Usually appearing within 6 to 12 months after the menarche, primary dysmenorrhea occurs almost invariably in ovulatory cycles. About 88% of adolescents with dysmenorrhea experience their first painful menstruation within the first 2 years after menarche.13 Dysmenorrhea occurring more than 2 years after the menarche is more likely to be secondary dysmenorrhea, and the underlying cause should be vigorously sought. Primary dysmenorrhea usually begins a few hours before or just after the onset of menstruation. The cramps are most severe on the first or second day of menstruation. Characteristically, the pains are spasmodic in nature and strongest over the lower abdomen, but they may also radiate to the back and the inner aspects of the thigh, and they are often described as labor-like pains. The cramp is commonly accompanied by one or more systemic symptoms, including nausea and vomiting (89%), fatigue (85%), diarrhea (60%), lower backache (60%), and headache (45%). Nervousness, dizziness, and in some severe cases, syncope and collapse can be associated with primary dysmenorrhea. Lasting a few hours to 1 day, the symptoms seldom persist for more than 2 to 3 days.
Primary dysmenorrhea should be diagnosed by its positive clinical features and not through exclusion of other causes of dysmenorrhea. The hallmarks of primary dysmenorrhea are
- Initial onset of primary dysmenorrhea. The condition should have begun
within a few months and at the very most within 2 years of menarche. Despite
such fairly rigid criteria, a diagnosis of endometriosis can be
extremely difficult to exclude because endometriosis-related dysmenorrhea
has a remarkable resemblance to primary dysmenorrhea. Typically, the
dysmenorrhea of endometriosis in adolescents begins at 2.9 years after
menarche.14
- Duration of the cramps. The cramps seldom last more than 48 to 72 hours. Usually
the pain lasts only 24 hours or less. The pain also starts a
few hours before, or more frequently, only after the onset of the menstrual
flow. Dysmenorrhea, which starts before the onset of menstrual
flow and extends into several days throughout the flow, is less likely
to be primary dysmenorrhea.
- Character of the pain. This is described as cramping or labor-like pain.
- Pelvic examination. No abnormal findings that could account for the primary
dysmenorrhea should be found during the examination (including rectovaginal).
Etiology
Behavioral and psychologic factors, uterine ischemia, cervical stenosis or narrowing, increased vasopressin release, increased uterine activity, and increased uterine prostanoid production and release have been implicated in the cause of primary dysmenorrhea. Evidence suggests that most women with primary dysmenorrhea have increased or abnormal uterine prostanoid production and release, giving rise to abnormal uterine activity and therefore to pain.15–20
Although psychologic factors have not been demonstrated convincingly to be the cause of primary dysmenorrhea, their contribution should be considered in patients who have not responded to medical therapy and in the absence of any visible pelvic pathology to account for the pain. Whereas many psychoanalytic formulations have been advanced to explain the basis of dysmenorrhea, none of these reports studied the patients before the development of the dysmenorrhea.7 Such observations may be an accompaniment or the result of the dysmenorrhea but do not generate primary dysmenorrhea.7 Psychologic factors can certainly influence the reactive component of pain and therefore the perception of apparent increased intensity of pain. Anticipation of severe dysmenorrhea each month can itself be expected to engender quite a bit of stress. Dysmenorrhea is more common in working women and in women who scored higher on the Hassle scale, which is a measure of the stresses or difficulties experienced.7 Such a stressful event as dysmenorrhea has been demonstrated to reduce the immune response of the woman on day 26 and days 1 and 2 of the cycle.21 Primary dysmenorrhea itself is not a psychologic disorder, but in its management, the health care provider can enhance the overall efficacy of pharmacotherapy with appropriate handling of the reactive component of pain.
Traditionally, cervical dilatation has been performed to relieve primary dysmenorrhea on the basis that the cervical canal is widened. However, there are no objective data to support the suggestion that women in primary dysmenorrhea have a relative stenosis or narrowing of the cervical canal. In a small number of women who do have true cervical stenosis associated with menstrual cramps, the condition is secondary dysmenorrhea.
Primary dysmenorrhea occurs only in ovulatory cycles.15,17 Dysmenorrhea occurring in anovulatory cycles is of the secondary type. Primary dysmenorrhea can be readily relieved with administration of oral contraceptive pills, which inhibit ovulation. Some preliminary studies suggest that there may be an increase in circulating vasopressin levels in women with dysmenorrhea during their menstruation.22 However, serial levels before and during menstruation have not been studied adequately. An increase in vasopressin levels, without an accompanying increase in oxytocin levels, can produce dysrhythmic uterine contractions that are more likely to produce uterine hypoxia and ischemia. Using these observations, a preliminary study indicates that the vasopressin antagonist is able to inhibit abnormal uterine contractions and relieve primary dysmenorrhea.23
There is ample evidence to indicate that increased or abnormal production and release of endometrial prostanoids and eicosanoids in many women with primary dysmenorrhea give rise to abnormal uterine activity and result in uterine hypoxia and ischemia.15–20 First, there is a striking similarity between the clinical manifestations of primary dysmenorrhea and the symptoms induced when exogenous prostaglandins E2 or F2α are administered. In both situations, uterine contractions occur, and diarrhea, vomiting, and nausea are common. Second, measurements of prostaglandin levels from luteal-phase endometrial biopsies, endometrial jet washings, and menstrual fluids have shown that there is a significantly higher than normal concentration of prostaglandins in women with painful primary dysmenorrhea. Secretory endometrium, under the influence of progesterone, has higher concentrations of prostaglandins than proliferative endometrium and with no increase in endometrial prostaglandin concentrations reported from endometrium of anovulatory cycles. Many nonsteroidal antiinflammatory drugs that are prostaglandin synthetase inhibitors have been shown to be effective in the treatment of primary dysmenorrhea. With some of these prostaglandin synthetase inhibitors, there are also objective data showing that the relief of primary dysmenorrhea is caused by and accompanied by a concomitant reduction in uterine prostaglandins released during menstruation.
In normal women with no dysmenorrhea, there are fluctuations in uterine activity during different phases of the menstrual cycle. These changes in the resting tone, active pressure, and frequency of contractions are caused by changes in ovarian steroid hormone levels that affect the sensitivity of the myometrium to uterotonic substances, changing concentrations of prostaglandins in the endometrium, and other uterotonic substances that may still be undefined. During menstruation in the nondysmenorrheic woman, the uterine resting tone is lowest (<10 mm Hg), the active pressure is maximum (120 mm Hg), and the number of contractions (3 to 4 per 10 minutes) is least, compared with the rest of the cycle.4,5,11,15,16 In dysmenorrheic women, the increased release of uterine prostaglandins produces a significant degree of myometrial hyperactivity that results in uterine hypoxia and ischemia. Four different types of abnormalities of uterine contraction have been observed in women with primary dysmenorrhea, including increased uterine resting tone, increased active pressure, increased number of contractions, and incoordinate or dysrhythmic uterine activity. Most patients with primary dysmenorrhea appear to have increased uterine resting tone. When more than one of these uterine contraction abnormalities are present, they tend to potentiate each other, and pain occurs with a much smaller change in the abnormality than when only one is present. When the uterine activity is abnormal and increased, uterine blood flow has been shown to be reduced. With suppression of the abnormal activity, the uterine blood flow is enhanced and symptoms disappear. One mechanism contributing to the pain of primary dysmenorrhea is uterine ischemia or uterine hypoxia.
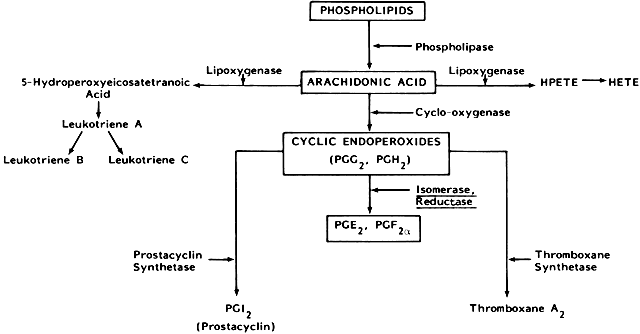
For a better understanding of the pathophysiology of primary dysmenorrhea and the mechanism of action of the nonsteroidal antiinflammatory drugs in the relief of this condition, it is essential to examine the biosynthesis of eicosanoids. Prostaglandins are C20 hydrocarbons with a cyclopentene ring and are present in most mammalian tissues, where they are produced locally under the control of microsomal enzymes collectively called prostaglandin synthetase. The pathway for the biosynthesis of prostaglandins, including prostacyclin and thromboxanes, is shown in Figure 1. Synthesized from free and unsaturated fatty acids, such as arachidonic acid and eicosatrienoic acid, which are often derived from conversion of phospholipids, triglycerides, and cholesterol esters by the enzyme acyl hydrolase, prostaglandins are produced under the influence of cyclooxygenase (COX), isomerase, and reductase, which are collectively referred to as prostaglandin synthetase.17 There are two isoforms of COX, COX-1 and COX-2, which are encoded by distinct genes.24,25 The gene for COX-1, which is constitutive and is a housekeeping enzyme, is located on chromosome 9.25 COX-2, which is inducible, is responsible for changes in prostaglandin production and is encoded by a gene on chromosome 1.25 Availability of arachidonic acid, endometrial cellular trauma, and availability and inducibility of COX, are important factors that stimulate prostaglandin production. In uterine tissues, arachidonic acid is usually produced from phospholipids through hydrolysis by the lysosomal enzyme phospholipase A2. Because this enzyme regulates the hydrolysis of phospholipids, phospholipase A2, rather than arachidonic acid availability, may be the more important rate-limiting factor in the biosynthesis of prostaglandins.
Progesterone exerts an important control on the stability of lysosomes; a high level of progesterone tends to stabilize lysosomes, and a declining level of progesterone labilizes it. At the end of the luteal phase of the menstrual cycle, if pregnancy does not occur, the corpus luteum undergoes regression leading to a decline in progesterone level and therefore lysosomal instability. Labilization of the lysosome is then accompanied by menstrual flow, and phospholipase A2 is released, causing hydrolysis of phospholipids from the cell membrane and generation of arachidonic acid. This continuing availability of increased arachidonic acid together with the intracellular destruction and trauma accompanying the onset of menstruation stimulates production of prostaglandins.
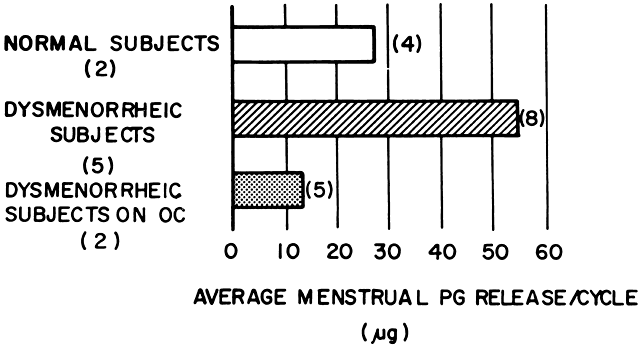
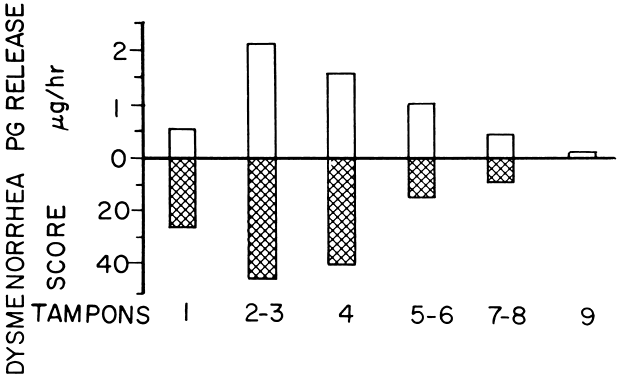
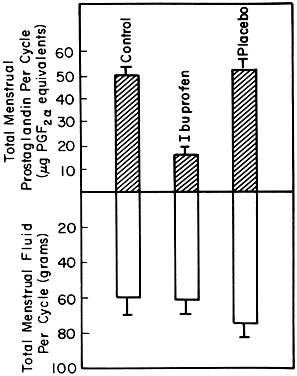
In women with primary dysmenorrhea, the endometrial tissues are capable of increased production and the release of prostaglandins during menstruation. Pickles and his colleagues26 were the first to quantitate prostaglandins in menstrual fluid and demonstrate that dysmenorrheic women produce 8 to 13 times more prostaglandin F than do nondysmenorrheic women. Significantly high quantities of prostaglandins are released per menstruation in women with primary dysmenorrhea (Fig. 2). Most of the production and release of prostaglandins occurs during the first 48 hours of menstrual flow, accounting for the intense pain experienced during the first or second day of menstruation in primary dysmenorrhea. The results of correlation between the amount of prostaglandins released in the menstrual fluid per hour and the clinical symptoms of the dysmenorrhea during the first 48 hours of the menstruation are shown in Figure 3. There is excellent correlation between the amount of prostaglandins released and the intensity of the pain for that duration. Luteal-phase human endometrium from dysmenorrheic women has been shown in vitro to produce seven times more prostaglandin F2α than luteal phase endometrium of normal women.27
|
Arachidonic acid or eicosatrienoic acid can also be converted through the 5-lipoxygenase pathway (Fig. 1) to 5-hydroxyperoxyeicosatetraenoic acid (5-HPETE) and leukotrienes (A4, B4, C4, D4). Leukotrienes and 5-HPETE stimulate uterine contraction. The factors regulating the 5-lipoxygenase pathway in the nonpregnant human uterus are unknown.
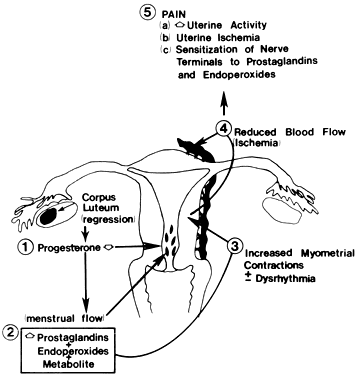
The postulated mechanisms for the generation of pain from pelvic structures in primary dysmenorrhea are summarized in Figure 4. The increased uterine production and release of prostaglandins at menstruation give rise to increased abnormal uterine activity, which then causes uterine hypoxia and pain. Increased uterine activity and uterine ischemia or hypoxia are two major factors in the causation of the pain. Prostaglandins, such as prostaglandin E2, and cyclic endoperoxides hypersensitize pain fibers in the pelvis and uterus to the action of pain-inducing substances or factors. This understanding of the pathophysiology of primary dysmenorrhea has enabled the rational use of nonsteroidal antiinflammatory drugs for the relief of primary dysmenorrhea rather than pharmacotherapy, which merely inhibits uterine contractions, such as with betamimetic agents.
The roles of prostanoids, such as thromboxane A2 and prostacyclin, and leukotrienes in primary dysmenorrhea are not well understood. Preliminary evidence suggests that prostacyclin is involved in the pathophysiology of primary dysmenorrhea.28 Increased uterine leukotriene may be responsible for some forms of primary dysmenorrhea29 that do not respond to therapy with nonsteroidal antiinflammatory drugs, because leukotrienes are produced through the 5-lipoxygenase enzyme pathway rather than the cyclooxygenase pathway (Fig. 1). Prostacyclin is a potent vasodilator that relaxes uterine muscle in vitro. A reduction of prostacyclin may potentiate enhancement of uterine activity and vasoconstriction, which gives rise to hypoxia, ischemia, and pain. In some women with primary dysmenorrhea, an imbalance in the concentrations of different prostaglandins, rather than absolute increases or decreases, may be responsible for the pain.
Differential Diagnosis
The differential diagnoses of primary dysmenorrhea include all the causes of secondary dysmenorrhea, such as endometriosis, presence of an intrauterine device, pelvic inflammatory disease and infections, adenomyosis, uterine myomas, polyps and adhesions, congenital malformation of the Müllerian system (e.g. bicornuate and septate uterus, transverse vaginal septum, rudimentary blind uterine horn), cervical strictures or stenosis, ovarian cysts, pelvic congestion syndrome, and defects in the broad ligament (Allen-Masters syndrome). Endometriosis must be especially considered as it can mimic primary dysmenorrhea very closely and can begin with the onset of menarche or shortly thereafter. Pelvic pain and dysmenorrhea due to endometriosis in teenage women have been shown to occur approximately 2.9 years after the menarche.14 Contrary to conventional thinking, endometriosis can occur in these teenagers and in black women, as shown with the increasingly frequent use of laparoscopy. In patients with strong indications for endometriosis, such as those with a familial history of endometriosis in their sisters or mothers (8% risk), laparoscopy should be undertaken fairly early during management after medical therapy has failed.
There are no special diagnostic tests to confirm the diagnosis of primary dysmenorrhea. Most of the investigations that are used are for confirming the presence of lesions responsible for secondary dysmenorrhea.
Management
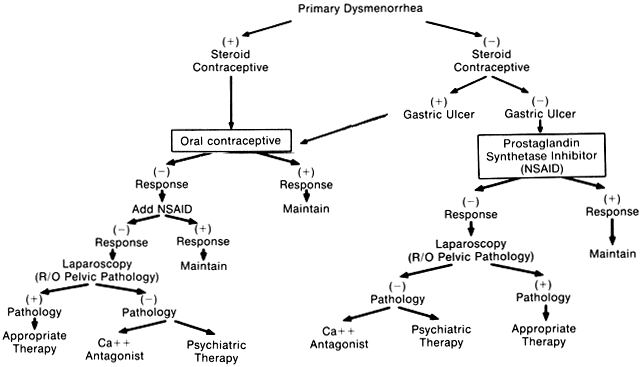
The overall approach to management should include skillful manipulation of the psychologic and behavioral factors and the specific pharmacotherapy.1–4,16 Careful assessment of the proportion contributed by the psychologic or reactive component of the pain in dysmenorrhea in each of the patients is essential to appropriate therapy or a combination of therapies. The efficacy of pharmacotherapy and other forms of therapy can be greatly enhanced by the simple psychotherapy that accompanies the doctor-patient dialogue, explanation, and reassurance given by the physician. Various modalities of treatment have been used in the treatment of primary dysmenorrhea and are summarized in Table 1. The most effective medications are oral contraceptives and the nonsteroidal antiinflammatory drugs, which are prostaglandin synthetase inhibitors. The drug of choice for the relief of primary dysmenorrhea is an effective nonsteroidal antiinflammatory agent. However, the choice of medication depends on whether the woman prefers an oral contraceptive for her birth control needs and whether there is any contraindication to the use of the combined oral contraceptive or the nonsteroidal antiinflammatory drug. The choice of medication and the medical management of primary dysmenorrhea is outlined in the algorithm in Figure 5.
TABLE 1. Modalities Available for the Treatment of Primary Dysmenorrhea
Approach | Specific Methods |
General measures | Psychotherapy, reassurance |
Surgery | Dilatation and curettage, presacral neurectomy |
Endocrine therapy | Oral contraceptive pills, inhibition of ovulation |
Tocolysis | Alcohol, β-receptor stimulators |
Analgesics | Nonnarcotics, narcotics |
Nerve block or stimulation | Alcohol or local anesthetic injection of uterosacral ligaments, transcutaneous electrical nerve stimulation |
Prostaglandin synthetase inhibitor | Cyclooxygenase blockers (indomethacin, ibuprofen, sodium naproxen, flufenamic acid) |
ORAL CONTRACEPTIVES.
If the patient desires contraception with the combined oral contraceptive pill, this is the method of choice because she can also get relief from her primary dysmenorrhea. Combination-type oral contraceptives are effective in the treatment of primary dysmenorrhea and were the main method of treatment until the advent of nonsteroidal antiinflammatory drugs. The menstrual fluid prostaglandin levels are reduced to below normal levels in women who use oral contraceptives.19 This reduction in menstrual fluid prostaglandins is brought about through two mechanisms. First, birth control pills reduce menstrual fluid volume through suppression of endometrial tissue growth, giving rise to reduced prostaglandin levels. Second, inhibition of ovulation with the birth control pill retards endometrial development and produces an anovulatory cycle with an endocrine milieu that is essentially similar to the early proliferative phase of the menstrual cycle when endometrial prostaglandin levels are low. The absence of luteal-phase progesterone levels that are necessary for increased endometrial prostaglandin biosynthesis also contributes to the anovulatory mechanism of reducing menstrual fluid prostaglandins. With birth control pills, more than 90% of dysmenorrheic women can be satisfactorily relieved of their primary dysmenorrhea. A trial of oral contraceptives for 3 to 4 months is useful. Women who respond well to oral contraceptives can be maintained on this regimen. However, if dysmenorrhea is not adequately relieved, an appropriate nonsteroidal antiinflammatory drug can be added. The response to the nonsteroidal antiinflammatory drugs can then be monitored as in the patient who is placed on such an agent initially.
NONSTEROIDAL ANTIINFLAMMATORY DRUGS.
Women with primary dysmenorrhea can be given nonsteroidal antiinflammatory drugs if they do not have any gastroduodenal ulcers or are hypersensitive to nonsteroidal antiinflammatory drugs. Such therapy can be allowed for 3 to 6 months, with adjustment in the doses and the type of nonsteroidal antiinflammatory drugs. If there is no relief, laparoscopy is indicated. If such measures are followed and a proper diagnosis of primary dysmenorrhea is made, 80% to 85% of patients obtain relief. Laparoscopy is necessary only in a small percentage of patients with dysmenorrhea in whom medical therapy has failed. In the event that pelvic disease is discovered at laparoscopy, the appropriate therapy directed to the underlying pathology is instituted, thereby alleviating the dysmenorrhea.
If no pelvic pathology is found, one of the newer methods of management, which is less established than using nonsteroidal antiinflammatory drugs, can be employed and becomes one of trial and error, because it is unclear whether any specific pharmacotherapy could be of help. A preliminary study30 found that a calcium channel blocker, such as nifedipine, provided some relief. In the investigator's clinical experience, calcium channel blockers were helpful in difficult cases of primary dysmenorrhea. Other agents that could be resorted to include the betamimetic drugs, but they are less effective than nonsteroidal antiinflammatory drugs and often carry side effects.1,2 These agents probably should not be used on a routine basis, and such attempts are best left to medical centers carrying out systematic studies of them. A noninvasive method of blocking the propagation of pain impulses from the pelvis using transcutaneous electrical nerve stimulation (TENS) has been employed.31 Psychiatric help in the management of these patients who have been relieved with nonsteroidal antiinflammatory drugs and who have no disease in the pelvis might also be appropriate. It should not be construed that dysmenorrhea is wholly psychosomatic, but in the absence of understanding of the basic pathophysiologic mechanisms of the dysmenorrhea, symptomatic treatment with analgesics and psychotherapy to modulate the psychologic factors that may be playing a significant or contributing role to the pain could ameliorate the dysmenorrhea to some extent. Few patients with primary dysmenorrhea fall into this category if a correct diagnosis has been made and appropriate steps in the management of the patient have been taken.
Unlike oral contraceptives, nonsteroidal antiinflammatory drugs are taken for the first 2 to 3 days of the menstrual flow, there is no significant suppression of the pituitary-ovarian axis, and there are none of the metabolic effects seen with oral contraceptives, which have to be taken for a minimum of 3 of every 4 weeks. Choosing the most suitable nonsteroidal antiinflammatory drug should be based on its proven clinical efficacy; rapid absorption to produce a quick onset of relief; wide margin of safety with a low ulcerogenecity index; minimal, tolerable, and inconsequential side effects; and long-term safety. The nonsteroidal antiinflammatory drugs should be given as soon as menstruation begins and should be taken on a continuing basis for the first 2 or 3 days and not on an as-needed basis. The latter method clearly aims at suppressing pain, whereas the former approach, which is more likely to be effective in most patients, acts by correction of the derangement in prostaglandin production. Nonsteroidal antiinflammatory drugs appear to relieve primary dysmenorrhea through suppression of menstrual fluid prostaglandins, as has been demonstrated in several studies (Fig. 6). These compounds also have direct analgesic properties.
|
Table 2 provides a summary of the clinical efficacy of nonsteroidal antiinflammatory drugs grouped according to the structural derivation. In contrast to oral contraceptives, which inhibit endometrial development and thereby reduce menstrual fluid prostaglandins (Fig. 6), nonsteroidal antiinflammatory drugs do not affect endometrial development; instead, they suppress menstrual fluid prostaglandin through enzymatic inhibition of prostaglandin production.
Prostaglandin Synthetase |
|
|
|
Inhibitor Group | Drug | Dose | Clinical Relief |
Benzoic acid derivatives | 500–600 mg 4×/day | No difference from placebo except in one study | |
Buterophenones | Phenylbutazone | No information available |
|
| Oxyphenbutazone |
|
|
Indole acetic acid | Indomethacin | 25 mg 3× to 6×/day | 73% to 90% relief |
Fenamates | Flutenamic acid | 100–200 mg 3×/day | 77% to 82% relief |
| Mefenamic acid | 250–500 mg 4×/day | 93% relief |
| Tolfenamic acid | 133 mg 3×/day | 88% relief |
Aryloproponic acid | 400 mg 4×/day | 66% to 100% relief | |
| 275 mg 4×/day | 78% to 90% relief | |
| 50 mg 3×/day | 90% relief | |
Miscellaneous | Suprofen | 200 mg 4×/day | 60% to 80% relief |
| Once/day | Effective |
From Dawood MY: Nonsteroidal antiinflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med 84 (Suppl 5A):23, 1988.
Contraindications to the use of nonsteroidal antiinflammatory drugs include gastroduodenal ulcers, previous gastric bleeding, and a previous history of bronchospasmatic type of reaction after the ingestion of aspirin or aspirin-like drugs. Side effects of nonsteroidal antiinflammatory drugs are relatively mild during therapy for primary dysmenorrhea, and are usually well tolerated. The known side effects of nonsteroidal antiinflammatory drugs are listed in Table 3.
TABLE 3. Side Effects of Prostaglandin Synthetase Inhibitors
Gastrointestinal symptoms
Indigestion
Heartburn
Nausea
Abdominal pain
Constipation
Vomiting
Anorexia
Diarrhea
Melena
CNS symptoms
Headache
Dizziness
Vertigo
Visual disturbances
Hearing disturbances
Irritability
Depression
Drowsiness
Sleepiness
Other symptoms
Allergic reactions
Rash
Edema
Bronchospasm
Hematologic abnormalities
Effects on the eyes
Fluid retention
Effects on liver and kidney
From Dawood MY: Overall approach to the management of dysmenorrhea. In Dawood MY (ed): Dysmenorrhea, p 261. Baltimore: Williams & Wilkins, 1981.
OTHER FORMS OF TREATMENT.
Although cervical dilatation as a primary method of treatment for primary dysmenorrhea is not warranted, dilatation of the cervix should be undertaken when laparoscopies are performed. This surgical manipulation does relieve primary dysmenorrhea temporarily, although with a progressive return of the symptoms. Relief may be caused by destruction of the paracervical nerve fibers and plexus with consequent neuropraxia or partial denervation of the cervix and a temporary increase in the diameter of the cervical canal, culminating in enhanced menstrual fluid flow with shorter contact time between the uterine wall and menstrual fluid containing the prostaglandins.
Among the betamimetic agents, only terbutaline has been found to be more effective than a placebo.1,2 The high incidence of side effects confirmed that this is not usually a practical approach. Alcohol, a tocolytic agent, diminishes dysmenorrhea, but to obtain complete relief, amounts must be taken that also produce inebriation and incapacitation. The progesterone-medicated intrauterine device (Progestasert) reduces menstrual fluid prostaglandin and relieves primary dysmenorrhea.32 It is not exactly prudent to recommend use of an intrauterine device, which itself gives rise to dysmenorrhea, as a method for treatment of women with primary dysmenorrhea.
Presacral neurectomy is rarely indicated for treatment of most forms of primary dysmenorrhea. Use of this procedure should be extremely limited and reserved for patients with chronic pelvic pain when other methods of pain relief have failed, for patients with pelvic malignancy, and for patients with pelvic pathologies, such as endometriosis, when there is impingement or involvement of the presacral plexus. Presacral neurectomy provided relief of dysmenorrhea in 53% to 72% of patients with different stages of endometriosis,33 much less than obtained with hormonal treatment.
For cases that have not responded to medical therapy and in which laparoscopic findings are negative, alcohol or local anesthetics can be injected into the uterosacral ligament to denervate the nerve supply to the paracervical region temporarily. Transection of the uterosacral ligaments through which these nerves pass has also been undertaken. With the advent of laser laparoscopy, this is being enthusiastically recommended by some without carefully considering the potential problems of this procedure, especially in young women with severe primary dysmenorrhea. Regardless of the methods used to denervate the uterosacral ligament surgically, the healing process may induce adhesions of the ovary and fallopian tube in this region and compromise the future reproductive potential of these women. Uterine prolapse has been reported in nulliparous women after uterosacral transection.
TENS has been studied for the relief of primary dysmenorrhea. The investigators found that high-frequency TENS significantly reduces the pain of primary dysmenorrhea and the amount of pain medication required.31 In a placebo-controlled TENS study, it was found that in 30% of cycles of women with severe primary dysmenorrhea TENS provided good relief without the need for any kind of backup pain medication.31 In the other women, the amount of pain medication required was significantly lower at all time intervals examined during the first 48 hours of menstruation. TENS appears to be a suitable method of relieving primary dysmenorrhea in women who may not wish to take medication, have medical contraindications to the use of nonsteroidal antiinflammatory drugs or birth control pills, have side effects from the use of nonsteroidal antiinflammatory drugs, or are unable to obtain total relief with maximum doses of nonsteroidal antiinflammatory drugs. TENS relieves primary dysmenorrhea through two likely mechanisms. First, with continuing transcutaneous electrical nerve stimulation, the preganglionic fibers are bombarded with impulses, which saturate the nerve cells of the dorsal horn and therefore block the propagation of pain impulses along these fibers (gate control theory). Second, TENS induces release of endorphin from these nerve cells that contributes to the relief of pain.