Premenstrual syndromes are diagnosed on the basis of the timing of the symptoms, which cross emotional, behavioral, and mood domains. For a diagnosis of PMS, the symptoms must occur during the 2 weeks before menses and subside during the menstrual flow. With remission of the symptoms, a symptom-free period occurs in the follicular phase between menses and ovulation. Clinically significant PMS is determined by the patient's seeking treatment and/or report of impaired functioning in relationships, work, or other activities. For a diagnosis of pure PMS, the symptoms must not be accounted for by other physical or psychiatric disorders. There are no hormonal measures, other laboratory tests, or physiologic measures that identify PMS. Such tests are useful only to identify or rule out other disorders that may be suspected in the diagnostic evaluation.
The most frequently reported symptoms of PMS include irritability, anxiety, nervous tension, mood swings, fatigue, depression, feeling overwhelmed or out of control, physical symptoms of swelling or bloating of the abdomen or extremities, appetite changes and food cravings, aches, and breast tenderness (see Table 2).
The specific symptoms appear to have little importance for the diagnosis. The critical elements are the relationship to menses, severity, and the degree of functional impairment. Women who seek medical treatment usually describe multiple symptoms, with the primary focus and most distressing problems typically focused on the mood and behavioral symptoms. Physical symptoms are widely experienced by women who do not perceive problems with premenstrual distress and are seldom sufficient for a clinical diagnosis of PMS without other mood or behavioral symptoms.
Premenstrual Syndrome
Diagnostic criteria for PMS are presented in the ACOG Practice Bulletin 15 of 2000.3 At least one of the listed affective or somatic symptoms must be experienced during the 5 days before menses in three prior menstrual cycles (Table 3) and relieved with the menstrual flow. The symptoms should be confirmed by two cycles of prospective reports. The symptoms cause identifiable impairment in the patient's functioning and are not accounted for by other physical or emotional disorders.
Table 3. ACOG Diagnosis of Premenstrual Syndrome
| Symptoms consistent with PMS that may include: | |
| Affective symptoms: | depression, angry outbursts, irritability, anxiety, confusion, social withdrawal |
| Somatic symptoms: | breast tenderness, abdominal bloating, headache, swelling of extremities |
| Restriction of these symptoms to the luteal phase of the menstrual cycle assessed prospectively | |
| Impairment of some facet of the woman's life | |
| Exclusion of other diagnoses that may better explain the symptoms | |
(American College of Obstetricians and Gynecologists. ACOG Practice Bulletin 15; 2000)
Premenstrual Dysphoric Disorder
The diagnostic criteria for PMDD are listed in the DSM-IV.1 These criteria were intended to diagnose a severe dysphoric form of PMS. The symptoms must be severe premenstrually and minimal or absent after menses. At least five of the 11 listed PMDD symptoms, including one or more of the mood symptoms, must meet these criteria. Physical symptoms, regardless of the number, are counted as a single symptom (Table 4). The symptoms must markedly interfere with functioning and not simply be an exacerbation of another physical or mental disorder. All criteria must be confirmed by prospective daily ratings for at least two menstrual cycles.
Table 4. Symptoms Required to Diagnose PMDD
| Depressed mood | Concentration difficulties |
| Anxiety/tension | Appetite changes/food cravings |
| Mood swings | Insomnia/hypersomnia |
| Irritability | Feeling out of control |
| Decreased interest | Physical symptoms |
| Fatigue |
PMDD, premenstrual dysphoric disorder
Five or more symptoms must be severe premenstrually and remit after menses (Adapted from American Psychiatric Association [1994])
There is no gold standard for operationalizing the premenstrual severity and postmenstrual remission level required for a PMDD diagnosis. The dependence of the diagnosis on subjective report of five specified symptoms without a standard method of determining severe and minimal levels results in marked differences among study samples identified as PMDD. Smith and associates demonstrated that the number of subjects identified by the PMDD criteria varies markedly by the rating scale used for symptom assessment.139 Increasing evidence indicates that approximately 5% to 8% meet stringent PMDD criteria.33,34 However, approximately 20% of women may experience severe PMS but not meet the PMDD criteria.33 There is no evidence that treatment response differs between a PMDD or PMS diagnosis in the few studies that have examined this issue.118,140
Differences Between Premenstrual Syndrome and Premenstrual Dysphoric Disorder
The most obvious difference between the PMS and PMDD diagnosis is the number of symptoms required. PMDD requires five of 11 identified symptoms; the ACOG diagnosis of PMS requires only one severe symptom consistent with PMS. The diagnoses are otherwise identical in their requirements for the menstrually related symptom pattern, confirmation by prospective ratings of the symptoms, impaired functioning and identification of other disorders that could account for the symptoms.
Daily Symptom Reports
The diagnosis of PMS and PMDD continues to be made from the patient's report of symptoms on a daily basis for at least two menstrual cycles. Numerous scales for daily symptom rating have been published and are available for use (Table 5). Daily symptom reports may be viewed as difficult to use in the primary care setting, but such reports can also help the busy clinician by swiftly demonstrating the patient's symptoms and indicating whether they are in fact linked to the menstrual cycle in the requisite pattern. Many women who report PMS will not confirm that the symptoms occur only premenstrually in daily symptom reports. Daily symptom reports are also helpful in indicating there may be other comorbidities when the reports show that symptoms occur randomly or throughout the menstrual cycle. The daily symptom reports are also an important educational tool for the patients. Patients can observe the patterns and severity of their symptoms over the menstrual cycle and gain an increased sense of control.
Table 5. Assessment Instruments for PMS/PMDD
| To Diagnose | ||||||
| Instrument | Name | # Items | PMS | PMDD | To Assess Symptoms | Reference |
| COPE | Calendar of premenstrual experiences | 22 | 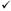 |
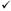 |
Mortola et al, 1990 | |
| DRSP | Daily record of severity of problems | 24 | 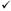 |
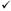 |
 |
Endicott & Harrison, 1996 |
| DSR | Penn daily symptom rating | 17 |  |
 |
 |
Freeman et al, 1996 |
| MDQ | Menstrual distress questionnaire | 48 | 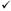 |
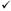 |
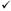 |
Moos, 1968 |
| PAF | Premenstrual assessment form | 96 | 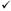 |
Halbreich et al, 1982 | ||
| PMSD | Premenstrual symptom diary | 17 |  |
 |
Thys-Jacobs et al, 1995 | |
| PMTO | Premenstrual tension scale-observer | 10 |  |
Steiner et al, 1980 | ||
| VAS | Visual analogue scales | Open |  |
 |
 |
Rubinow et al, 1984; Steiner et al, 1999 |
Diagnostic Procedures
A medical history (with emphasis on reproductive events, mood disorders, and family history of PMS and other mood disorders), complete physical examination, and a gynecologic examination are important for the diagnosis. By definition, PMS occurs with regular ovulatory menstrual cycles in the normal range; irregular cycles require further gynecologic investigation.
Comorbid conditions should be identified, including dysmenorrhea, endometriosis, ovarian cysts, uterine fibroids, pelvic inflammatory disease, seizure disorders, migraine, thyroid disorders, asthma, allergies, diabetes, hepatic dysfunction, cancer, lupus, anemia, and infections. Psychiatric conditions that must be considered include mood disorders, substance abuse, eating disorder, anxiety disorders, and personality disorders (Table 6). It may be difficult to determine whether the symptoms are an exacerbation of a comorbid condition or PMS symptoms superimposed on another condition. In either case, the usual recommendation is to treat the underlying condition first, and then reassess and possibly add treatment for the symptoms that arise premenstrually.
Table 6. Differential Diagnosis of PMS
| Medical Disorders | Psychiatric Disorders |
| Dysmenorrhea | Major depression |
| Endometriosis | Dysthymia |
| Ovarian cysts | Bipolar illness |
| Uterine fibroids | Generalized anxiety |
| Hypothyroidism | Panic |
| Seizure disorders | Substance abuse |
| Autoimmune disorders | Eating disorders |
| Asthma | |
| Allergies | |
| Diabetes | |
| Anemia |
PMS, premenstrual syndrome
At least two visits should be considered for the diagnostic evaluation. Evaluating the patient when she is premenstrual usually confirms the severity of the symptoms, but it is difficult to determine their overlap with other conditions. It is diagnostically useful to see the patient after menses when symptoms have abated. If symptoms are absent, it provides convincing evidence for the diagnosis. However, if symptoms are present in the follicular phase, the type and severity of the symptoms provide important diagnostic information for other comorbid conditions.
 -[
-[