Anorexia Nervosa and Bulimia
Authors
INTRODUCTION
Anorexia nervosa is a syndrome that combines bizarre behavioral manifestations with self-induced weight loss and amenorrhea. This illness is occurring with increasing frequency.1 Although the incidence is 0.242 to 1.63 per 100,000 people, it differs greatly among different population groups.4,5 One in 100 girls age 16 to 18 years were diagnosed to have this syndrome in Great Britain, and partially recovered anorexia nervosa was the most common cause of amenorrhea in another report.3 An at-risk population appears to exist. This syndrome occurs in 1 of every 100 middle-class adolescent girls.1
Professional ballet dancers have an incidence ranging from 1 in 206 to 1 in 5,7 depending on the competitive level of the company from which the survey originated. The striking incidence of this disorder in ballet dancers may relate to the rigid standards for thinness and the significantly greater number of hours of exercise performed,7 particularly in the highly competitive ballet companies. These environmental factors may be fertile ground for the development of anorexia nervosa. Animal studies suggest that increased levels of activity and restricted eating can induce self-starvation in rats, indicating an animal model for an activity-based anorexia.8 Studies also indicate that some ethnic groups have a much lower incidence of the syndrome anorexia nervosa; for instance, it is very rare among blacks, including black ballet dancers who are exposed to the same rigid standards of competition and weight restriction.7 The low incidence of this problem among blacks may relate to different sociocultural influences, or, conceivably, this group may possess more efficient metabolic mechanisms for dealing with the high activity level and lowered caloric intake. Some reports are appearing on the disorder in blacks, but it is generally thought to be rare.9 Studies on monozygous twins have indicated a genetic factor in the emergence of the syndrome and a risk for female siblings of 6%,10 suggesting that inborn metabolic factors may be contributing to the risk of the syndrome. There is also an increased incidence of this problem in association with Turner's syndrome,11 diabetes mellitus,12,13 and Cushing's disease,14 suggesting that factors associated with these conditions may predispose to the development of the illness. Although rare in men (male/female ratio is 1:9),15 this syndrome has been reported in men who are training for competitive activity while restricting their weight.16 Data indicate that the incidence of this syndrome is definitely increasing.17
Bulimia is often related to previous anorectic behavior. Bulimics gorge themselves and use artificial means to purge themselves of calories; the gorging episodes may alternate with periods of severe food restriction. Bulimia occurs among high school and college students (incidence varies from 2% in community-based samples to 4% to 13% in college age-groups)18,19,20 and may be more common than anorexia nervosa in males. In addition, others have described a separate condition known as bulimia nervosa (see The Bulimic Syndrome).21,22 Bulimics have a wide variety of medical problems that may be superimposed on the anoretic syndrome, including severe tooth decay, parotid enlargement, stomach rupture, metabolic alkalosis, carpopedal spasm, hypercarotenemia, and pancreatitis.23,24,25,26,27
The frequency of weight-loss “diet”-related amenorrhea among young women suggests that amenorrhea in this setting may represent a very mild form of the severe hypothalamic disorder seen in anorexia nervosa.
Anorexia nervosa occurs almost invariably in young, white, middle- to upper-class girls younger than 25 years of age; the syndrome manifests itself by weight loss to a pathologic degree accompanied by a self-imposed food restriction and a distorted mental image of the body. Occasionally there are bizarre food habits (hiding of food, mastication and spitting, vomiting) and hyperactivity (running or jogging, gymnastics).28 The precipitating factors are variable and are sometimes difficult to differentiate from the hallmarks of a normal adolescence. Deep-seated psychologic problems have been implicated and are the source of much literature.29 A family history of depression, feelings of ineffectiveness, and poor interceptive awareness have all been cited as risk factors for the disorder among adolescents. A family history of alcohol and drug abuse or dependence also seems to be implicated.29A The endocrine manifestations suggest that this syndrome may be a primary hypothalamic problem rather than psychogenic in nature. Accumulating evidence, both direct and indirect, indicates that the hypothalamus is malfunctioning. Whether the hypothalamic dysfunction is a primary problem or secondary to the starvation and weight loss is still unclear. There are no observed data on the early stages of the evolution of this disorder, so the mechanism is unknown. The interrelationships among the psychologic disturbance, the hypothalamic dysfunction, and the effects of weight loss and starvation are the subject of considerable debate in the literature.
CLINICAL SYNDROME
Criteria for the diagnosis of anorexia nervosa differ,28,29 but in general a classic triad of a psychiatric disturbance, weight loss, and amenorrhea is always recognized. The amenorrhea occasionally occurs before the onset of weight loss, but in general it can be pinpointed to the onset of food restriction even if weight loss has been only slight.15,30,31 Occasionally this condition may occur before menarche, and the patient may present with primary amenorrhea. With progressive weight loss, a familiar syndrome ensues.
Signs and Symptoms
There is no blood test or specific physical finding that makes the diagnosis of anorexia nervosa. The clinician makes the diagnosis based on a symptom complex32 that includes a severe weight loss (usually down to less than 20% of ideal body weight); behavioral changes, such as hyperactivity and preoccupation with food; and perceptual changes, in particular a distorted view of the body, generally with an unreasonable concern about being “too fat.” The physical and metabolic adaptations to a self-induced semistarved state are thought to bring about the diverse physical changes.
Some of the most common manifestations are listed in Table 1. Most of the signs and symptoms have been noted in starvation.15 Amenorrhea may be the presenting complaint and the symptom that causes the patient or family to seek medical advice. Constipation may be extreme (bowel movements once a week) and may be accompanied by severe abdominal pain. Preoccupation with food may manifest as a fanatic interest in calories, tabulations of intake, and large intakes of lettuce, raw vegetables, diet sodas, and other foods thought to be low in caloric value. Patients occasionally develop an aversion to specific types of food, such as meat or butter.
TABLE 1. Symptoms and Signs of Anorexia Nervosa
| Total No. | % | Reported in Starvation |
Amenorrhea (22 postpubertal girls) | 22/22 | 100 | Yes |
Constipation | 26/42 | 61.9 | Yes |
Preoccupation with food | 19/42 | 45.2 | Yes |
Abdominal pain | 8/42 | 19 | Yes |
Intolerance to cold | 8/42 | 19 | Yes |
Vomiting | 5/42 | 4.9 | No |
Hypotension | 36/42 | 85.7 | Yes |
Hypothermia | 27/42 | 64.3 | Yes |
Dry skin | 26/42 | 61.9 | Yes |
Lanugo-type hair | 22/42 | 52.4 | Yes |
Bradycardia | 11/42 | 26.2 | Yes |
Edema | 11/42 | 26.2 | Yes |
Systolic murmur | 6/42 | 14.3 | No |
Petechiae | 4/42 | 9.5 | Yes |
(Adapted from Warren MP, Vande Wiele RL: Clinical and Metabolic features of anorexia nervosa. Am J Obstet Gynecol 117:435, 1973)
Hypercarotenemia has been reported in anorexia nervosa, and increased levels of carotenes in the blood give a yellow cast to the skin. This is only in part a result of an increased intake of raw vegetables. Investigations have suggested that a metabolic deficit that occurs in the setting of dieting prevents the normal metabolism of carotene, a precursor of vitamin A.33 This yellow substance is deposited in the subcutaneous layer of the skin and is seen in patients with hypothalamic amenorrhea related to dieting. The yellowish hue can be seen in the palms and soles, but the sclerae remain clear. One study suggests that limiting the intake of foods high in vitamin A, and thereby lowering carotene intake, may reverse the amenorrhea.34 In addition, hyperactivity may set in early in the illness, with hours spent on gymnastics, jogging, tennis, or other active pursuits.
Clinical Laboratory Findings
Hypotension can be extreme, and a pediatric cuff is sometimes necessary to measure the blood pressure in an emaciated extremity. A marked hypothermia develops, and patients may have a resting basal temperature lower than 96°F (35.6°C). The skin becomes rough and dry, and a soft, downy, lanugo-type hair may grow on the back and buttocks. Bradycardia can be extreme, and pulses as low as 30 bpm have been recorded during rest. Changes noted in the electrocardiogram include low voltage and low and inverted T waves. Edema is usually pitting and pretibial and may occur during weight gain. Hypoalbuminemia usually is not seen. Petechiae may occur and may be associated with a hypoplastic bone marrow. Common clinical laboratory findings are shown in Table 2. The azotemia is generally caused by dehydration, because the creatinine is normal and values return to normal with therapy. Rarely, true renal disease is seen in a patient who has resorted to long-term diuretic and laxative abuse and has persistent hypokalemia. The leukopenia may be as low as 2000 cells/mm3 and is usually accompanied by a relative lymphocytosis. The elevation of the serum carotene concentration may cause a yellowish coloration of the skin, well seen in the palms and other skin creases. This can be associated with a large intake of raw yellow vegetables, although its persistence in the face of a change in diet suggests that an acquired defect in the utilization of vitamin A may be present.35 A few patients are noted to have delayed emptying of barium from the stomach and considerable slowing of the passage of barium from the small bowel. In contrast to other forms of cachexia, anemia is rare.
TABLE 2. Clinical Laboratory Findings in Anorexia Nervosa
| Total No. | % | Reported in Starvation |
Abnormal electrocardiogram | 13/25 | 52.2 | Yes |
Hypoplastic marrow | 6/13 | 46.2 | Yes |
Blood urea nitrogen >20 | 17/42 | 40.5 | Yes |
White blood cell count <5000 cu mm | 16/42 | 38.1 | Yes |
Relative lymphocytosis (>49%) | 6/42 | 14.3 | Yes |
Diabetic glucose tolerance test | 6/16 | 37.5 | Yes |
Flat glucose tolerance test | 4/16 | 25.0 | Yes |
Thrombocytopenia | 1/4 | 25.0 | No* |
D -Xylose absorption | 8/18 | 22.5 | No* |
Increased serum carotene | 5/13 | 38.0 | Yes |
Hypomobile uper gastrointestinal tract | 3/42 | 7.1 | Yes |
Anemia | 3/42 | 7.1 | Yes |
*These parameters have not been studied in starvation.
(Adapted from Warren MP, Vande Wiele RL: Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 117:435, 1973)
Numerous medical problems have also been reported in this syndrome, including salivary gland enlargement,36 cardiac arrhythmias,37 pericardial effusion,38 pancreatitis,39,40,41,42 pancreatic insufficiency,43,44 liver dysfunction,43 pneumomediastinum,44,45 kidney stones,46 trace metal deficiencies including hypoproteinemia and zinc deficiency,47 thiamine deficiency,48 coagulopathies,37,49 electrolyte imbalance,50,51 hypophosphatemia,52 and bilateral peroneal nerve palsies.53 Marked vasoconstriction of the extremities may also occur in this syndrome as a heat-conserving mechanism,54 and if the weight loss occurs at or before the growth spurt, documented and permanent growth deficiencies may occur.55 Hematologic abnormalities, such as decreased counts of total leukocytes, neutrophils, monocytes, and platelets, seem to be correlated with total body fat mass depletion.55A Although there is a marked leukopenia, increased risk of infection has not been documented and cell-mediated immunity appears to remain intact.56,57,58 The hypoglycemia in this syndrome has been severe enough to cause coma.59 Ulcers can occur with anorexia,60 as can acute vascular compression of the duodenum and the superior mesenteric artery syndrome,61,62,63 so it is unwise to dismiss complaints of abdominal pain without a detailed history and appropriate work-up. Osteoporosis and fractures are also being reported, problems that are most likely multicausal and the result of long-term poor nutritional intake as well as prolonged estrogen deficiency.64,65,66
Endocrinopathy
The endocrine changes seen in this syndrome, summarized in Table 3, have fascinated scientists because they represent strong indirect evidence for a hypothalamic dysfunction. The typical hormone secretory patterns include low levels of plasma luteinizing hormone (LH) and follicle-stimulating hormone (FSH), accompanied by a profound estrogen deficiency.15,67 LH secretion is particularly affected, and the hormone may be undetectable. Some of the lowest LH values seen in secondary amenorrhea have been observed in this syndrome. The clinical pattern is that of a hypogonadotropic hypogonadism, which is at least in part caused by the weight loss. The gonadotropin levels return to normal with weight gain, although the amenorrhea may persist. The apparently reversible, selective, acquired deficiency of LH, and often FSH, seen in this syndrome presents some unique aspects. The low LH and FSH levels can be accompanied by low thyroxine and high plasma cortisol concentrations; the latter finding differentiates anorexia nervosa from pituitary insufficiency.15
TABLE 3. Endocrine Changes in Anorexia Nervosa
| Total No. | % | Reported in Starvation |
Low basal metabolic rate-5% | 9/9 | 100 | Yes |
Atrophic vaginal smear | 13/13 | 100 | Yes |
Low plasma LH (postpubertal) | 13/15 | 87 | Yes |
| (Total urinary) | ||
Low plasma FSH (postpubertal) | 7/15 | 47 | Yes |
| (Total urinary) | ||
Low urinary 17-ketosteroids | 13/34 | 38 | Yes |
Low urinary 17-ketogenic steroids | 13/34 | 38 | Yes |
Low thyroxine | 11/32 | 34 | Yes |
High plasma corticoids | 3/23 | 13 | Yes |
Hyperresponsive adrenocorticotropic hormone test | 2/5 | 40 | Yes |
(Adapted from Warren MP, Vande Wiele RL: Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 117:435, 1973)
EVIDENCE FOR HYPOTHALAMIC DYSFUNCTION
Studies of the gonadotropin deficiency in anorexia nervosa reveal a number of qualitative and quantitative abnormalities suggestive of a hypothalamic dysfunction. The response of luteinizing hormone-releasing hormone (LHRH) is reduced by a factor that is directly correlated with weight loss. In addition, the pattern of response in these underweight patients is more akin to the pattern seen in prepubertal children; that is, the FSH response is greater than the LH response. The opposite is usually found in the postpubertal state. The return of LH responsiveness is correlated with weight gain68,69 or can be induced by repeated injections of LHRH,70 suggesting that the pituitary gonadotropes have become sluggish owing to the lack of endogenous stimulation with LHRH. Moreover, patients who are partially recovered from anorexia nervosa tend to have exaggerated responses to LHRH.67,69 These changes have been seen in children in early puberty, suggesting that in anorexia nervosa the hypothalamic signals of the central nervous system revert to a prepubertal or a pubertal state.
Attempts to explain the neurotransmitter defect that most probably underlies the gonadotropin-releasing hormone (GnRH) abnormalities have been confusing. Naloxone, which inhibits endogenous opiates, causes increases in gonadotropins in some71 but not all72,73 patients with anorexia nervosa and in one study caused further decrease in gonadotropin levels.74 Dopaminergic mechanisms have not been specifically examined in anorexia nervosa, although in other types of hypothalamic amenorrhea an increase in LH pulse frequency has been noted with metoclopramide, a dopamine receptor blocker.75 Stress and activation of the hypothalamic-pituitary-adrenal axis has also been suggested as a possible mechanism. Cortisol is elevated, and responses to corticotropin-releasing hormone (CRH) are abnormal.76,77,78 Increased CRH is also found in cerebrospinal fluid of patients with anorexia nervosa. CRH is known to inhibit GnRH secretion. CRH may also augment dopaminergic and opiodergic inhibition of GnRH.79,80,81 The defect therefore is still uncertain, but all of these pathways are undoubtedly linked to cause dysfunction of the GnRH pulse generator.
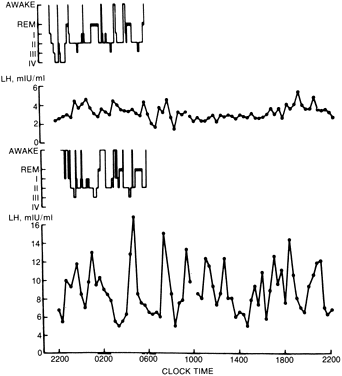
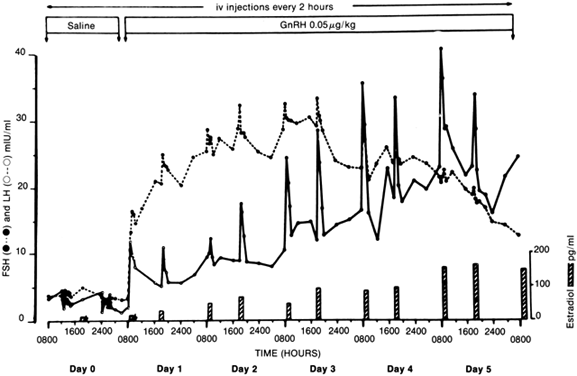
Patients with anorexia nervosa have persistent low levels of LH and FSH throughout the day and night, similar to prepubertal children. With recovery (weight gain), sleep-associated episodic secretion of LH appears, similar to that of the early pubertal child. With full recovery, the 24-hour pattern resembles that of a normal adult, with fluctuating levels without sleep-associated spikes82,83 (Fig. 1). It appears that the central nervous system mechanisms responsible for these patterns regress to a prepubertal state during the illness and reverse with weight gain. Artificially, the pattern of gonadotropin secretion can be made to revert to a normal adult-like pattern by the administration of pulsatile GnRH. If the drug is given intravenously or subcutaneously every 2 hours, a normal adult-like pattern of gonadotropin secretion results and menstrual bleeding and ovulation can be induced84 (Fig. 2). In one study, baseline plasma gonadotropin levels were lower both in patients with anorexia nervosa and in normal-weight, refed anorexia nervosa patients, compared with controls. Percentages of total gonadotropin not bound to concanavalin A (complex carbohydrate chains) were higher in anorexia nervosa patients than in controls, and in refed patients the percentage of unbound FSH was higher than in controls. On administration of GnRH, these respective percentages decreased. Although administration increased LH and FSH levels for anorexic and recovering patients, these increases were lower than they were for controls. The administration of GnRH appears to induce changes in circulating gonadotropin isoforms in subjects with and without anorexia nervosa. Furthermore, the LH response in recovering subjects and in controls is associated with the same glycosylation pattern. Therefore, the biologic activity of gonadotropin may be affected by changes produced by the administration of GnRH.84A Research indicates that neurons containing GnRH are situated in the arcuate nucleus of the hypothalamus and that axons descend into the median eminence, where they terminate. From this vascular area, a message is sent from the nerve terminals by way of the hypophyseal portal vein to pituitary gonadotropes situated in the anterior pituitary.85 These findings suggest that the amenorrhea seen in anorexia nervosa is probably caused by faulty signals reaching the medial central hypothalamus from the arcuate nucleus, the center most likely to be responsible for the important episodic stimulation of GnRH.
Despite the return of normal gonadotropin secretory patterns, amenorrhea persists in almost 30% of patients with anorexia nervosa.15,86 Sonographic visualization of the ovaries suggests cystic ovaries similar to those seen at adolescence.87,88 A 10-year follow-up study suggested that amenorrhea persists in 49% of patients overall, but in only 10% of those who have a normalization of weight.89
Therefore, other mechanisms, yet to be elucidated, are at fault. A likely mechanism is leptin, a hormone produced by the obese gene in mice and secreted by the adipocyte that has received considerable attention since its discovery. One reason leptin is so fascinating is that it seems to be a critical link between metabolic and reproductive pathways. Numerous studies in rodents have demonstrated that ob/ob rodents born without an active form of leptin tend to be overweight and, intriguingly, are also amenorrheic and infertile. In humans, leptin appears to modulate food intake by affecting appetite, energy requirements, and eating behavior.89A In addition, low leptin levels have been reported in patients with hypothalamic amenorrhea.89B There appear to be critical leptin levels needed to maintain menstruation.89C Furthermore, low leptin levels have been linked with low bone mass.89L
Hypoleptinemia has consistently been observed in patients with anorexia nervosa, but leptin levels increase significantly with partial weight recovery.89D,89E,89F,89G Although leptin levels correlate linearly with body mass index in recovering and normal subjects, levels uncouple in a nonlinear fashion in untreated anorexics, suggesting a threshold effect at lowest body weights.89D By comparing serum leptin levels among patients with various eating disorders, researchers have found that leptin levels are not correlated to a specific pathology but rather to individual body mass index. Therefore, leptin levels cannot be used to diagnose or develop a prognosis for anorexia.89H Although there is a high correlation between absolute body fat mass and leptin concentration, fasting behavior, regardless of body fat level, may also be correlated with low leptin.89I Several studies have shown a rapid, disproportional lowering of leptin concentration with starvation.89J,89K Anorexic patients have two factors working to lower their leptin concentration: low body fat and starvation itself. Leptin levels have been shown to normalize and perhaps peak at higher than normal values with incomplete weight gain.89E,89F This underlies the importance of leptin in the pathology of anorexia: because leptin acts to reduce food intake, the restoration of leptin concentration before full weight recovery can be extremely detrimental to the patient with anorexia nervosa. When coupled with a threshold effect at lowest weights, this characteristic response to weight gain in anorexic patients serves to perpetuate the disorder.
Of interest also are the low estradiol levels; they are partially a result of lack of ovarian stimulation, but estrogen metabolism appears to be altered. With weight loss, the metabolism of estradiol, which normally proceeds with 16α-hydroxylation, is decreased in favor of 2-hydroxylation and the formation of catechol estrogen (2-hydroxyesterone).90 The latter compound has features of an antiestrogen in that it has no intrinsic biologic activity.91 Therefore, the extraordinarily low estrogen levels seen in this syndrome are compounded by the presence of an endogenously produced antiestrogen, and, in addition, the lack of fat tissue may deny the patient extraovarian sources of estrogen (i.e. a source of estrone conversion from androstenedione).
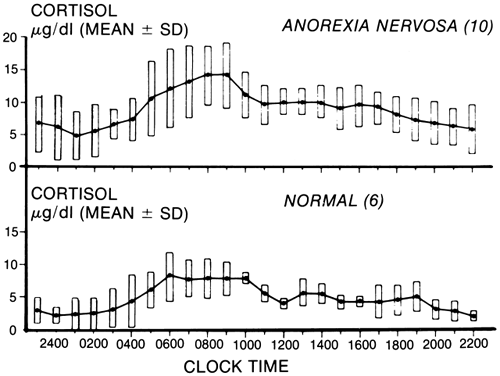
Major alterations have also been reported. Mean levels of cortisol are elevated, and random sampling may suggest an absence or even a reversal of the normal circadian rhythm. Twenty-four-hour studies have shown that both the episodic and the circadian rhythm are normal, but the cortisol levels are considerably higher than normal ( Fig. 3).92 This change, which has also been seen in malnutrition, results from a prolonged half-life of the cortisol caused by reduced metabolic clearance. As would be expected, the levels of the urinary steroids, including the 17-hydroxysteroids and the 17-ketosteroids, are usually low. Other studies have suggested that the production rates may be elevated,93 and the cortisol concentrations may exceed the binding capacity of cortisol-binding globulin (CBG). There appears also to be a decreased affinity of CBG for cortisol. Therefore, unbound cortisol may be significantly increased and available to the tissues in patients with anorexia nervosa. One would expect the higher levels of cortisol measured in these patients to suppress adrenocorticotropic hormone (ACTH). The observation that a new circadian rhythm at the higher cortisol level is established suggests that a new set point is determined by the hypothalamic-pituitary-adrenal axis. ACTH responses to CRH show blunting, and increased CRH in cerebrospinal fluid suggests activation of this axis.79,80
Despite their marked cachexia, patients with anorexia nervosa have clinical and metabolic signs suggestive of hypothyroidism. These include constipation, cold intolerance, bradycardia, hypotension, dry skin, prolonged ankle reflex, low basal metabolic rate, and hypercarotenemia.15,94 Some of these changes do suggest a compensatory hypometabolism, and studies on the circulatory system show that during maximal exercise the attainable oxygen uptake and heart rate are low in children with anorexia nervosa. The maximum oxygen consumption (V®O2max) appears to be decreased out of proportion to the circulatory and body dimensions, indicating an adaptation to the low caloric intake.95
Serum levels of thyroxine (T4), triiodothyronine (T3), and thyroid-stimulating hormone (TSH) in patients with anorexia nervosa are significantly lower than in normal persons, particularly the level of T3. Abnormal thyroid tests include low basal T3, T4, and TSH. T3 appears to increase with weight gain, but T4 and TSH remain low, and all three hormones are lower than in controls.96 The low T3 levels may be explained by an alteration in T4 conversion. In anorexia nervosa, as in starvation, the peripheral deiodination conversion of T4 is diverted from formation of the active T3 to production of reverse T3, an inactive metabolite.97 Evidence also indicates that fasting decreases hepatic uptake of T4, with a proportionate decrease in T3 production.98 The low T4 value is somewhat more difficult to explain. Low T4 euthyroidism has been seen in seriously ill patients,99,100 in whom studies indicate there is a unique dysfunctional state of deficient T4 binding with a normal free T4 availability to peripheral tissue sites. Presumably, a similar mechanism may be operative in anorexia nervosa. Secretion of TSH appears to be normal, but the peak TSH response to thyroid-releasing factor (TRF) stimulation is delayed, from 30 or 60 to 120 minutes.101 This may also reflect an altered set point for endogenous TRF regulation. Another study showed that the T4 and T3 response to thyroid-releasing hormone (TRH) is subnormal, although the TSH response is normal, suggesting chronic understimulation of the thyroid, as occurs in hypothalamic hypothyroidism.96
Other parameters of pituitary-hypothalamic function have been studied. Growth hormone (GH) levels are known to be increased in starvation or in any situation with restriction of food beyond the normal 12- to 15-hour “overnight” fast. In general, basal GH levels are significantly higher than normal in anorexia nervosa but respond normally to one or more provocative stimuli. The enhanced GH secretion in anorexia nervosa may be caused by a heightened frequency of secretory pulses superimposed on augmented tonic GH secretion. When anorexics are compared with subjects of normal weight and with obese subjects, the enhancement of GH secretion appears to reflect a complex hypothalamic dysregulation of GH release, as opposed to simply a malnutrition-induced impairment of the production of insulin-like growth factor I.96A Occasionally, low levels are seen with blunted responses to insulin hypoglycemia.15 The GH levels associated with delta-wave sleep are normal.102 The basal prolactin level is indistinguishable from normal. TRF-stimulated prolactin levels are also normal, although the time of peak prolactin is delayed.102
In addition to the endocrine abnormalities described, a number of other physiologic changes strongly suggest a hypothalamic dysfunction. One of the most impressive is the disorder of thermoregulation reported in this condition.103 When exposed to acute hypothermia or hyperthermia under controlled conditions, persons with anorexia nervosa do not show the normal adjustments. For instance, in the cold, no shivering or vasoconstriction occurs, and core temperature continues to fall. In the heat, no vasodilation occurs, and eventually the core temperature rises with no stabilization. The rate of change of core temperature per hour also correlates with the percent below ideal body weight. In addition, the absence of vasoconstriction and vasodilation prevents the “paradoxic” initial decrease in temperature seen in the heat and the rise seen in the cold. These responses, including shivering, vasoconstriction, and vasodilation, indicating loss of central temperature, are dysfunctional in the anorexic. The thermosensitive neurons in the anterior hypothalamus (which mediate vasomotor responses) and its interconnectives with a motor center for shivering in the posterior hypothalamus may be affected. Studies of thermoregulatory responses to a given metabolic heat load resulting from exercise in anorexia nervosa indicate that the altered response may involve other mechanisms. In particular, the low sweating capacity seen in this syndrome and the loss of body fat, which reduces thermal insulation, alter the ambient temperatures over which the body temperature can be maintained during exercise.104
Patients with anorexia nervosa also appear to have abnormalities of water conservation that are diagnostic of partial diabetes insipidus. This was judged by the ability to concentrate the urine maximally after 15 hours of dehydration and a further response to exogenous vasopressin. A further increase in urine osmolarity after administration of exogenous antidiuretic hormone was seen in the majority of patients with anorexia nervosa and was diagnostic of partial diabetes insipidus.103
The altered behavior patterns in this syndrome are also distinctive and include preoccupation with food and hyperactivity. Sometimes periods of gorging (bulimia) alternate with starvation and food avoidance. Altered food intake, combined with activity changes, has been seen in hypothalamic tumors and has been documented in rats with lesions of the ventromedial nucleus of the hypothalamus. These lesions have led investigators to conclude that the ventromedial nucleus can inhibit food intake and promote activity, as seen in anorexia nervosa.
Other behavioral changes include a perceptual distortion in which patients with anorexia nervosa perceive themselves to be too fat. This body image misperception can be quantitated by the use of a special apparatus consisting of two movable lights equidistant from a central point. The subject can move the lights until they appear to approximate what she perceives to be specific body dimensions or even a piece of wood. Experiments of this nature have shown that there is a direct association between the degree of the perceptual disorder and that of malnutrition and that patients with anorexia nervosa consistently overestimate body and other dimensions.105 These perceptual abnormalities may be aggravated by a high carbohydrate meal and tend to disappear with weight gain. This suggests that when the patient is severely underweight, the illness tends to be self-perpetuating, and that weight gain in itself is an important goal to break this vicious cycle. Other investigators have found, however, that these abnormalities are complexly determined and can be demonstrated in nonanorectic persons in their teens and in obese subjects. Generally, the prognosis is thought to be better in those patients whose self-reported estimates are more or less accurate after restoration of weight and are no longer influenced by an ordinary carbohydrate meal. Gross overestimation of body widths is associated with premorbid obesity, bulimia and vomiting.106
Studies have also been performed to evaluate the behavior disorder seen in anorexia as well as the perceptual abnormality. Anorexic behavior scales have been devised to aid in differentiating persons with anorexia nervosa from those who have secondary amenorrhea due to other causes. The scale may consist of a psychologic profile that is self-administered or administered by a doctor, nurse, or clinical psychologist. One study indicated that the anorectic behavior scale was very useful and accurate in distinguishing healthy female subjects from those with anorectic behavior. These scales may therefore be useful in the early diagnosis of anorexia nervosa, particularly in patients presenting with only secondary amenorrhea and little or no weight loss. In contrast to the perceptual disorder, the degree of anorectic behavior does not always correlate with the malnutrition, suggesting that the anorectic behavior is more pronounced in the better-nourished patients.107
Along with the behavioral disorder, objective changes in the brain have been demonstrated by computed tomography. Enlargement of the cortical sulci and subarachnoid spaces, as well as cerebral atrophy, has been demonstrated in a small number of cases, with reversal of the atrophy with weight gain in one.108,109 A number of recent studies suggest that changes in the brain resulting from anorexia nervosa may be irreversible. Gray matter volume deficits, unilateral temporal lobe hypoperfusion, and various localized brain dysfunctions appear to persist despite weight recovery.109A,109B,109C
Anorexia nervosa continues to be a perplexing syndrome, most likely psychogenic in nature. The large preponderance of girls from upper socioeconomic backgrounds who are affected at or near puberty suggests the importance of environmental influences. The fact that the amenorrhea may precede the development of the full-blown syndrome has often been emphasized, although in most studies amenorrhea has most often coincided with the onset of food restriction. Many of the signs and symptoms (including psychologic changes) seen in anorexia nervosa have been reported in starvation, and it is difficult to tell when “anorexia” leaves off and starvation begins to predominates.
Some of the metabolic changes—including lowered metabolic rate, a decrease in attainable oxygen uptake and maximal aerobic power (V O2max), an increase in cortisol (which stimulates gluconeogenesis and decreases peripheral glucose utilization), and a decrease in gonadotropins (with a loss of fertility)—are appropriate adaptations to starvation.
On the other hand, the early development of amenorrhea and behavioral symptoms also suggests a primary hypothalamic syndrome. The abnormalities of gonadotropin release and activity are associated with the medial central hypothalamus; those of thermoregulatory control, with the anterior and posterior hypothalamus; and those of water conservation, with the supraoptic area. The involvement of all these areas suggests a lesion too diffuse to be anything but perhaps metabolic. Although anorexia nervosa may be a primary hypothalamic syndrome, it is much more likely that this dysfunction is a result of the diffuse metabolic changes associated with starvation (Table 4).
TABLE 4. Abnormalities Suggestive of Hypothalamic Dysfunction in Anorexia Nervosa
Abnormality | Area of Dysfunction |
Amenorrhea | |
Gonadotropin release and control | Medial central |
Thermoregulatory control | Anterior |
| Posterior |
Water conservation | Supraoptic |
| Paraventricular |
Behavioral | Ventromedial |
Altered food intake | |
Activity | |
The association of psychologic and neuroendocrine changes in patients with anorexia nervosa has led investigators to speculate that abnormalities of neurotransmission may be involved in the pathogenesis of the syndrome. Excessive dopamine and norepinephrine in particular have well-documented effects on behavior and appetite.110 In view of strong evidence depicting anorexia nervosa as a hypothalamic syndrome, it is possible to hypothesize that some of the neuropeptides, such as beta-endorphin, may have roles in mediating the abnormalities seen. These peptides have been isolated from other areas than the brain, including the gut, and it is not unreasonable to consider that the messages mediated by these neuropeptides may play a role in the alterations seen in anorexia nervosa.111,112
Evidence also indicates that some estrogens are formed in the brain and that their synthesis is favored in anorexia nervosa; an example is the catechol estrogen, 2-hydroxyestrone. Catechol estrogens may have the potential for interacting with both the catecholamine and the estrogen-mediated system in the central nervous system, thereby influencing dopamine and norepinephrine synthesis.113
ANTECEDENTS OF ANOREXIA NERVOSA
The striking incidence of anorexia nervosa in professional ballet dancers suggests that the chronic dieting behavior followed by persons in this profession to achieve a thin body image may be important in the pathogenesis of anorexia nervosa. A number of investigators have been interested in the concept that anorexia nervosa occurs as a continuum of chronic dieting behavior. Weight loss-related amenorrhea and exercise-induced amenorrhea have similar endocrine profiles, suggesting that all three entities have similar hypothalamic mechanisms.114 Regression to prepubertal patterns of gonadotropin secretion and nocturnal LH spurting have been seen in weight loss-related amenorrhea, similar to patterns seen in amenorrhea with anorexia nervosa.115 Ballet dancers with amenorrhea also have similar endocrine profiles, although their 24-hour and nocturnal patterns have not been studied.114 Investigations of the menstrual patterns of ballet dancers indicate that amenorrhea (or absence of menses lasting longer than 5 months) is more significantly related to abnormal eating patterns and a history of anorexia nervosa than to exercise training.116 In this group, the incidence of anorexia nervosa was 20%, and the incidence was significantly higher in the more competitive companies.7 The contributions of exercise training, weight loss, lowered body fat, dieting, and anorexia nervosa to the genesis of amenorrhea seen in exercise training remain unresolved. The fact that some of these activities are fertile breeding ground for the development of anorexia nervosa is becoming evident. Recent research indicates that excessive exercise is an integral aspect of the disorder in its acute phases. Findings on comorbidity of excessive exercise and anorexia nervosa highlight the detrimental effects of combining strenuous physical activity with immoderate dieting.116A The recognition of the syndrome in its early stages and a study of these syndromes to determine significant abnormalities have particular appeal because early intervention results in a more favorable outcome.
THE BULIMIC SYNDROME
Bulimia, although more common in slightly older age groups, is also generally a condition of young women and is often related to previous anorectic behavior. Bulimics gorge themselves and use artificial means to purge themselves of calories. These means include vomiting and abuse of laxatives or diuretics. Gorging episodes may alternate with periods of severe food restriction. This syndrome occurs among high school and college students (with an incidence varying from 2% in community-based samples to 4% to 13% in college age-groups)18,19,20 and may be more common than anorexia nervosa in men. The bulimic's weight may fluctuate, but usually not to dangerously low levels. There is often a history of other impulsive behaviors, such as alcohol or drug use, and features of this disorder are not unlike those seen with drug addiction. Depression is also common. Stealing and shoplifting, as well as unrestrained promiscuity, may be part of the syndrome, in contrast to the restrictive anorectic, who remains generally asexual. The patients tend to be older than anorectics, usually between 17 and 25 years of age. A separate condition, known as bulimia nervosa, has been described in which the bulimic behavior evolves from the completely restricting, anorexia nervosa-type pattern. Bulimics have a wide variety of medical problems that may be superimposed on the anorectic syndrome. These include severe tooth decay, parotid enlargement, stomach rupture, metabolic alkalosis, carpopedal spasm, hypercarotenemia, and pancreatitis.23,24,25,26,27 The bulimic person may also present with menstrual irregularity, but the incidence is highly variable. These persons may also have adequate estrogen secretion and present with an anovulatory syndrome. This type of problem may be difficult to diagnose because the menstrual disorders and the amenorrhea can develop even when the weight remains normal.117 Bulimic behavior is often secretive, and patients will not admit to these patterns even when questioned directly. The behavior is often chronic, and increased anxiety, irritability, depression, and poor social functioning are common.118 The weight may remain within a reasonable range, but protein-calorie malnutrition must certainly supervene and most likely contributes to the development of the reproductive disorder.
A number of neurologic problems have been seen in association with bulimia, including Huntington's chorea, schizophrenia, and seizure disorders associated with a postictal phenomenon. Bulimic behavior has also been described after amygdalectomy, with frontal lobe tumors, and after prefrontal lobotomy. Bilateral destruction of the ventromedial hypothalamus has been associated with hyperphasia and obesity and has been a sequela to encephalitis. Bulimia can also be seen in association with hypersomnia in cases of Kleine-Levin syndrome and in patients with parkinsonism, who have been reported to improve in their eating patterns after treatment.119
Furthermore, bulimia may have an organic cause; hypothalamic diseases may be associated with excessive appetite and lack of satiety inhibition. An underlying neurophysiologic dysfunction may be present in patients with bulimia. Electroencephalographic abnormalities have been reported in response to bulimic behavior. The association of neurologic abnormalities raises the intriguing possibility of underlying neuroendocrine dysfunction as a cause of both the nutritional and the menstrual disorder. A high incidence of substance abuse and alcoholism is seen in association with eating disorders, particularly bulimia. Some data suggest that diagnosis of one of these disorders in women is an indicator for close monitoring and counseling for both.120,121,122,123 As with anorexia nervosa, there is an associated history of substance abuse and or alcoholism in the family.124,125
TREATMENT AND PROGNOSIS
Management and therapy for these syndromes is still a subject of wide debate. Treatment includes various combinations of psychotherapy, including family therapy, psychoanalysis, and, occasionally, drug therapy. Chlorpromazine, insulin therapy, tricyclic antidepressants, cyproheptadine, L -dopa, and metoclopramide have all been considered but have met with only transitory or no success. It is disconcerting to both physician and patient that, despite impressive studies on the cause and psychogenesis of anorexia nervosa, few specific or new therapeutic modalities are available. Emphasis has been placed on early diagnosis and the recognition of anorectic behavior, so that the patient may be treated before the full-blown syndrome sets in. Because amenorrhea in the early part of the disease process is usually the first symptom that causes the patient to seek help, gynecologists should be particularly attentive to a history of dieting and weight loss in their young patients.
Behavioral modification is used with some success, although opinions on its efficacy vary.126 Extreme methods such as frontal lobotomy and leukotomy may be associated with a higher rate of suicide. Some workers maintain that the inclusion of psychotherapeutic measures127 and family therapy128 improves the late prognosis by more specifically correcting the psychopathology of anorexia nervosa. The first essential goal is to restore the patient's weight to normal. This can be accomplished by a variety of methods. Management has included hospitalization, forced fluids, and nasogastric feedings. The latter should be regarded with some caution because of the decreased motility of the gut seen in this syndrome. Generally, patients who are 75% of ideal body weight or lower need immediate and aggressive intervention. Dietary therapy is important, because response to psychotherapy is improved with nutritional rehabilitation.129,130 Usually, this is best done in a hospital setting by a team consisting of psychiatrists or psychologists, internists or pediatricians, and, if possible, a nutritionist with special interest and expertise in anorexia nervosa and related eating disorders.131 A larger series on the use of hyperalimentation has not been reported. Recent research highlights the dangers associated with refeeding in severely malnourished patients. Rapid nutritional rehabilitation of patients who are less than 70% of ideal body weight may result in hypophosphatemia, cardiac arrest, and delirium. Gradual dietary intervention should proceed only in combination with close monitoring of cardiac status and phosphorus, serum, and electrolyte levels.131A Precautions should also be taken to monitor patients' levels of exercise, because refeeding appears to restore muscle performance long before normal nutritional status is achieved. This improved muscle performance allows high levels of physical activity concomitant with sustained malnourishment.131B
The prognosis in anorexia nervosa is good to intermediate in almost 75% of patients. Generally, with weight gain, all of the metabolic parameters revert to normal. There is evidence that in some cases the perceptual disorder and the preoccupation with food persist. This is generally associated with a poorer prognosis. A poorer prognosis is also associated with bulimia.132 A fatal or chronic prognosis in anorexia nervosa may be predicted by laboratory findings obtained on initial diagnosis. Abnormally low levels of serum albumin and a low weight at initial examination may predict a lethal prognosis. A chronic course may be predicted by high serum creatinine and uric acid concentrations.132A The reproductive system remains particularly sensitive to fluctuations in weight, and this should be avoided. Ongoing research suggests that bulimia in particular may respond to antidepressant therapy.132 One study with a long-term follow-up period (4 to 8 years) showed a poor prognosis, which was also associated with the following: older age at onset; lower weight during illness and at presentation; the presence of bulimia, vomiting, or anxiety when eating with others; and poor childhood social adjustment. Environmental influences, such as a poor parental relationship and a lower socioeconomic status, also appear to play a role.133 The relapse rate for bulimia nervosa can be as high as 30%, although risk of relapse declines after approximately 4 years. In a recent study, almost 50% of bulimia nervosa patients were fully recovered 5 to 10 years after presentation, whereas 20% continued to exhibit full-fledged symptoms.133A Concomitant obsessive-compulsive disorder does not seem to indicate a significantly poorer prognosis for patients with anorexia or bulimia nervosa.133B Mortality rates vary from 2% to 10%, and death is often a result of unknown causes. Electrolyte imbalance, sepsis, and aspiration have all been reported.15 The importance of medical as well as psychiatric care is reinforced by this high mortality rate.
Anorexia nervosa is rare in men, although the syndrome, when it occurs, shows the same characteristic psychopathologic features of a relentless “pursuit of thinness” and a fear of becoming obese. In general, the prognosis tends to be worse among men, and when the condition does develop it appears to be within the context of overwhelming psychopathology or premorbid massive obesity. In male patients, it is particularly important to exclude the rare tumors of the hypothalamus that mimic anorexia nervosa.134,135
Recovery from anorexia nervosa can take several years. One study found that 50% of patients showed no improvement for at least 6 years after initial inpatient treatment. Patients with a restriction-type anorexia nervosa and low serum creatinine levels tend to recover earlier, whereas patients exhibiting purging behavior in combination with social disturbances may have a lower chance of recovery.135A Similar work indicates that approximately 30% of restricter-type patients develop binge eating within 5 years after intake. After initial hospital discharge, almost 30% of patients relapse, although relapse after full recovery is unlikely. After 10 to 15 years, most patients should have achieved normal weight and regular menstruation. Recovery time may range from 57 to 79 months after treatment commencement, depending on the definition of recovery.135B Longitudinal studies of this kind demonstrate the importance of tailoring treatment of anorexia nervosa to the specific symptoms and history of the patient. More long-term follow-up studies should eventually help improve the chances of recovery.
Amenorrhea persists in 40% to 50% of patients. The resumption of normal cyclic menses depends first on the return to normal weight. Ninety percent of standard body weight may be a reasonable target weight for treatment because it appears to be an average weight for the resumption of menses. Research suggests that 86% of patients who reach this weight resume menstruating within 6 months. Resumption of menses does not appear to depend on amount of body fat, but rather on restoration of hypothalamic-pituitary-ovarian function.135C In addition, evidence indicates that a large energy drain compounds the effects of a low body weight, and patients with recovered anorexia nervosa may need to weigh even more if they continue to be highly active.136
For those who do not have return of menstrual function, fertility may be restored by induction of ovulation. Agents such as clomiphene citrate or human menopausal gonadotropins can induce ovulation in almost all cases. The patient should have an evaluation to rule out other causes of amenorrhea, such as pituitary tumors or ovarian failure, before induction of ovulation; with these causes definitely eliminated, the patient can be reassured of future fertility. Research indicates that cyclic menstrual function can be restored in weight-related hypothalamic amenorrhea (e.g. anorexia nervosa) with pulsatile administration of GnRH using a pump and the subcutaneous or intravenous route. These therapeutic modalities hold much promise for the future, because weight loss-related amenorrheas represent a group of disorders that respond particularly well to this form of therapy. These and other therapeutic modalities will soon be available to treat the deficiencies that remain after the illness is cured.
Despite the return of weight, some patients continue to have permanent problems. Preoccupation with food and persistent dieting behavior are not uncommon in weight-recovered patients.15 For those patients with recovered anorexia nervosa who never menstruate again, estrogen replacement is indicated to circumvent premature osteoporosis. Recent work suggests, however, that estrogen replacement does not restore bone mass in women with anorexia nervosa.136A,136B Reports of osteoporosis-related problems in the patient with anorexia nervosa are appearing in the literature and should be of some concern.65,66 Evidence suggests that hypoestrogenism of a prolonged nature (longer than 6 months) in young women may predispose to osteopenia and premature osteoporosis.117 One study found that, after 12 years, 67% of patients diagnosed with chronic anorexia nervosa and 27% of those in a good to intermediate outcome group presented with medical comorbidity for the most part due to osteoporosis and renal disease.132A Unfortunately, many patients who remain underweight refuse estrogen therapy because of anxiety about gaining weight.
As would be expected, a severe nutritional deficiency with prolonged hypoestrogenism places patients with eating disorders at risk for osteoporosis.137,138,139,140,141,142,143,144,145 Decreased bone mass has been documented in anorexia nervosa, as have increased fractures.144,145 Bone mass does not appear to return to normal after recovery, even with weight gain, calcium supplementation, and exercise.145 In a recent study, 14 out of 18 recovered anorexia nervosa patients exhibited osteopenia.145A Most likely these patients will not reach their peak bone mass, which makes them at risk for severe osteoporosis in late life. Severe skeletal complications, such as collapse of the femoral head and hip fracture, have been reported in young patients.145,146 Studies have shown that osteoporosis and, in general, parameters of mineral metabolism have been normal, including serum calcium, phosphorus, 25-hydroxyvitamin D, and parathyroid hormone. Occasionally the 25-hydrox-yvitamin D level is low, but values for 25-dihydroxycholecalciferol (1,25(OH)2D) are normal.145,147 Subnormal osteocalcin reflects diminished osteoblastic activity.147 Increased cortisol levels seen in anorexia nervosa have been suggested as a mechanism of the osteo-penia.148,149
Further study of this syndrome, particularly in its early stages, may lead to a greater understanding of its evolution. Until then, recognition of the disorder depends on a number of factors, both psychiatric and metabolic, the first being weight loss. It is generally contended that the weight loss is secondary to a psychologic disturbance and that the physiologic changes are also secondary. The ensuing malnutrition produces a distinctive syndrome seen only in starvation.
REFERENCES
Crisp AH, Palmer RL, Kalucy RS: How common is anorexia nervosa? A prevalence study. Br J Psychiatry 128: 549, 1976 |
|
Theander S: Anorexia nervosa. Acta Psychiatr Scand 214: (Suppl) 1, 1970 |
|
Kendell RE, Hall DJ, Harley A et al: The epidemiology of anorexia nervosa. Psychol Med 2: 200, 1973 |
|
Kalucy RC, Crisp AH, Lacy JH et al: Prevalence and prognosis in anorexia nervosa. Aust N Z J Psychiatry 11: 251, 1977 |
|
Jacobs HS, Hall MGR, Murray MAF et al: Therapy-oriented diagnosis of secondary amenorrhea. Horm Res 6: 268, 1975 |
|
Garner DM, Garfinkel PE: Sociocultural factors in anorexia nervosa. Lancet 2: 674, 1978 |
|
Homek LH, Brooks-Gunn J, Warren MP: Sociocultural influences on eating disorders in professional female ballet dancers. Int J Eat Disord 4: 465, 1985 |
|
Epling WF, Pierce WD, Stephan L: A theory of activity-based anorexia. Int J Eat Disord 3: 27, 1983 |
|
Aumariega AJ, Edwards P, Mitchell CB: Anorexia nervosa in black adolescents. J Am Acad Child Psychiatry 1: 111, 1984 |
|
Askevold F, Heiberg A: Anorexia nervosa: Two cases in discordant MZ twins. Psychother Psychosom 32: 223, 1979 |
|
Darby PL, Garfinkel PE, Vale JM et al: Anorexia nervosa and “Turner syndrome”: Cause or coincidence? Psychol Med 1191: 141, 1981 |
|
Brooks SA: Diabetes mellitus and anorexia nervosa: Another view. Br J Psychiatry 144: 640, 1984 |
|
Roland JM, Bhanji S: Anorexia nervosa occurring in patients with diabetes mellitus. Postgrad Med J 58: 354, 1982 |
|
Kontula K, Mustajoik P, Paetau A et al: Development of Cushing's disease in patient with anorexia nervosa. J Endocrinol Invest 7: 35, 1984 |
|
Warren MP, Vande Wiele RL: Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 117: 435, 1973 |
|
Smith NJ: Excessive weight loss and food aversion in athletes stimulating anorexia nervosa. Pediatrics 66: 139, 1980 |
|
Willi J, Grossmann S: Epidemiology of anorexia nervosa in a defined region of Switzerland. Am J Psychiatry 140: 564, 1983 |
|
Pyle RL, Mitchell JE, Eckert ED et al: The incidence of bulimia in freshman college students. Int J Eat Disord 2: 75, 1983 |
|
Stangler RS, Printz AM: DSM-III: Psychiatric diagnosis in a university population. Am J Psychiatry 137: 937, 1980 |
|
Halmi KA, Falk JR: Binge-eating and vomiting: A survey of a college population. Psychol Med 4: 697, 1981 |
|
Russell GFM: Bulimia nervosa: An ominous variant of anorexia nervosa. Psychol Med 9: 429, 1979 |
|
Fairburn CG, Cooper PJ: Self-induced vomiting and bulimia nervosa: An undetected problem. Br Med J 284: 1153, 1982 |
|
Mitchell JE, Pyle RL: The bulimic syndrome in normal weight individuals: A review. Int J Eat Disord 1: 61, 1982 |
|
Pyle RL, Mitchell JE: Bulimia: A report of 34 cases. J Clin Psychiatry 42: 60, 1981 |
|
Harris RT: Bulimarexia and related serious eating disorders with medical complications. Ann Intern Med 99: 800, 1983 |
|
Hasler JF: Parotid enlargement: A presenting sign in anorexia nervosa. Oral Surg 53: 567, 1982 |
|
Marano AR, Sangree MH: Acute pancreatitis associated with bulimia. J Clin Gastroenterol 6: 245, 1984 |
|
Freighner JP, Robins E, Guze SB et al: Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 26: 57, 1972 |
|
Brush H: Psychologic antecedents of anorexia nervosa. In Vigersky R (ed): Anorexia Nervosa, p 1. New York: Raven Press, 1977 |
|
Lyon M, Chatoor I, Atkins D et al: Testing the hypothesis of the multidimensional model of anorexia nervosa in adolescents. Adolescence 32: 101, 1997 |
|
Cris AH: Clinical and therapeutic aspects of anorexia nervosa: A study of 30 cases. J Psychosom Res 9: 67, 1965 |
|
Halmi KA: Anorexia nervosa: Demographic and clinical features in 94 cases. Psychosom Med 36: 18, 1974 |
|
Freighner JP, Robins E, Guze SB et al: Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 26: 57, 1972 |
|
Frumar AM, Meldrum DR, Judd HL: Hypercarotenemia in hypothalamic amenorrhea. Fertil Steril 32: 261, 1979 |
|
Kemmann E, Pasquale SA, Skaf R: Amenorrhea associated with carotenemia. JAMA 249: 926, 1983 |
|
Robboy MS, Sato AS, Schwabe AD: The hypercarotenemia in anorexia nervosa: A comparison of vitamin A and carotene levels in various forms of menstrual dysfunction and cachexia. Am J Clin Nutr 27: 362, 1974 |
|
Walsh BT, Croft CB, Katz JL: Anorexia nervosa and salivary gland enlargement. Int J Psychiatry Med 11: 255, 1981 |
|
Brotman AW, Stern TA: Case report of cardiovascular abnormalities in anorexia nervosa. Am J Psychiatry 140: 1227, 1983 |
|
Silverman JA, Krongad E: Anorexia nervosa: A cause of pericardial effusion? Pediatr Cardiol 4: 125, 1983 |
|
Schoettle UC: Pancreatitis: A complication, a concomitant, or a cause of an anorexia nervosalike syndrome. J Am Acad Child Psychiatry 18: 384, 1979 |
|
Rampling D: Acute pancreatitis in anorexia nervosa. Med J Aust 2: 194, 1982 |
|
Cox KL, Cannon RA, Ament ME et al: Biochemical and ultrasonic abnormalities of the pancreas in anorexia nervosa. Dig Dis Sci 28: 225, 1983 |
|
Nordgren L, von Scheele C: Hepatic and pancreatic dysfunction in anorexia nervosa: A report of two cases. Biol Psychiatry 12: 681, 1977 |
|
Luthi M, Zurbrugg RP: A puzzling triad: Anorexia nervosa, high sweat electrolytes and indication to partial exocrine pancreatic insufficiency. Helv Paediatr Acta 38: 149, 1983 |
|
Al-Mufty NA, Bevan DH: A case of subcutaneous emphysema, pneumomediastinum and pneumoretroperitoneum associated with functional anorexia. Br J Clin Pract 31: 160, 1977 |
|
Brooks AP, Martyn C: Letter: Pneumomediastinum in anorexia nervosa. Br Med J 1: 125, 1979 |
|
Silber TJ, Kass EJ: Anorexia nervosa and nephrolithiasis. J Adolesc Health Care 5: 50, 1985 |
|
Esca SA, Brenner W, Mach K et al: Kwashiorkor-like zinc deficiency syndrome in anorexia nervosa. Acta Derm Venereol 59: 361, 1979 |
|
Smith DK, Ovesen L, Chu R et al: Hypothermia in a patient with anorexia nervosa. Metabolism 32: 1151, 1983 |
|
Niiya K, Kitagawa T, Fujishita M et al: Bulimia nervosa complicated by deficiency of vitamin K-dependent coagulation factors. JAMA 250: 792, 1983 |
|
Warren SE, Steinberg SM: Acid-base and electrolyte disturbances in anorexia nervosa. Am J Psychiatry 136: 415, 1979 |
|
Lefebvre J: Treatment of undernutrition and electrolyte disturbances in anorexia nervosa. Acta Psychiatr Belg 80: 551, 1980 |
|
Sheridan PH, Collins M: Potentially life-threatening hypophosphatemia in anorexia nervosa. J Adolesc Health Care 491: 44, 1983 |
|
Schott GD: Anorexia nervosa presenting as foot drop. Postgrad Med J 55: 58, 1979 |
|
Freyschuss U, Fohlin L, Thoren C: Limb circulation in anorexia nervosa. Acta Paediatr Scand 67: 225, 1978 |
|
Rappaport R, Prevot C, Czernichow P: Somatomedin activity and growth hormone secretion: I. Changes related to body weight in anorexia nervosa. Acta Paediatr Scand 69: 37, 1980 |
|
Lambert M, Hubert C, Depresseux G et al: Hematological changes in anorexia nervosa are correlated with total body fat mass depletion. Int J Eat Disord 21: 329, 1997 |
|
Bowers TK, Eckert E: Leukopenia in anorexia nervosa: Lack of increased risk of infection. Arch Intern Med 1389: 1520, 1978 |
|
Golla JA, Larson LA, Anderson CF et al: An immunological assessment of patients with anorexia nervosa. Am J Clin Nutr 34: 2756, 1981 |
|
Pertschuk MJ, Crosby LD, Barot L et al: Immunocompetency in anorexia nervosa. Am J Clin Nutr 35: 968, 1982 |
|
Zalin AM, Lant AF: Anorexia nervosa presenting as reversible hypoglycaemic coma. J R Soc Med 77: 193, 1984 |
|
Kline CL: Anorexia nervosa: Death from complications of ruptured gastric ulcer. Can J Psychiatry 24: 153, 1979 |
|
Pentlow BD, Dent RG: Acute vascular compression of the duodenum in anorexia nervosa. Br J Surg 68: 665, 1981 |
|
Sours JA, Vorhaus LJ: Superior mesenteric artery syndrome in anorexia nervosa: A case report. Am J Psychiatry 138: 519, 1981 |
|
Froese AP, Szmuilowicz J, Bailey JD: The superior-mesenteric-artery syndrome: Cause or complication of anorexia nervosa? Can Psychiatr Assoc J 23: 325, 1978 |
|
McAnarney ER, Greydanus DE, Campanella VA et al: Rib fractures and anorexia nervosa. J Adolesc Health Care 4: 40, 1983 |
|
Ayers JW, Gidwani GP, Schmidt IM et al: Osteopenia in hypoestrogenic young women with anorexia nervosa. Fertil Steril 41: 224, 1984 |
|
Rigotti NA, Nussbaum SR, Herzog DB et al: Osteoporosis in women with anorexia nervosa. N Engl J Med 311: 1601, 1984 |
|
Beumont PJV, George GCW, Pimstone BL et al: Body weight and pituitary response to hypothalamic releasing hormone in patients with anorexia nervosa. J Clin Endocrinol Metab 43: 487, 1976 |
|
Sherman BM, Halmi KA, Zamudio R: LH and FSH response to gonadotropin-releasing hormone in anorexia nervosa: Effect of nutritional rehabilitation. J Clin Endocrinol Metab 41: 135, 1975 |
|
Warren MP, Jewelewicz R, Dyrenfurth I et al: The significance of weight loss in the evaluation of pituitary response to LH-RH in women with secondary amenorrhea. J Clin Endocrinol Metab 40: 601, 1975 |
|
Yoshimoto Y, Moridera K, Imura H: Restoration of normal pituitary gonadotropin reserve by administration of luteinizing hormone releasing hormone in patients with hypogonadotropic hypogonadism. N Engl J Med 292: 242, 1975 |
|
Baranowska B, Rozbicka G, Jeske W et al: The role of endogenous opiates in the mechanisms of inhibited luteinizing hormone (LH) secretion in women with anorexia nervosa: The effect of naloxone on LH, follicle-stimulating hormone, prolactin, and beta-endorphin secretion. J Clin Endocrinol Metab 59: 412, 1984 |
|
Giusti M, Delitala G, Mazzocchi G et al: The role of opioid receptors on the secretion of LH, FSH and PRL in anorexia nervosa. In Endroczi E, Angelucci L, Scapagnini U et al (eds): Neuropeptides and Psychosomatic Processes, p 701. Budapest: Akademiai Kiado, 1983 |
|
Grossman A, Moulte PJA, McIntyre H et al: Opiate mediation of amenorrhea in hyper-prolactinemia and in weight loss-related amenorrhea. Clin Endocrinol 17: 379, 1983 |
|
Giusti M, Cavagnaro P, Torre R et al: Endogenous opioid blockade and gonadotropin secretion: Role of pulsatile luteinizing hormone-releasing hormone administration in anorexia nervosa and weight loss amenorrhea. Fertil Steril 49: 797, 1988 |
|
Berga SL, Loucks AB, Rossmanith WG et al: Acceleration of luteinizing hormone pulse frequency in functional hypothalamic amenorrhea by dopaminergic blockade. J Clin Endocrinol Metab 72: 151, 1991 |
|
Boyar R, Hellman L, Roffwarg H et al: Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med 296: 190, 1977 |
|
Gold P, Gwirtsman H, Avgerinos P et al: Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. N Engl J Med 314: 1335, 1986 |
|
Hotta I, Shebasoki K, Masuda A et al: The responses of plasma adrenocorticotropin and cortisol to corticotropin releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab 62: 319, 1986 |
|
Sapolsky RM, Krey LC: Stress-induced suppression of luteinizing hormone concentrations in wild baboons: Role of opiates. J Clin Endocrinol Metab 66: 722, 1988 |
|
Petraglia F, Vale W, Rivier C: Opioids act centrally to modulate stress-induced decrease in luteinizing hormone in the rat. Endocrinology 119: 2445, 1986 |
|
Xiao E, Luckhaus J, Niemann W et al: Acute inhibition of gonadotropin secretion by CRH in the primate: Are the adrenal glands involved? Endocrinology 124: 1632, 1989 |
|
Boyar RM, Finkelstein J, Roffwarg H et al: Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287: 582, 1972 |
|
Boyar RM, Katz J, Finkelstein JW et al: Anorexia nervosa: Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med 291: 861, 1974 |
|
Marshall JC, Kelch RP: Low dose pulsatile gonadotropin-releasing hormone in anorexia nervosa: A model of human pubertal development. J Clin Endocrinol Metab 49: 712, 1979 |
|
Savastano S, Tommaselli AP, Valentino R et al: Changes in the glycocylation pattern of circulating gonadotropins after acute administration of gonadotropin-releasing hormone in patients with anorexia nervosa. Eur J Endocrinol 138: 76, 1998 |
|
Fritz MA, Speroff L: Current concepts of the endocrine characteristics of normal menstrual function: The key to diagnosis and management of menstrual disorders. Clin Obstet Gynecol 26: 647, 1983 |
|
Starkey TA, Lee RA: Menstruation and fertility in anorexia nervosa. Am J Obstet Gynecol 105: 374, 1969 |
|
Adams J, Franks S, Polson DW et al: Multifollicular ovaries: Clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 2: 1375, 1985 |
|
Treasure JL, Gordon PAL, King EA et al: Cystic ovaries: A phase of anorexia nervosa. Lancet 2: 1379, 1985 |
|
Kohmura H, Miyake A, Aono T et al: Recovery of reproductive function in patients with anorexia nervosa: A 10-year follow up study. Eur J Obstet Gynecol Biol 22: 293, 1986 |
|
Fishman J, Boyar RM, Hellman L: Influence of body weight on estradiol metabolism in young women. J Clin Endocrinol Metab 41: 989, 1975 |
|
Gordon S, Cantrall EW, Leklenick WP et al: Steroid and lipid metabolism: The hypocholesterolaemic effects of estrogen metabolites. Steroids 4: 267, 1964 |
|
Boyar RM, Hellman LD, Roffwarg H et al: Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med 296: 190, 1977 |
|
Casper RC, Chatterton RT, Davis JM: Alterations in serum cortisol and its binding characteristics in anorexia nervosa. J Clin Endocrinol Metab 49: 406, 1979 |
|
Fowler PBS, Banim SO, Ikram H: Prolonged ankle reflex in anorexia nervosa. Lancet 2: 307, 1972 |
|
Fohlin L: Exercise, performance, and body dimensions in anorexia nervosa before and after rehabilitation. Acta Med Scand 204: 61, 1975 |
|
Kiyohara K, Tamai H, Takaichi Y et al: Decreased thyroidal triiodothyronine secretion in patients with anorexia nervosa: Influence of weight recovery. Am J Clin Nutr 50: 767, 1989 |
|
Scacchi M, Pincelli AI, Caumo A et al: Spontaneous nocturnal growth hormone secretion in anorexia nervosa. J Clin Endocrinol Metab 82: 3225, 1997 |
|
Moshang T Jr, Parks JS, Baker L et al: Low serum triiodothyronine in patients with anorexia nervosa. J Clin Endocrinol Metab 40: 470, 1975 |
|
Jennings AS, Ferguson DC, Utiger RD: Regulation of the conversion of thyroxine to triiodothyronine in the perfused rat liver. J Clin Invest 64: 1614, 1979 |
|
Cyrus J, Wood D, Samols E et al: Low T4 and FT4I in Seriously Ill Patients. Presented at the 61st Annual Meeting of the Endocrine Society, Anaheim, California, June 13–15, 1979, p 297 |
|
Kaptein EM, Wheeler WS, Spencer CA et al: Thyroid Hormone Economy in Critical Illness. Presented at the 61st Annual Meeting of the Endocrine Society, Anaheim, California, June 13–15, 1979, p 240 |
|
Vigersky RA, Loriaux DL, Anderson AE et al: Anorexia nervosa: Behavioral and hypothalamic aspects. J Clin Endocrinol Metab 5: 517, 1976 |
|
Vigersky RA, Loriaux D: Anorexia nervosa as a model of hypothalamic dysfunction. In Vigersky R (ed): Anorexia Nervosa, p 109. New York: Raven Press, 1977 |
|
Mecklenburg RS, Loriaux DL, Thompson RH et al: Hypothalamic dysfunction in patients with anorexia nervosa. Medicine (Baltimore) 53: 147, 1974 |
|
Davies CTM, Fohlin L, Thosen C: Temperature regulation in anorexia nervosa: Patients during prolonged exercise. Acta Med Scand 205: 257, 1979 |
|
Slade RD, Russell GFM: Awareness of body dimensions in anorexia nervosa: Cross-sectional and longitudinal studies. Psychol Med 3: 188, 1973 |
|
Crisp AH, Kalucy RS: Aspects of the perceptual disorder in anorexia nervosa. Br J Med Psychol 47: 349, 1974 |
|
Fries H: Studies on secondary amenorrhea, anorectic behavior and body image perception: Importance for the early recognition of anorexia nervosa. In Vigersky R (ed): Anorexia Nervosa, p 163. New York: Raven Press, 1977 |
|
Enzmann DR, Lane B: Cranial computed tomography findings in anorexia nervosa. J Comput Assist Tomogr 4: 410, 1977 |
|
Heinz RE, Martinez J, Haenggeli A: Reversibility of cerebral atrophy in anorexia nervosa and Cushing's syndrome. J Comput Assist Tomogr 4: 415, 1977 |
|
Barry VC, Klawans HL: On the role of dopamine in the pathophysiology of anorexia nervosa. J Neural Transm 38: 107, 1976 |
|
Lord JA, Waterfield AA, Hughes J et al: Endogenous opioid peptides: Multiple agonists and receptors. Nature 267: 495, 1977 |
|
Margules DL, Lewis MJ, Shibuya H et al: β-Endorphin is associated with overeating in genetically obese mice (ob/ob) and rats (fa/fa). Science 202: 988, 1978 |
|
Paul SM, Axelrod J: Catecholestrogens: Presence in brain and endocrine tissue. Science 197: 657, 1977 |
|
Warren MP: The effects of exercise on pubertal progression and reproductive function in girls. J Clin Endocrinol Metab 51: 1150, 1980 |
|
Kapen S, Sternthal E, Braverman L: Case report: A pubertal 24-hour luteinizing hormone (LH) secretory pattern following weight loss in the absence of anorexia nervosa. Psychosom Med 43: 177, 1981 |
|
Brooks-Gunn J, Warren MP, Hamilton LH: The relationship of eating problems and amenorrhea in ballet dancers. Med Sci Sports Exerc 19: 41, 1987 |
|
Davis C, Katzman DK, Kaptein S et al: The prevalence of high-level exercise in the eating disorders: Etiological implications. Compr Psychiatry 38: 321, 1997 |
|
Warren MP: Anorexia nervosa. In DeGroot L (ed): Endocrinology, 3rd ed, p 2679. Philadelphia: WB Saunders, 1994 |
|
Herzog DB, Copeland PM: Medical progress: Eating disorders. N Engl J Med 313: 295, 1985 |
|
Warren MP: The effects of undernutrition on reproductive function in the human. Endocr Rev 4: 363, 1983 |
|
Hatsukami D, Eckert E, Mitchell J et al: Affective disorder and substance abuse in women with bulimia. Psychol Med 14: 701, 1984 |
|
Jonas J, Gold M: Naltrexone treatment of bulimia: Clinical and theoretical findings linking eating disorders and substance abuse. Adv Alcohol Subst Abuse 7: 29, 1987 |
|
Killen J, Taylor C, Telch M et al: Depressive symptoms and substance use among adolescent binge eaters and purgers: A defined population study. Am J Public Health 77: 1539, 1987 |
|
Lacey J, Moureli E: Bulimic alcoholics: Some features of a clinical sub-group. Br J Addict 81: 389, 1986 |
|
Halmi KA, Loney J: Familial alcoholism in anorexia nervosa. Br J Psychiatry 123: 53, 1973 |
|
Collins GB, Kotz M, Janesz JW et al: Alcoholism in the families of bulimic anorexics. Cleve Clin Q 52: 65, 1985 |
|
Agras S, Werne T: Behavior modification in anorexia nervosa: Research foundations. In Vigersky R (ed): Anorexia Nervosa, p 291. New York: Raven Press, 1977 |
|
Bruch H: The Eating Disorders: Obesity, Anorexia Nervosa and the Person Within. London: Routledge and Kegan Paul, 1974 |
|
Minuchin S, Baker L, Rosman B et al: A conceptual model of psychosomatic illness in children. Arch Gen Psychiatry 32: 1031, 1975 |
|
Walker J, Roberts SL, Halmi KA et al: Caloric requirements for weight gain in anorexia nervosa. Am J Clin Nutr 32: 1396, 1979 |
|
Huse DA, Lucas AR: Dietary treatment of anorexia nervosa. J Am Diet Assoc 83: 687, 1983 |
|
Liebman R, Minuchin S, Baker L: An integrated treatment program for anorexia nervosa. Am J Psychiatry 4: 431, 1974 |
|
Kohn MR, Golden NH, Shenker IR: Cardiac arrest and delirium: presentations of the refeeding syndrome in severely malnourished adolescents with anorexia nervosa. J Adolesc Health 22: 239, 1998 |
|
Rigaud D, Moukaddem M, Cohen B et al: Refeeding improves muscle performance without normalization of muscle mass and oxygen consumption in anorexia nervosa patients. Am J Clin Nutr 65: 1845, 1997 |
|
Johnson C, Stuckey M, Mitchell J: Psychopharmacological treatment of anorexia nervosa and bulimia. J Nerv Ment Dis 9: 524, 1983 |
|
Herzog W, Deter HC, Fiehn W et al: Medical findings and predictors of long-term physical outcome in anorexia nervosa: A prospective, 12-year follow-up study. Psychol Med 27: 269, 1997 |
|
Hsu LKG, Crisp AH, Harding B: Outcome of anorexia nervosa. Lancet 1: 61, 1979 |
|
Keel PK, Mitchell JE: Outcome in bulimia nervosa. Am J Psychiatry 154: 313, 1997 |
|
Thiel A, Zuger M, Jacoby GE et al: Thirty-month outcome in patients with anorexia or bulimia nervosa and concomitant obsessive-compulsive disorder. Am J Psychiatry 155: 244, 1998 |
|
Hay GG, Leonard JC: Anorexia nervosa in males. Lancet 2: 575, 1979 |
|
Crisp AH, Kalucy RS, Lacey TH et al: The long term prognosis in anorexia nervosa: Some factors predictive of outcome. In Vigersky R (ed): Anorexia Nervosa, p 55. New York: Raven Press, 1977 |
|
Herzog W, Schellberg D, Deter HC: First recovery in anorexia nervosa patients in the long-term course: A discrete-time survival analysis. J Consult Clin Psychol 65: 169, 1997 |
|
Strober M, Freeman R, Morrell W: The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord 22: 339, 1997 |
|
Golden NH, Jacobson MS, Schebendach J et al: Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 151: 16, 1997 |
|
Warren MP: The Effects of Exercise on Pubertal Progression and Reproductive Function in Girls. Presented at the 61st Annual Meeting of the Endocrine Society, Anaheim, California, June 13–15, 1979, p 239 |
|
Kreipe RE, Hicks DG, Rosier RN et al: Preliminary findings on the effects of sex hormones on bone metabolism in anorexia nervosa. J Adolesc Health 14: 319, 1993 |
|
Klibanski A, Biller BMK, Schoenfeld DA et al: The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab 80: 898, 1995 |
|
Rigotti NA, Nussbaum SR, Herzog DB et al: Osteoporosis in women in anorexia nervosa. N Engl J Med 311: 601, 1984 |
|
Ayers JWT, Gidwani GP, Schmidt IMV et al: Osteopenia in hypoestrogenic young women with anorexia nervosa. Fertil Steril 41: 224, 1984 |
|
Szmukler GI, Brown SW, Parsons V et al: Premature loss of bone in chronic anorexia nervosa. Br Med J 290: 26, 1985 |
|
Treasure JL, Russell GFM, Fogelman I et al: Reversible bone loss in anorexia nervosa. Br Med J 295: 474, 1987 |
|
Crosby LO, Kaplan FS, Pertschuk MJ et al: The effect of anorexia nervosa on bone morphometry in young women. Clin Orthop 201: 271, 1985 |
|
Biller BMK, Saxe V, Herzog DB et al: Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 68: 548, 1989 |
|
Kaplan FS, Pertschuk M, Fallon M et al: Osteoporosis and hip fracture in a young woman with anorexia nervosa. Clin Orthop 212: 250, 1986 |
|
Bachrach LK, Guido D, Katzman D et al: Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics 86: 440, 1990 |
|
Rigotti NA, Neer RM, Skates SJ et al: The clinical course of osteoporosis in anorexia nervosa: A longitudinal study of cortical bone mass. JAMA 265: 1133, 1991 |
|
Ward A, Brown N, Treasure J: Persistent osteopenia after recovery from anorexia nervosa. Int J Eat Disord 22: 71, 1997 |
|
Warren MP, Shane E, Lee MJ et al: Femoral head collapse associated with anorexia nervosa in a twenty-year-old ballet dancer. Clin Orthop 251: 171, 1990 |
|
Fonseca VA, D'Souza V, Houlder S et al: Vitamin D deficiency and low osteocalcin concentrations in anorexia nervosa. J Clin Pathol 41: 195, 1988 |
|
Newman MM, Halmi KA: Relationship of bone density to estradiol and cortisol in anorexia nervosa and bulimia. Psychiatry Res 29: 105, 1989 |
|
Biller BMK, Saxe V, Herzog DB et al: Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 68: 548, 1989 |