Vaginal Hysterectomy
Authors
INTRODUCTION
Hysterectomy is the second most frequently performed surgical procedure in the United States, with approximately 650,000 performed annually.1 According to the Centers for Disease Control in Atlanta, the current ratio of abdominal to vaginal hysterectomy is 3:1. It has been suggested that approximately 50% of these abdominal hysterectomies could have been performed by the vaginal route. The benefits of vaginal hysterectomy are a shorter, less complicated postoperative course and a nationwide savings as it relates to hospital days and hospital bed fees.2,3 This chapter presents the preoperative indications, surgical considerations, surgical technique, and postoperative considerations of vaginal hysterectomy.
PREOPERATIVE INDICATIONS
The majority of vaginal hysterectomies are performed for benign conditions. Rarely does one perform a vaginal hysterectomy as an emergent procedure. The indications for vaginal hysterectomy are those conditions that cannot be successfully managed with conservative medical or surgical therapy. The more common indications for vaginal hysterectomy include abnormal or dysfunctional uterine bleeding, dysmenorrhea and/or dyspareunia of presumed uterine cause, complex endometrial hyperplasia, symptomatic leiomyoma, symptomatic pelvic organ prolapse, and cervical intraepithelial neoplasia or microinvasive carcinoma of the cervix. Each indication should be thoroughly evaluated in the preoperative stage. The decision as to whether a nonsurgical therapy is effective will determine whether the vaginal hysterectomy may be the procedure of choice.
The selection of patients begins in the preoperative period. Factors such as menopausal status, adnexal pathology, uterine mobility and pelvic size, obesity, and the ability to tolerate anesthesia, as defined by a patient's cardiac status, all must be taken into consideration. The vaginal hysterectomy may be performed using either regional or general anesthesia. The choice of anesthetic will be decided jointly by the patient, anesthesiologist, and surgeon.
With abdominal hysterectomy, morbidly obese patients have a higher risk of wound disruption, infection, ileus, and deep vein thrombosis. These complications are less likely to occur when a vaginal hysterectomy is performed. Vaginal hysterectomy should not be attempted in the face of significant adnexal pathology (i.e., ovarian neoplasm, severe endometriosis, or severe adhesive disease). A history of prior pelvic surgery is not a contraindication to vaginal hysterectomy. In cases where adnexal pathology or previous complex surgery has been performed, the use of laparoscopic visualization permits accurate diagnosis and aids in the decision-making process of whether to proceed with the vaginal hysterectomy.4 We recommend prophylactic oophorectomy in perimenopausal and menopausal women. Prospective studies to date indicate that more than 90% of the adnexa can be removed vaginally, and more recent work shows that this can be effective in 94–99% of all ovaries attempted to be removed transvaginally.5
Adequate evaluation of pelvic support is paramount when the decision is being made as to the route of hysterectomy. An important observation is uterine mobility. A vaginal approach is the treatment of choice if the uterus is mobile. Uterine mobility can be assessed during an office pelvic examination, with the patient demonstrating descensus of the pelvic organs while performing a Valsalva maneuver. The Valsalva maneuver, in effect, allows any of the vaginal defects to be brought out and shown at their worst possible rating stage. In addition, physicians should have some organized prolapse classification. To date, there is no standard classification, but the half-way system by Baden and Walker6 and the one proposed by the International Continence Society are becoming popular and are anatomically correct.
After assessing the degree and number of vaginal fascial defects, the bony pelvis is evaluated. While not a hard and fast rule, a pubic arch of 90° or greater, an adequate vaginal canal, and a deep and wide posterior vaginal fornix will make the operation easier to perform. The gynecoid pelvis usually has the above characteristics, which makes it easier to access the operative site with increased exposure.
Certain laboratory studies may be indicated when performing a vaginal hysterectomy. These include hemoglobin/hematocrit, pregnancy test, urinalysis, and blood type and screening for possible use intraoperatively. The patient should have had a normal Papanicolaou smear within the last 12 months. Other studies and documentations may be necessary, depending on the indication for the hysterectomy. In a patient older than 35, an electrocardiogram and a preoperative chest x-ray are usually performed for medical indications only. Last, but not least, in patients who have had longstanding, complete prolapse/procidentia, an intravenous (IV) pyelogram will help to rule out hydronephrosis and assess the ureteral anatomy.7, 8
PROPHYLACTIC ANTIBIOTICS AND VAGINAL PREPARATION
It is well established that preoperative antibiotics decrease the chance of postoperative, posthysterectomy infection. The risk of postoperative morbidity and infection can be reduced if antibiotics are given before the initial surgical incision. Adequate, broad-spectrum antibiotics should be given in a single dose immediately prior to surgery. To be effective, the antibiotic must be in the tissue at the time of surgery. The routine choice of any specific one would not be appropriate. However, the antibiotic chosen should have the ability to diffuse into the tissue fields being operated on.
Immediately before the operation, the patient is positioned in the dorsal lithotomy position with the buttocks just over the table's edge. Guidelines for positioning the patient to avoid neurologic injury may include adequate leg padding, avoidance of marked flexion of the thigh, and avoidance of pressure points, particularly laterally, where the peroneal nerve circles around the head of the fibula. Trendelenburg (10–15°) positioning aids, along with the intravaginal exposure, are needed for the procedure. In addition, it is entirely appropriate to cleanse the vagina before the initial incision. The purpose of this is to decrease the number of bacteria present. It is very important to make sure that the patient is not allergic to the cleansing solution being used. If this has not been satisfactorily determined, the vagina can be lavaged and swabbed with normal saline to decrease the number of bacteria in the inoculum that would be present with the initial incision.
After preparing the vagina and draining the bladder, the urinary catheter may or may not be removed (depending on the surgeon's preference) before the procedure. The patient is then draped according to the surgeon's preference. There is no reason to shave the pubic hair.
Other considerations to be taken into account are appropriate instrumentation, lighting, and suture material. In addition, the patient's risk factors for developing deep vein thrombosis or pulmonary embolus are evaluated, and appropriate prophylactic measures are taken.
THE SURGICAL TECHNIQUE
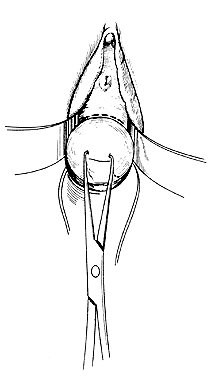
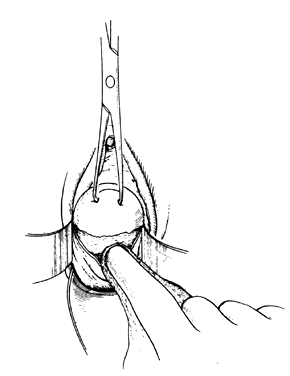
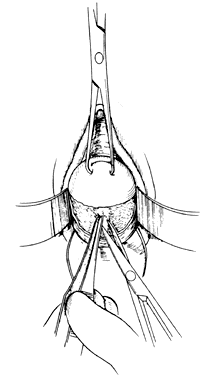
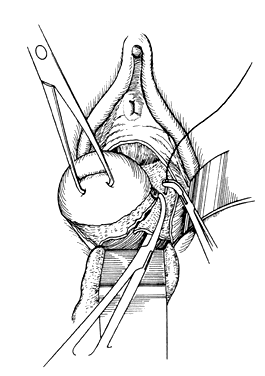
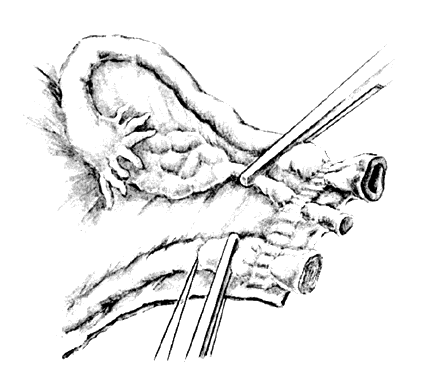
The following step-by-step vaginal hysterectomy technique may not be suitable for all surgeons in all circumstances; however, it may be used as a basis on which to build one's own surgical technique. An examination under anesthesia is performed once the patient has been prepared and draped, in dorsal lithotomy position, to assess descensus of the pelvic organs and respective defects, mobility, and uterine size. The final decision is made at this time4 as to whether the procedure may be done vaginally or abdominally. The cervix is then grasped on the anterior or posterior lip with a tenaculum or a vulsellum forceps. Sounding the uterus at this time will help to confirm the suspected size and contour of the uterus found in the office examination and in the examination under anesthesia. Gentle traction in all directions with the vulsellum enables the surgeon to visualize the cervical-vaginal junction, the area where the initial incision will be made. At this time, a paracervical and submucosal injection of 1/2% lidocaine with 1:200,000 or a dilute solution of vasopressin9 may be used to help decrease operative blood loss, decrease postoperative pain, and as some believe, delineate the surgical planes. Areas to be injected include the paracervical tissue, the area around the uterosacral ligaments, the lower portion of the cardinal ligament, and the bladder pillars. The use of vasoconstrictive infiltration is not necessary in all instances. The majority of time, blood loss is controlled with clamps and cautery. I believe that the surgical planes are not made easier to identify by injection. Some believe that the spasm of the small vessels brought on by vasoconstrictive agents may also mask bleeding of a small vessel until the medication wears off, when it shows up as a postoperative hemorrhage. With adequate traction on the anterior and posterior lips of the cervix, a circumferential incision is made through the full thickness of vaginal epithelium at the cervical-vaginal junction (Fig. 1 and Fig. 2). As this is being performed, adequate counter-traction against the respective lateral vaginal wall will prove to be helpful.
|
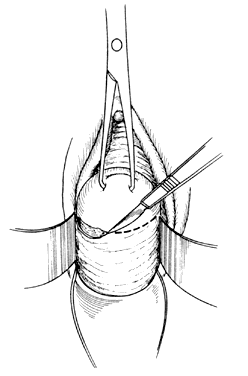
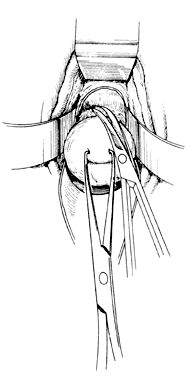
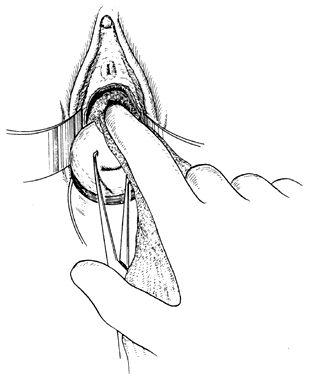
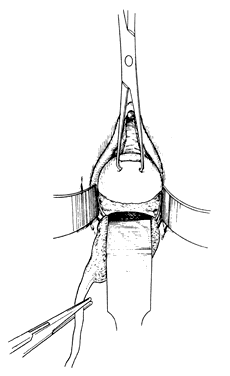
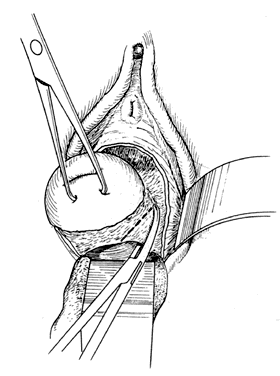
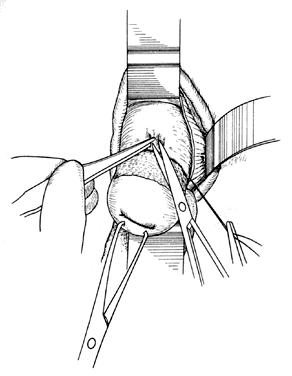
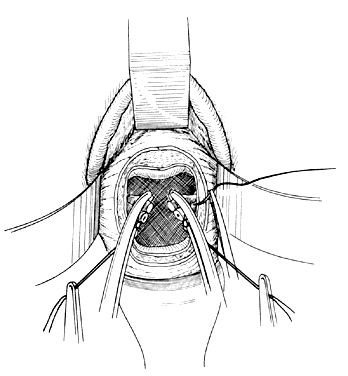
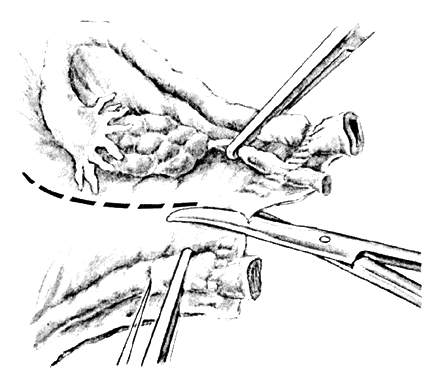
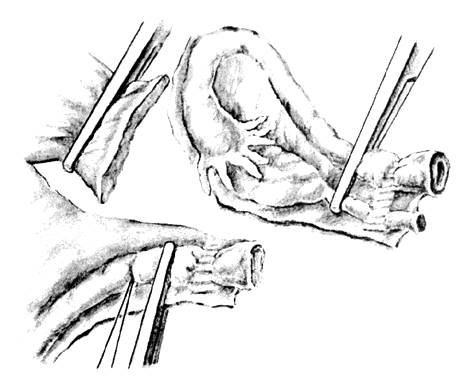
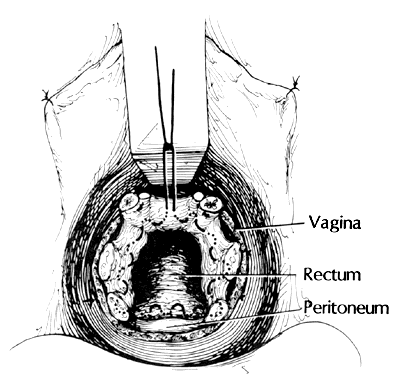
Once the initial incision is made through the full thickness of the vaginal epithelium, the vagina may be dissected sharply from the underlying tissue or may be accomplished with the operator's finger. It is important to remember that this blunt dissection should be done gently so that the tissues being displaced have excellent tactile sensation (Fig. 3 and Fig. 4). If the initial incision is made below the cervical-vaginal junction and too close to the external os, a greater amount of dissection will be needed to displace the vaginal mucosa. In addition, there is usually an increased amount of bleeding when an incision is made too low on the cervix. Therefore, the selection of the level should be at the cervical-vaginal junction, a point that is just below the bladder reflection. Should the initial circumferential incision be made too deep, the dissection extends into the cervix, leading to increased blood loss and technical difficulty. It is most important to dissect through the full thickness of the vaginal epithelium to identify the anterior and posterior cul-de-sac. The next step is entry into the posterior cul-de-sac of Douglas. This can be identified by placing the posterior vaginal mucosa and underlying tissue on stretch with either forceps or sponge over the operator's finger (Fig. 5). Once the posterior peritoneum has been identified, it is grasped with forceps and then opened with scissors (Fig. 6). The incision line is then extended to either side just short of the uterosacral ligaments. An interrupted suture is then placed at the 3, 6, and 9 o'clock positions to approximate the peritoneum to the vaginal cuff, aiding in hemostasis and traction and helping define the peritoneum at the time of vaginal cuff and intra-abdominal closure. Once the pelvic cavity has been opened, an intra-abdominal pelvic examination is performed to rule out any other pelvic disease of the uterus or adhesive disease of the cul-de-sac and adnexa. At that time, the weighted speculum, or a retractor with a long blade (Fig. 7), is placed into the posterior cul-de-sac to help keep the rectum safely out the way during the operation.
|
|
|
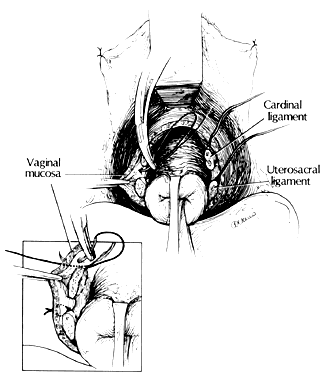
With retraction of the lateral vaginal walls and countertraction on the cervix, the uterosacral ligaments are clamped with the tip of the clamp incorporating the lower portion of the cardinal ligaments, if possible. The clamp is placed perpendicular to the uterine axis, and the pedicle is cut and sutured (Fig. 8). The pedicles taken during vaginal hysterectomy are cut close to the clamps so that there is a decreased chance of their becoming necrotic, sloughing, or providing a culture medium for bacterial infection. When suturing a pedicle, the needle point is placed at the posterior tip of the clamp and passed through the tissue by a rolling motion of the operator's wrist (Fig. 9).
Once ligated, the uterosacral ligaments may be immediately transfixed to the posterolateral vaginal mucosa (Fig. 10) or held long for use at the end of the case. Either way, the suture is used to help support the vagina to prevent posthysterectomy prolapse, provide hemostasis at the angle of the vagina (a common place for postoperative bleeding), and if done as an initial step, help the surgeons not to cut the sutures accidentally before their transfixation to the vaginal mucosa at the end of the procedure. If the suture is held long, it will help facilitate the location and inspection of all the pedicles at the end of the procedure.
The next step involves deciding whether to enter the vesicovaginal space and the anterior cul-de-sac. With downward traction on the cervix, a pair of scissors with the points directed toward the uterus is used to advance the bladder (Fig. 11). Entry into the vesicouterine space will allow the bladder to be displaced upward, helping the ureters to be elevated and displaced somewhat laterally. This helps to pull them away from the midline and aids in the safe exposure of the areas where the uterine arteries will be serially clamped, cut, and ligated. Should the vesicovaginal peritoneal reflection not be easily identified, entry into the space can be delayed. As long as the bladder anatomy has been identified by the operator, there is no danger in doing this as long as it is advanced appropriately. Once the bladder is advanced, a retractor is placed in the midline, holding the bladder out of the operative field. This process proceeds each step during the hysterectomy until the vesicovaginal space is entered.
With continued traction on the cervix, the cardinal ligaments are identified, clamped, cut, and suture-ligated. These are attached to the vaginal mucosa as the uterosacral ligaments were to the vaginal mucosa to lend support and aid hemostasis (see Fig. 10).
Continued attention to the bladder is noted. If the anterior cul-de-sac has not been opened with the retractor elevating the bladder and ureters out of harm's way, the bladder is advanced with each step of the hysterectomy. Blunt dissection is commonly used, again remembering to have adequate tactile sensation to the tissue so that blunt trauma does not cause entry into the bladder. Sharp dissection is the treatment of choice in this instance and is also a particularly good choice when the patient has had previous surgery, such as a cesarean section.
The anterior peritoneal fold can be identified before or just after clamping on suture ligation of the arteries. Opening the peritoneal cavity anteriorly should not be done blindly so as to prevent bladder injury. The peritoneum is grasped with forceps, tented, and opened with scissors with the tips, again pointing toward the uterus. A Heaney or a Deaver retractor is then placed, and the peritoneal contents are identified. To reiterate, this retractor serves to elevate the bladder out of the operative field as further pedicles are secured along the uterine arteries and infundibulopelvic ligament (Fig. 12).
|
Contralateral and downward traction is placed in a serial fashion on the cervix. An effort is made to incorporate the anterior and posterior leaves of the vesicouterine peritoneum in the clamp such that the uterine vessels are identified, clamped, cut, and suture-ligated. A single clamp, single suture method is completely adequate. However, in a training institution a two-suture technique has been used. The first is the simple ligature with the second being a Heaney-type transfixation suture. While we are not advocates of double-tying, double-tying of all pedicles throughout the hysterectomy can be performed as long as other structures such as the ureters are identified and kept out of harm's way. The uterus can be removed with the cervix presenting first or by delivering the uterine fundus posteriorly. To assist in the removal of the uterus, a tenaculum is placed on the posterior fundus, in a successive fashion, to deliver the fundus posteriorly. Whether delivering posteriorly or bringing the uterus out with the cervix presentation, the operator's index finger is used to identify the utero-ovarian ligament and to aid in clamp placement. With both the anterior and posterior peritoneum opened, the remainder of the broad ligament and the utero-ovarian ligaments are clamped, cut, and ligated. This pedicle will usually include the round ligament as well, but the round ligament may also be taken as a separate piece (Fig. 13). Double ligation of the utero-ovarian and round ligament complex is preferred, although the single ligation technique can be used. When the two clamp technique is used, the first clamp is effected with a suture ligature followed by a second suture ligature medial to the first. As stated, this pedicle includes the proximal portions of the round ligament and the fallopian tube, as well as the utero-ovarian ligament. At all times, clamps on the “adnexal pedicle” should be pointed away from the pelvic sidewall toward the midline to avoid having the pelvic sidewall structures at risk during the hysterectomy.
|
The fallopian tubes and ovaries are now inspected by drawing them into the operative field. If the adnexal removal had been a planned step, the round ligaments would have been taken separately as adnexal structures (Fig. 14). Traction is then placed on the utero-ovarian pedicle and the ovary drawn into the operative field with a Babcock clamp. The peritoneum between the round ligament and the fallopian tube is then excised (Fig. 15) with the scissors. This maneuver will separate the fallopian tube and ovary from the round ligament so that the infundibulopelvic ligament can be clamped, cut, and tied (see Fig. 15; Fig. 16). It is entirely appropriate to take the fallopian tube and ovary as separate pedicles to enable good visualization should the ureter or nearby blood vessels be in such a position that they are at risk. This will allow better visualization of the entire sidewall area.
|
|
It is my bias that reperitonealization should be performed. I use a permanent suture and begin with the anterior peritoneal edge. A continued purse-string suture is begun at 12 o'clock and continues in a clockwise fashion to incorporate the distal portion of the left upper pedicle and the left uterosacral and cardinal ligaments. The suture then incorporates the posterior peritoneum as high as possible, if not the muscularis of the anterior wall of the rectum. The suture is then carried through the uterosacral and cardinal ligament on the patient's right side, as well as through the right upper pedicle. This type of suture with high peritoneal closure helps to prevent future enterocele formation. It is entirely appropriate to use a delayed, absorbable suture, but I believe that permanent suture should be used to prevent any type of herniation. Our colleagues in general surgery use permanent suture for any type of hernia, and this has become my suture of choice to close off the cul-de-sac (Fig. 17). This type of suture, in essence, creates a posterior culdoplasty of one type as a routine measure to prevent enterocele formation. However, there are other types of culdoplasties that may be used in an attempt to achieve cul-de-sac closure and enterocele prevention. The Halban vertical closure, the modified Moschcowitz procedure through the vagina, and the McCall culdoplasty have all been heralded as methods to help prevent future enterocele formation.
Once cul-de-sac peritonealization has been accomplished, the vaginal mucosa can be approximated in one of several ways: either in a vertical or horizontal fashion, using either interrupted or continuous sutures.10 These sutures will be placed through the entire thickness of the vaginal epithelium, with care taken not to enter the bladder anteriorly. The purpose of the sutures is to obliterate the underlying dead space and to produce an anatomic approximation of the vaginal epithelium. The vaginal cuff may also be left open to promote drainage, helping to prevent blood and serous fluid collection postoperatively.
After the procedure, I prefer to leave an indwelling catheter in the bladder for the first 24 hours, especially if the patient has been under a general anesthetic and will not be moving too much during the initial 24 hours except for turning in bed. In addition, at the end of the procedure I place a vaginal pack soaked with a cleansing solution. The cleansing solution does not decrease infection, but merely makes it easier to place the pack and remove it in the next 24 hours. My feeling, although controversial, is that this pack acts much like any other type of pressure dressing, and will help tamponade any small vessels that may begin to bleed once the dilute vasoconstrictive solutions have worn off. Both urinary catheter and pack are removed 24 hours after the procedure. The following are other indications for postoperative bladder drainage 24 hours after hysterectomy: patient unable to void spontaneously, significant pelvic pain, concurrent vaginal reparative procedures, surgical procedures for stress incontinence, vaginal packing, and patient anxiety.
POSTOPERATIVE CARE
Although minimal bowel manipulation takes place during vaginal hysterectomy, there is a slowing of gastrointestinal motility. However, this should not prevent some form of oral intake after surgery. Nausea, in combination with drowsiness from analgesics, will tend to make the patient disinterested in food on the evening of the surgery. However, a clear liquid diet is suitable during the first 12–24 hours after surgery. A regular diet is then resumed on the first full postoperative day. It is well known that the patient will be the best judge as to whether she wants to eat.
Fluid management is also considered in the postoperative period. Initially, fluids should be administered to correct any deficits and then used as maintenance fluids only. Following an uncomplicated vaginal hysterectomy, a fluid deficit usually will not exist and the maintenance volume can be begun. Initially, physiologic fluids such as 5% dextrose and lactated Ringer's solution should be administered and maintained at approximately 125 mL/hour. When to stop the IV maintenance fluids will depend on the patient's dietary tolerances.
Postoperative analgesia is an important aspect in achieving patient ambulation and compliance with coughing, deep breathing, and turning in bed. Pain is typically managed initially with parenteral narcotics. The choice will vary from surgeon to surgeon but usually includes morphine sulfate or meperidine sulfate. One of the newer forms of postoperative analgesia is termed patient controlled analgesia (PCA), in which the patient controls the rate of narcotic by using a bedside pump. For the motivated patient, this is an excellent form of analgesia. Intramuscular or IV analgesics are rarely needed beyond the first 48 hours after surgery. At that point, oral analgesics (e.g., codeine, oxycodone, hydrocodone) are all that are required. In some instances, nonsteroidal anti-inflammatory agents will suffice.
POSTOPERATIVE COMPLICATIONS
Early postoperative hemorrhage after vaginal hysterectomy may present in one of two ways. First, bleeding from the vagina may be noted by the nursing staff or physician within the first few hours after surgery. Second, and less commonly, the patient may be noted to have little bleeding from the vagina, but have deteriorating vital signs, as manifested by low blood pressure and rapid pulse, falling hematocrit, and flank or abdominal pain.
The first presentation usually represents bleeding from the vaginal cuff or one of the vascular pedicles. The second presentation may represent a retroperitoneal hemorrhage. Each situation is approached differently in its evaluation and treatment, but both involve the same general principles of rapid diagnosis, stabilization of vital signs, appropriate fluid and blood replacement, and constant surveillance of the patient's condition.
Once vital signs are assessed, attention should be directed to the amount of bleeding. A small amount of bleeding is expected after any vaginal hysterectomy. However, steady bleeding 2 or 3 hours after surgery suggests lack of hemostasis. The patient should be taken promptly to the examining room, where the operative site is viewed using a large speculum and good lighting. If bleeding is not excessive, the vaginal cuff can be inspected; in many instances, bleeding from the cuff edge will be found. Hemostasis may be achieved with one or two sutures placed through the mucosa.
If bleeding is excessive or appears to come from above the cuff or if the patient is too uncomfortable to tolerate an adequate examination, she should be taken into the operating room. General anesthesia should be administered and the vaginal operative site thoroughly explored. Bleeding points may be sutured or ligated. Vaginal bleeding can usually be controlled vaginally. After opening the lateral sutures, the pedicles are usually available for inspection and control. All the vaginal pedicles should have been extraperitonealized during the course of the operation. Occasionally, a vessel will retract out of a tie or ligature and result in bleeding retroperitoneally or intraperitoneally. However, if bleeding is coming from above or is extremely brisk, it is unlikely that this can be controlled through the vaginal route. An exploratory laparotomy will be necessary to examine the pelvis, identify and isolate the bleeding vessel, and achieve hemostasis. The ovarian vessels and uterine arteries should be thoroughly inspected, as they are often the source of excessive vaginal bleeding. If it is difficult to localize bleeding to a specific pelvic vessel, ligation of the hypogastric artery or arteries may be necessary.
In the patient with little vaginal bleeding but deteriorating vital signs, retroperitoneal hemorrhage should be suspected. Input and output should be monitored. A hematocrit should be ordered immediately, along with cross-matching of blood. Examination may reveal tenderness and dullness in the flank. In cases of intraperitoneal bleeding, abdominal distension may occur. Diagnostic radiologic studies can be used to confirm the presence of retroperitoneal or intra-abdominal bleeding. Ultrasonography is one option for looking at low-pelvic hematomas. Computerized tomography, however, gives better visualization of retroperitoneal spaces and can delineate a hematoma.
If the patient's condition stabilizes rapidly with IV fluids, one of two approaches may be used for continued care. The first, and the one we prefer, is to transfuse the patient and follow serial hematocrits and vital signs. In many instances, retroperitoneal bleeding will tamponade and stop, forming a hematoma. This hematoma will eventually be resorbed. The risk with this approach is that the hematoma will eventually be infected, necessitating additional surgery. An additional nonsurgical intervention for brisk bleeding is selective angiographic embolization.
The surgical option is to perform an abdominal exploration while the patient is stable and in good condition. This approach brings the added morbidity of a second anesthesia and an abdominal incision, but avoids the possibility of the patient's condition deteriorating with continued delay or the possibility of a pelvic abscess later. Once adequate exposure is obtained in the pelvis, the peritoneum over the hematoma should be opened and the blood evacuated. All bleeding vessels should be identified and ligated. Again, if control of bleeding is difficult, thought should be given to unilateral or bilateral ligation of the anterior division of the internal iliac artery. Once hemostasis is achieved, the pelvis should be drained using a closed system.
The definition of postoperative pelvic infection must first be clarified. Clinically, a temperature of 38°C and clinical symptoms of lower abdominal and pelvic pain satisfy the criteria. The incidence of pelvic infection after vaginal hysterectomy has been reported to reach 65% without antibiotic prophylaxis and to remain as low as 6–7% with a single dose of preoperative cephalosporin.
When infection after vaginal hysterectomy is suspected, other sources of fever should be ruled out before instituting therapy for a pelvic infection. A routine evaluation including examination of the pelvis, lungs, and urine should be performed. In many instances, the diagnosis of a pelvic infection is one of exclusion. In addition to fever, patients may experience lower abdominal pain, pelvic pain, and induration and tenderness of the vaginal cuff. These physical findings may also be present in the routine postoperative patient who does not have fever. The several forms of infection after vaginal hysterectomy are classified as follows: vaginal cuff cellulitis, pelvic cellulitis, pelvic abscess, infected pelvic hematoma, and adnexal abscess.
Some degree of vaginal cuff cellulitis probably occurs after every hysterectomy. Usually, however, temperature elevation does not accompany this short-term infection. Antibiotics are not required if a temperature elevation alone occurs without symptoms or significant physical findings. If fever persists, accompanied by findings of pelvic pain and abdominal tenderness, antibiotic therapy should be instituted. An infection of this degree is better classified as pelvic cellulitis. Pelvic cellulitis usually manifests itself around the third day after surgery. It is a progression of infection into the soft tissue of the surrounding pelvic structures.
In most cases, response to parenteral antibiotic therapy is rapid. If response is poor after 48–72 hours, a pelvic abscess should be suspected. Pelvic examination should be repeated to document the presence or absence of a pelvic mass. If it is difficult to perform the examination, or the clinician is unsure as to the findings, ultrasonography may aid in confirming the diagnosis. Antibiotic therapy is initiated and typically extends 3–4 days. If the patient still does not respond, the pelvic examination should be repeated and the vaginal cuff explored with an instrument such as a Kelly clamp, hopefully allowing drainage of any purulent material. Rarely is repeat surgery necessary to treat an abscess.
An early pelvic hematoma may also become infected and form an abscess (infected pelvic hematoma). Its presentation is similar to that of a pelvic abscess but is usually accompanied by a significant fall in hematocrit. Antibiotic therapy is identical to that for a pelvic abscess, but exploration and drainage may need to be instituted earlier.
Antibiotic treatment for postoperative pelvic infection (adnexal abscess) should consist of broad-spectrum coverage. Institution of therapy is empiric in most instances, as these infections are usually polymicrobial. The combination of choice is clindamycin (900 mg IV every 8 hours) and gentamicin (5 mg/kg/day IV every 8 hours). These two drugs can be administered in the same IV bag. Metronidazole hydrochloride is a suitable alternative to clindamycin. The addition of penicillin or ampicillin to this regimen rarely improves the results.
Antibiotics should be continued 24–48 hours after the resolution of fever and symptoms. Oral antibiotics are not necessary once the patient is discharged.
REFERENCES
Ling FW, Stovall TG, Cruikshank SH et al: Vaginal Hysterectomy, pp 1 - 29. New York, Churchill Livingstone, 1995 |
|
Bolsen B: Study suggests vaginal hysterectomy is safer. JAMA 247: 13, 1982 |
|
Kovac SR: Guidelines to determine the route of hysterectomy. Obstet Gynecol 85: 1823, 1995 |
|
Kovac SR, Cruikshank SH, Retto HF: Laparoscopy-assisted vaginal hysterectomy. J Gynecol Survey 6: 185, 1990 |
|
Kovac SR, Cruikshank SH: Guidelines to determine route of oophorectomy with hysterectomy. Am J Obstet Gynecol 175, 1996 |
|
Baden WF, Walker T: Fundamentals, symptoms, and classification. In Baden WF, Walker T (eds): Surgical Repair of Vaginal Defects, pp 9 - 23. Philadelphia, JB Lippincott, 1992 |
|
Cruikshank SH, Pixley RL: Urinary tract gas formation in a woman with primary genital prolapse symptoms. Infect Surg 6: 429, 1987 |
|
Cruikshank SH, Kovac SR: Role of the uterosacral-cardinal ligament in protecting the ureter during transvaginal hysterectomy. Int J Obstet Gynecol 40: 141, 1993 |
|
Julian TM, Johnson GW, Gosewehr JA, O'Connell BJ: Vasopressin as a chemical tourniquet during vaginal surgery. J Gynecol Surg 9: 161, 1993 |
|
Cruikshank SH, Pixley RL: Methods of vaginal cuff closure and preservation of vaginal depth during transvaginal hysterectomy. Obstet Gynecol 70: 61, 1987 |