JG et al: Fetal biophysical profile and the effect of premature rupture of the membranes. Obstet Gynecol 67:818, 1986 |
|
Vintzileos AM, Campbell WA, Nochimson DJ et al: The fetal biophysical profile in patients with premature rupture of the membranes: An early predictor of fetal infection. Am J Obstet GynecoI 152:510, 1985 |
|
Vintzileos AM, Bors Koefoed R, Pelegano IF et al: The use of fetal biophysical profile improves pregnancy out come in premature rupture of the membranes. Am J Obstet GynecoI 157:236, 1987 |
|
Goldstein I, Romero R, Merrill S et al: Fetal body and breathing movements as predictors of intraamniotic infection in preterm premature rupture of membranes. Am J Obstet GynecoI 159:363, 1988 |
|
Del Valle GO, Joffe GM, lzquierdo LA et al: The biophysical profile and the nonstress test: Poor predictors of chorioamnionitis and fetal infection in prolonged preterm premature rupture of membranes. Obstet Gynecol 80:106, 1992 |
|
Miller JM Jr, Kho MS, Brown HL et al: Clinical chorioamnionitis is not predicted by an ultrasonic biophysical profile in patients with premature rupture of membranes. Obstet Gynecol 76:1051, 1990 |
|
Centers for Disease Control and Prevention: Prevention of group B streptococcal disease: A public health perspective. MMWR Morb Mortal Wkly Rep 45:RR-7:1, 1996 |
|
Fiore Mitchell T, Pearlman MD, Chapman RL et al: Maternal and transplacental pharmacokinetics of cefazolin. Obstet Gynecol 1075:98, 2001 |
|
Silverman NS, Morgan M, Nichols WS: Antibiotic resistance patterns of group B streptococcus in antenatal genital cultures. J Reprod Med 979:45, 2000 |
|
Schrag S, Gorwitz R, Fultz-Butts K et al: Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC MMWR Recomm Rep 51:RR-11:1, 2002 |
|
American College of Obstetricians and Gynecologists. ACOG Committee Opinion: number 279, December 2002. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2002;100:1405-12 |
|
Mercer B: Antibiotic therapy for preterm premature rupture of membranes. Clin Obstet Gynecol 461:41, 1998 |
|
Egarter C, Leitich H, Karas H et al: Antibiotic treatment in premature rupture of membranes and neonatal morbidity: A meta-analysis. Am J Obstet Gynecol 589:174, 1996 |
|
Cox SM, Leveno KJ, Sherman ML et al: Ruptured membranes at 24 to 29 weeks: a randomized double blind trial of antimicrobials versus placebo. Am J Obstet Gynecol 412:172, 1995 |
|
Ovalle-Salas A, Gomez R, Martinez MA et al: Antibiotic therapy in patients with preterm premature rupture of membranes: a prospective, randomized, placebo-controlled study with microbiological assessment of the amniotic cavity and lower genital tract. Prenat Neonat Med 213:2, 1997 |
|
Svare J: Preterm delivery and subclinical uro-genital infection [thesis]. Denmark: Department of Obstetrics and Gynaecology Rigshospitalet, University of Copenhagen, 1997. summarized in Kenyon S, Boulvain M. Antibiotics for preterm premature rupture of membranes (Cochrane Review). In: The Cochrane Library, Issue 2, 2001. Oxford, Update software |
|
Mercer B, Miodovnik M, Thurnau G et al: Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. JAMA 989:278, 1997 |
|
Kenyon SL, Taylor DJ, Tarnow-Mordi W et al: Broad spectrum antibiotics for preterm, prelabor rupture of fetal membranes: the ORACLE I Randomized trial. Lancet 979:357, 2001 |
|
Amon E, Lewis SV, Sibai BM et al: Ampicillin prophylaxis in preterm premature rupture of the membranes: A prospective randomized study. Am J Obstet Gynecol 539:159, 1988 |
|
Johnston MM, Sanchez-Ramos L, Vaughn AJ et al: Antibiotic therapy in preterm premature rupture of membranes: A randomized, prospective, double-blind trial. Am J Obstet Gynecol 743:163, 1990 |
|
Christmas JT, Cox SM, Andrews W et al: Expectant management of preterm ruptured membranes: Effects of antimicrobial therapy. Obstet Gynecol 759:80, 1992 |
|
McCaul JF, Perry KG Jr, Moore JL Jr et al: Adjunctive antibiotic treatment of women with preterm rupture of membranes or preterm labor. Int J Gynaecol Obstet 19:38, 1992 |
|
Lockwood CJ, Costigan K, Ghidini A et al: Double-blind; placebo-controlled trial of piperacillin prophylaxis in preterm membrane rupture. Am J Obstet Gynecol 970:169, 1993 |
|
Morales WJ, Angel JL, O'Brien WF et al: Use of ampicillin and corticosteroids in premature rupture of membranes: a randomized study. Obstet Gynecol. 721:73, 1989 |
|
Owen J, Groome LJ, Hauth JC: Randomized trial of prophylactic antibiotic therapy after preterm amnion rupture. Am J Obstet Gynecol 976:169, 1993 |
|
Lewis DF, Adair CD, Robichaux AG, Jaekle RK, Moore JA, Evans AT, Fontenot MT. Antibiotic therapy in preterm premature rupture of membranes: Are seven days necessary? A preliminary, randomized clinical trial. Am J Obstet Gynecol. 2003;188:1413-6; discussion 1416-7. |
|
Segel SY, Miles AM, Clothier B, Parry S, Macones GA. Duration of antibiotic therapy after preterm premature rupture of fetal membranes. Am J Obstet Gynecol. 2003;189:799-802. |
|
Romero R, Scioscia AL, Edberg SC et al: Use of parenteral antibiotic therapy to eradicate bacterial colonization of amniotic fluid in premature rupture of membranes. Obstet Gynecol 67:S:15S, 1986 |
|
National Institute of Health. NIH Consensus Development Conference Statement: Effect of corticosteroids for fetal maturation on perinatal outcomes, February 28–March 2, 1994 Am J Obstet Gynecol 246:173, 1995 |
|
Lewis DF, Fontenot MT, Brooks GG et al: Latency period after preterm premature rupture of membranes. A comparison of ampicillin with and without sulbactam Obstet Gynecol 392:86, 1995 |
|
Pattinson RC, Makin JD, Funk M et al: The use of dexamethasone in women with preterm premature rupture of membranes-a multicentre, double-blind, placebo-controlled, randomised trial. Dexiprom Study Group S Afr Med J 865:89, 1999 |
|
Harding JE, Pang J, Knight DB et al: Do antenatal corticosteroids help in the setting of preterm rupture of membranes. ? Am J Obstet Gynecol 131:184, 2001 |
|
Christensen KK, Ingemarsson I, Leideman T et al: Effect of ritodrine on labor after premature rupture of the membranes. Obstet Gynecol 55:187, 1980 |
|
Garite TJ, Keegan KA, Freeman RK et al: A randomized trial of ritodrine tocolysis vs. expectant management in patients with premature rupture of membranes at 25–30 weeks of gestation Am J Obstet Gynecol 157:388, 1987 |
|
Miller JM Jr, Brazy JE, Gall SA et al: Premature rupture of the membranes: Maternal and neonatal infectious morbidity related to betamethasone and antibiotic therapy. J Reprod Med 25:173, 1980 |
|
Levy DL, Warsof SL: Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol 66:621, 1985 |
|
Weiner CP, Renk K, Klugman M: The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol 159:216, 1988 |
|
How HY, Cook CR, Cook VD et al: Preterm premature rupture of membranes: Aggressive tocolysis versus expectant management. J Matern Fetal Med. 8:7, 1998 |
|
Combs CA, McCune M, Clark R, Fishman A. Aggressive tocolysis does not prolong pregnancy or reduce neonatal morbidity after preterm premature rupture of the membranes. Am J Obstet Gynecol. 2004;190:1723-31. |
|
Curet LB, Rao AV, Zachman RD, Morrison JC, Burkett G, Poole WK, Bauer C. Association between ruptured membranes, tocolytic therapy, and respiratory distress syndrome. Am J Obstet Gynecol 1984;148:263-8 |
|
Dewan H, Morris JM. A systematic review of pregnancy outcome following preterm premature rupture of membranes at a previable gestational age. Aust N Z J Obstet Gynecol 2001-41:389-94. |
|
Moretti M, Sibai B: Maternal and perinatal outcome of expectant management of premature rupture of the membranes in midtrimester. Am J Obstet Gynecol 159:390, 396, 1988 |
|
Bottoms SF, Paul RH, Mercer BM et al: Obstetric determinants of neonatal survival: Antenatal predictors of neonatal survival and morbidity in extremely low birth weight infants. Am J Obstet Gynecol 665:180, 1999 |
|
Tyson JE, Younes N, Verter J et al: Viability, morbidity, and resource use among newborns of 501- to 800-g birth weight. National Institute of Child Health and Human Development Neonatal Research Network JAMA 1645:276, 1996 |
|
Wood NS, Marlow N, Costeloe K et al: Neurologic and developmental disability after extremely preterm birth. EPICure Study Group N Engl J Med 378:343, 2000 |
|
Fanaroff AA, Wright LL, Stevenson DK et al: Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991 through December 1992. Am J Obstet Gynecol 1423:173, 1995 |
|
D'Alton M, Mercer B, Riddick E et al: Serial thoracic versus abdominal circumference ratios for the prediction of pulmonary hypoplasia in premature rupture of the membranes remote from term. Am J Obstet Gynecol 166:658, 1992 |
|
Johnson A, Callan NA, Bhutani VK et al: Ultrasonic ratio of fetal thoracic to abdominal circumference: An association with fetal pulmonary hypoplasia. Am J Obstet GynecoI 157:764, 1987 |
|
Nimrod C, Varela-Gettings F, Machin G et al: The effect of very prolonged membrane rupture on fetal development. Am J Obstet Gynecol 148:540, 1984 |
|
Rotschild A, Ling EW, Puterman ML et al: Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet GynecoI 162:46, 1990 |
|
Tibboel D, Gallard ILL, Spritzer R et al: Pulmonary hypoplasia secondary to oligohydramnios with very premature rupture of fetal membranes. Eur J Pediatr 149:496, 1990 |
|
Vintzileos AM, Campbell WA, Rodis JF et al: Comparison of six different ultrasonographic methods for predicting lethal fetal pulmonary hypoplasia. Am J Obstet Gynecol 161:606, 1989 |
|
Moessinger AC, Collins MH, Baau WA et al: Oligohydramnios induced lung hypoplasia: The influence of timing and duration in gestation. Pediatr Res 20:951, 1986 |
|
van Eyck J, van der Mooren K, Wladimiroff JW: Ductus arteriosus flow velocity modulation by fetal breathing movements as a measure of fetal lung development. Am J Obstet Gynecol 163:558, 1990 |
|
Vilos GA, McLeod WI, Carmichael L et al: Absence or impaired response of fetal breathing to intravenous glucose is associated with pulmonary hypoplasia in congenital myotonic dystrophy. Am J Obstet Gynecol 148:558, 1984 |
|
Adzick NS, Harrison MR, Glick PL et al: Experimental pulmonary hypoplasia and oligohydramnios: Relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg 19:658, 1984 |
|
Blott M, Greenough A, Nicolaides KH et al: The ultrasonographic assessment of the fetal thorax and fetal breathing movements in the prediction of pulmonary hypoplasia. Early Hum Dev 21:143, 1990 |
|
Blott M, Greenough A, Nicolaides KH et al: Fetal breathing movements as predictor of favorable pregnancy out come after oligohydramnios due to membrane rupture in second trimester. Lancet 18:129, 1987 |
|
Blott M, Greenough A, Nicolaides K: Fetal breathing movements in pregnancies complicated by premature membrane rupture in the second trimester. Early Hum Dev 21:41, 1990 |
|
Greenough A, Blott M, Nicolaides K et al: Interpretation of fetal breathing movements in oligohydramnios due to membrane rupture. Lancet 23:182, 1988 |
|
Kilbride JW, Thibeault DW, Yeast J et al: Fetal breathing is not a predictor of pulmonary hypoplasia in pregnancies complicated by oligohydramnios. Lancet 6:305, 1988 |
|
Roberts AB, Goldstein I, Romero R et al: Fetal breathing movements after preterm premature rupture of membranes. Am J Obstet Gynecol 164:821, 1991 |
|
Nicolini U, Fisk NM, Rodeck CH et al: Low amniotic pressure in oligohydramnios: Is this the cause of pulmonary hypoplasia. ? Am J Obstet Gynecol 161:1098, 1989 |
|
Laudy JA, Tibboel D, Robben SG, de Krijger RR, de Ridder MA, Wladimiroff JW. Prenatal prediction of pulmonary hypoplasia: clinical, biometric, and Doppler velocity correlates. Pediatrics. 2002;109:250-8. |
|
Callan NA, Colmorgen GH, Weiner S: Lung hypoplasia and prolonged preterm ruptured membranes. A case report with implications for possible prenatal ultrasonic diagnosis. Am J Obstet Gynecol 151:756, 1985 |
|
Fong K, Ohlsson A, Zalev A: Fetal thoracic circumference: A prospective cross-sectional study with real-time ultra sound. Am J Obstet Gynecol 158:1154, 1988 |
|
Nimrod C, Davies D, Iwanicki S et al: Ultrasound prediction of pulmonary hypoplasia. Am J Obstet Gynecol 68:495, 1986 |
|
Nimrod C, Nicholson S, Davies D et al: Pulmonary hypoplasia testing in clinical obstetrics. Am J Obstet Gynecol 158:277, 1988 |
|
Roberts AB, Mitchell JM: Direct ultrasonographic measurement of fetal lung length in normal pregnancies and pregnancies complicated by prolonged rupture of membranes. Am J Obstet Gynecol 163:1560, 1990 |
|
Songster GS, Gray DL, Crane JP: Prenatal prediction of lethal pulmonary hypoplasia using ultrasonic fetal chest circumference. Obstet Gynecol 73:261, 1989 |
|
Baumgarten K, Moser S: The technique of fibrin adhesion for premature rupture of the membranes during pregnancy. J Perinat Med 14:43, 1986 |
|
Quintero RA: New horizons in the treatment of preterm premature rupture of membranes. Clin Perinatol 861:28, 2001 |
|
Sciscione AC, Manley JS, Pollock M et al: Intracervical fibrin sealants: a potential treatment for early preterm premature rupture of the membranes. Am J Obstet Gynecol 368:184, 2001 |
|
O'Brien JM, Milligan DA, Barton JR: Gelatin sponge embolization. a method for the management of iatrogenic preterm premature rupture of the membranes Fetal Diagn Ther 17:8-10, 2002 |
|
R, Millar LK, Bryant-Greenwood G, Lewi L, Deprest JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: a review of current evidence. Am J Obstet Gynecol. 2006;195:1512-20. |
|
Imanaka M, Ogita S, Sugawa T: Saline solution amnioinfusion for oligohydramnios after premature rupture of the membranes: A preliminary report. Am J Obstet Gynecol 161:102, 1989 |
|
Ogita S, Masahiko M, Yoshihiko T et al: Clinical effective ness of a new cervical indwelling catheter in the management of premature rupture of the membranes: A Japanese collaborative study. Am J Obstet Gynecol 159:336, 1988 |
|
Ogita S, Imanaka M, Matsumoto M et al: Transcervical amnioinfusion of antibiotics: A basic study for managing premature rupture of membranes. Am J Obstet Gynecol 158:23, 1988 |
|
Perlman M, Williams J, Hirsch M: Neonatal pulmonary hypoplasia after prolonged leakage of amniotic fluid. Arch Dis Child 51:349, 1976 |
|
Blaustein A, (ed): Pathology of the Female Genital Tract. p 771, 2nd ed.. New York, Springer-Verlag, 1984 |
|
Fanaroff AA, Martin RJ, (eds): Viral Infections in Neonatal-Perinatal Medicine. p 662, Vol 2:St. Louis, Mosby Year Book, 1992 |
|
Brown ZA, Vontver LA, Benedetti I et al: Genital herpes in pregnancy: Risk factors associated with recurrences and asymptomatic shedding. Am J Obstet Gynecol 153:24, 1985 |
|
Harger JH, Amortegui AI, Meyer MP et al: Characteristics of recurrent genital herpes simplex virus in pregnant women. Obstet Gynecol 73:367, 1989 |
|
Vontver LA, Hickok DE, Brown Z et al: Recurrent genital herpes simplex virus in pregnancy: Infant outcome and frequency of asymptomatic recurrences. Am J Obstet GynecoI 143:75, 1982 |
|
Arvin AM, Hensleigh PA, Prober CG et al: Failure of antepartum cultures to predict the infant's risk of exposure to herpes simplex virus at delivery. N Engl J Med 314:749, 1986 |
|
Catalano PM, Merritt AO, Mead PB: Incidence of genital herpes simplex virus at the time of delivery in women with known risk factors. Am J Obstet Gynecol 164:1303, 1991 |
|
Prober CG, Hensleigh PA, Boucher FD et al: Viral infections in neonatal-perinatal medicine. Use of routine viral cultures at delivery to identify neonates exposed to herpes simplex virus N Engl J Med 318:887, 1988 |
|
Chuang T: Neonatal herpes: Incidence, prevention and consequences. Am J Prev Med 4:47, 1988 |
|
Sullivan-Boyai J, Hull HF, Wilson C et al: Neonatal herpes simplex virus infection in King County, Washington. Increasing incidence and epidemiological correlates JAMA 315:796, 1983 |
|
American Academy of Pediatrics: Committee on Fetus and Newborn, Committee on Infectious Disease. Perinatal herpes simplex virus infections Pediatrics 66:147, 1980 |
|
Baldwin S, Whitley RJ: Teratogen update: Intrauterine herpes simplex virus infection. Teratology 39:1, 1989 |
|
Corey L, Adams HG, Brown ZA et al: Genital herpes simplex virus infections: Clinical manifestations, clinical course and complications. Ann Intern Med 98:958, 1983 |
|
Yeager AS, Arvin AM: Reasons for the absence of a history of recurrent genital infections in mothers of neonates infected with herpes simplex virus. Pediatrics 73:188, 1984 |
|
Brown ZA, Vontver LA, Benedetti J et al: Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med 317:1246, 1987 |
|
Stone KM, Brooks CA, Guinan ME et al: National surveillance for neonatal herpes simplex virus infections. Sex Transm Dis 16:152, 1989 |
|
Whitley RJ, Corey L, Arvin A et al: Changing presentation of herpes simplex virus infection in neonates. J Infect Dis 158:109, 1989 |
|
Amstey MS: Management of pregnancy complicated by genital herpes virus infection. Obstet Gynecol 37:515, 1971 |
|
Nahmias AI, Josey WE, Naib ZM: Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol 110:825, 1971 |
|
Grossman JH III: Herpes simplex virus HSV infections. Clin Obstet Gynecol 25:555, 1985 |
|
Ray DA, Evans AT, Elliot IP et al: Maternal herpes infection complicated by prolonged premature rupture of membranes. Am J Perinatol 2:96, 1985 |
|
Utley K, Bromberger P, Wagner L et al: Management of primary herpes in pregnancy complicated by ruptured membranes and extreme prematurity: Case report. Obstet Gynecol 69:471, 1987 |
|
Major CA, Towers CV, Lewis DF et al: Expectant management of patients with both preterm premature rupture of membranes and genital herpes (abstr 16). Am J Obstet Gynecol 164:248, 1991 |
|
Mckeown T, Record RG: Observations in fetal growth in multiple pregnancy in man. J Endocrinol 5:387, 1952 |
|
Caspi E, Ronen J, Schreyer P et al: The outcome of pregnancy after gonadotropin therapy. Br J Obstet Gynecol 83:967, 1976 |
|
Naeye RL, Tafari N, Judge D et al: Twins: Causes of perinatal death in 12 United States cities and one African city. Am J Obstet Gynecol 131:267, 1978 |
|
Mercer BM, Crocker LG, Pierce WF et al: Clinical characteristics and outcome of twin gestation complicated by preterm premature rupture of the membranes. Am J Obstet Gynecol 168:1467, 1993 |
|
Montgomery DM, Perlow JH, Asrat T et al: Preterm pre mature ruptured membranes in the twin gestation: A case control study (abstr 310). Am J Obstet Gynecol 166:360, 1992 |
|
Borenstein R, Shoham Z: Premature rupture of the membranes in a single twin gestational sac: A case report. J Reprod Med 35:270, 1990 |
|
Treadwell MC, Bronsteen RA, Bottoms SF: Prognostic factors and complication rates for cervical cerclage: A review of 482 cases. Am J Obstet Gynecol 165:555, 1991 |
|
Harger JH, Hsing AW, Tuomala RE et al: Risk factors for preterm premature rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol. 163:130, 1990 |
|
Blickstein J, Katz Z, Lancet M et al: The outcome of pregnancies complicated by preterm rupture of the membranes with and without cerclage. Int J Obstet Gynecol 237:28, 1989 |
|
Yeast JD, Garite TJ: The role of cervical cerclage in the management of preterm premature rupture of the membranes. Am J Obstet Gynecol 106:158, 1988 |
|
Ludmir J, Bader T, Chen L, al: Poor perinatal outcome associated with retained cerclage in patients with premature rupture of membranes. Obstet Gynecol 823:84, 1994 |
|
Jenkins TM, Berghella V, Shlossman PA et al: Timing of cerclage removal after preterm premature rupture of membranes: Maternal and neonatal outcomes. Am J Obstet Gynecol. 847:183, 2000 |
|
McElrath TF, Norwitz ER, Lieberman ES et al: Management of cervical cerclage and preterm premature rupture of the membranes: should the stitch be removed. ? Am J Obstet Gynecol 840:183, 2000 |