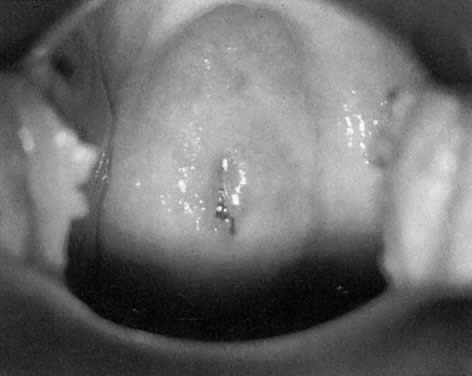
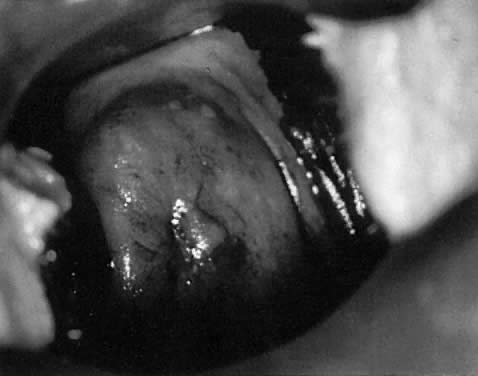
In a patient with an abnormal Pap smear, colposcopy can be expected to
identify an abnormal area in the TZ that corresponds to the Pap smear
findings. In most cases, the diagnosis of an abnormal TZ is based on the
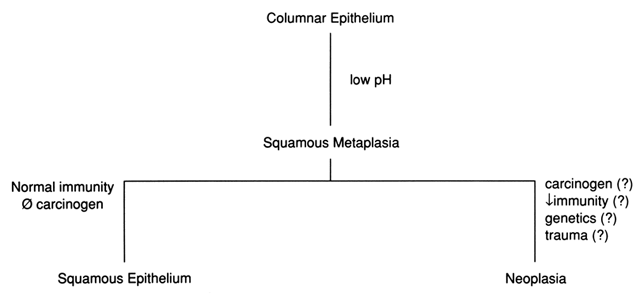
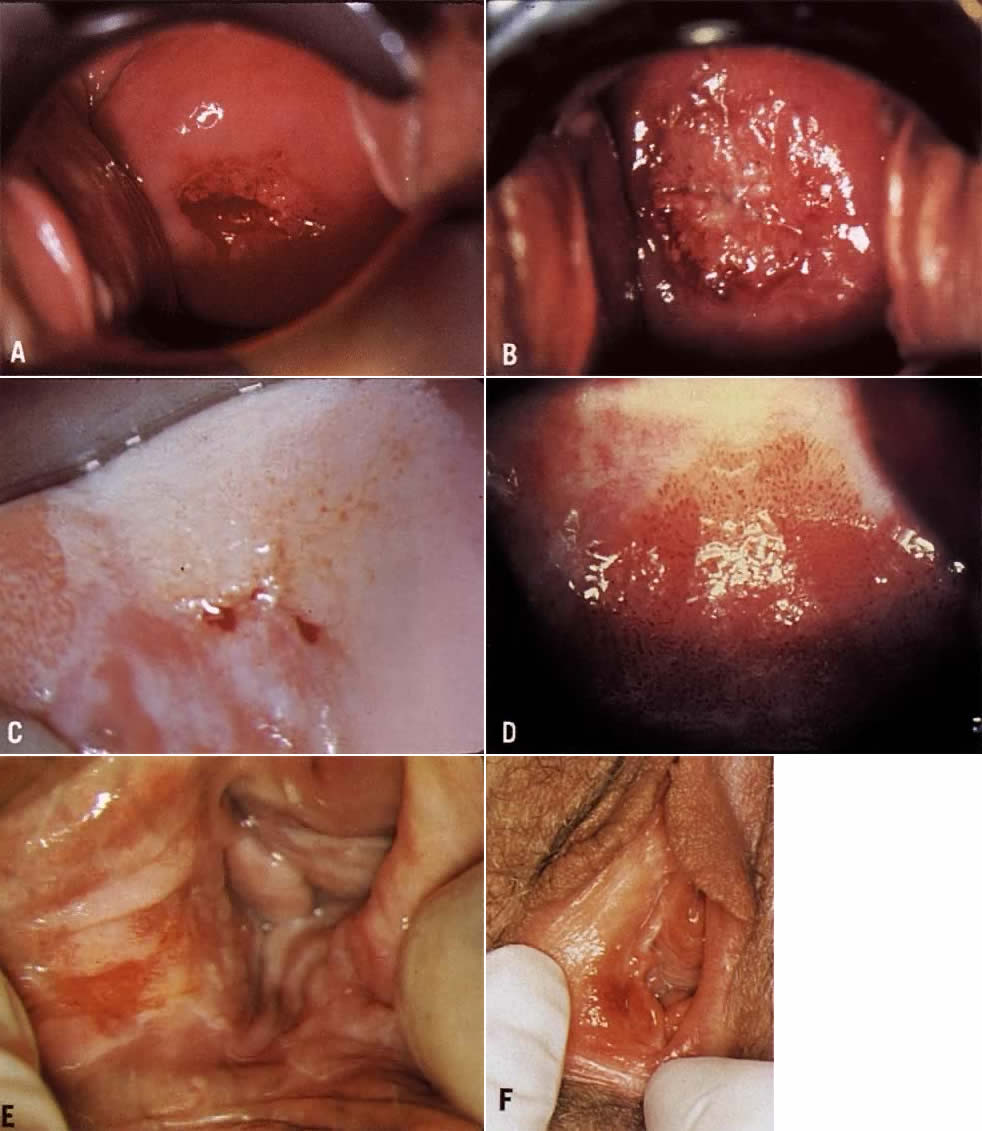
identification of vascular patterns, which suggest cervical neoplasia (Fig. 8). Most of these patterns are not visible to the naked eye and, indeed, are
not visible at the time of colposcopic examination unless a solution
of 3% to 5% acetic acid is first applied to the cervix. Although the
exact mechanism of action of acetic acid is not known, any area with
an increased nuclear/cytoplasmic ratio will appear whiter than the surrounding
area after the application of dilute acetic acid. Because squamous
metaplasia has an increased nuclear/cytoplasmic ratio, it will
appear faintly white. However, neoplasia is much more distinct from the
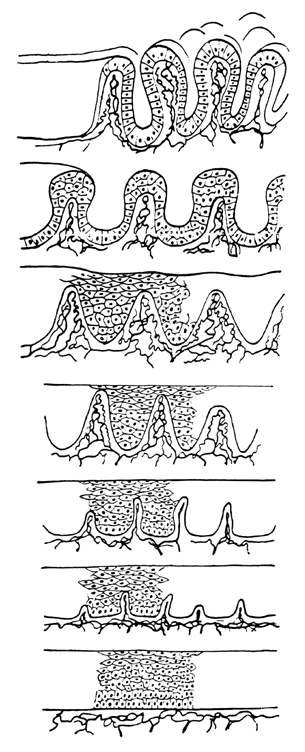
background than is squamous metaplasia. 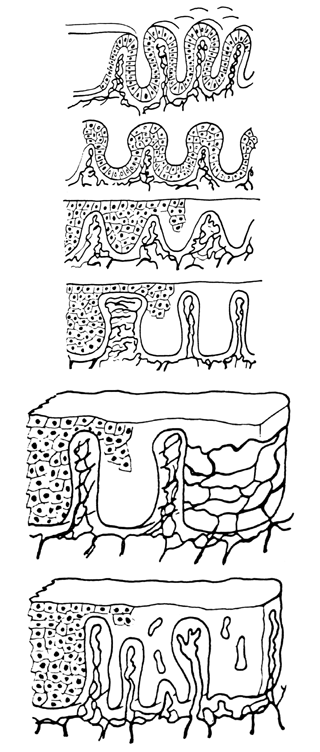 Fig. 8. Atypical squamous metaplasia. The metaplastic epithelium for some unknown
reason starts to grow in buds or blocks. The individual cells become
indistinguishable from those of frank invasive carcinoma. The central
vascular network of the villi of columnar epithelium remains as punctate
or mosaic vessels that extend close to the surface of the epithelium. The
mosaic vessels form basketlike structures around the blocks of
abnormal cells.(Kolstad P, Stafl A: Atlas of Colposcopy. Baltimore, University Park Press, 1972) Fig. 8. Atypical squamous metaplasia. The metaplastic epithelium for some unknown
reason starts to grow in buds or blocks. The individual cells become
indistinguishable from those of frank invasive carcinoma. The central
vascular network of the villi of columnar epithelium remains as punctate
or mosaic vessels that extend close to the surface of the epithelium. The
mosaic vessels form basketlike structures around the blocks of
abnormal cells.(Kolstad P, Stafl A: Atlas of Colposcopy. Baltimore, University Park Press, 1972)
|
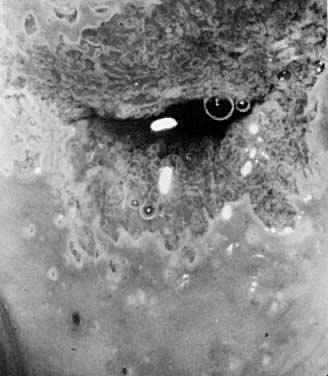
Acetowhite Epithelium (White Epithelium) Areas that appear white after the application of acetic acid are called
acetowhite epithelium or white epithelium. These areas may be observed
either inside or outside of the TZ. When located outside of the TZ, they
often represent areas of HPV infection, trauma, or repair (Fig. 9). 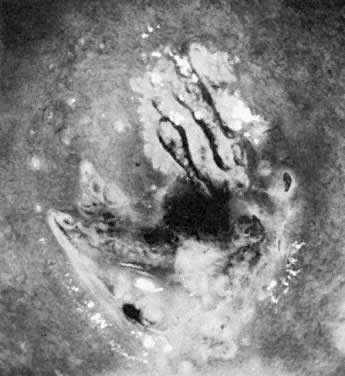 Fig. 9. The anterior lip of the cervix demonstrates gland openings, squamous metaplasia, and
a localized area of white epithelium with a very fine vascular
mosaic. Biopsy revealed only chronic cervicitis and squamous metaplasia. Fig. 9. The anterior lip of the cervix demonstrates gland openings, squamous metaplasia, and
a localized area of white epithelium with a very fine vascular
mosaic. Biopsy revealed only chronic cervicitis and squamous metaplasia.
|
White epithelium can be graded based on its surface contour, its whiteness, and
its border with surrounding tissues. In general, neoplasia has
a distinct border, is grayish-white rather than pearly-white, and is
somewhat raised from the surrounding tissues. Areas of HPV infection
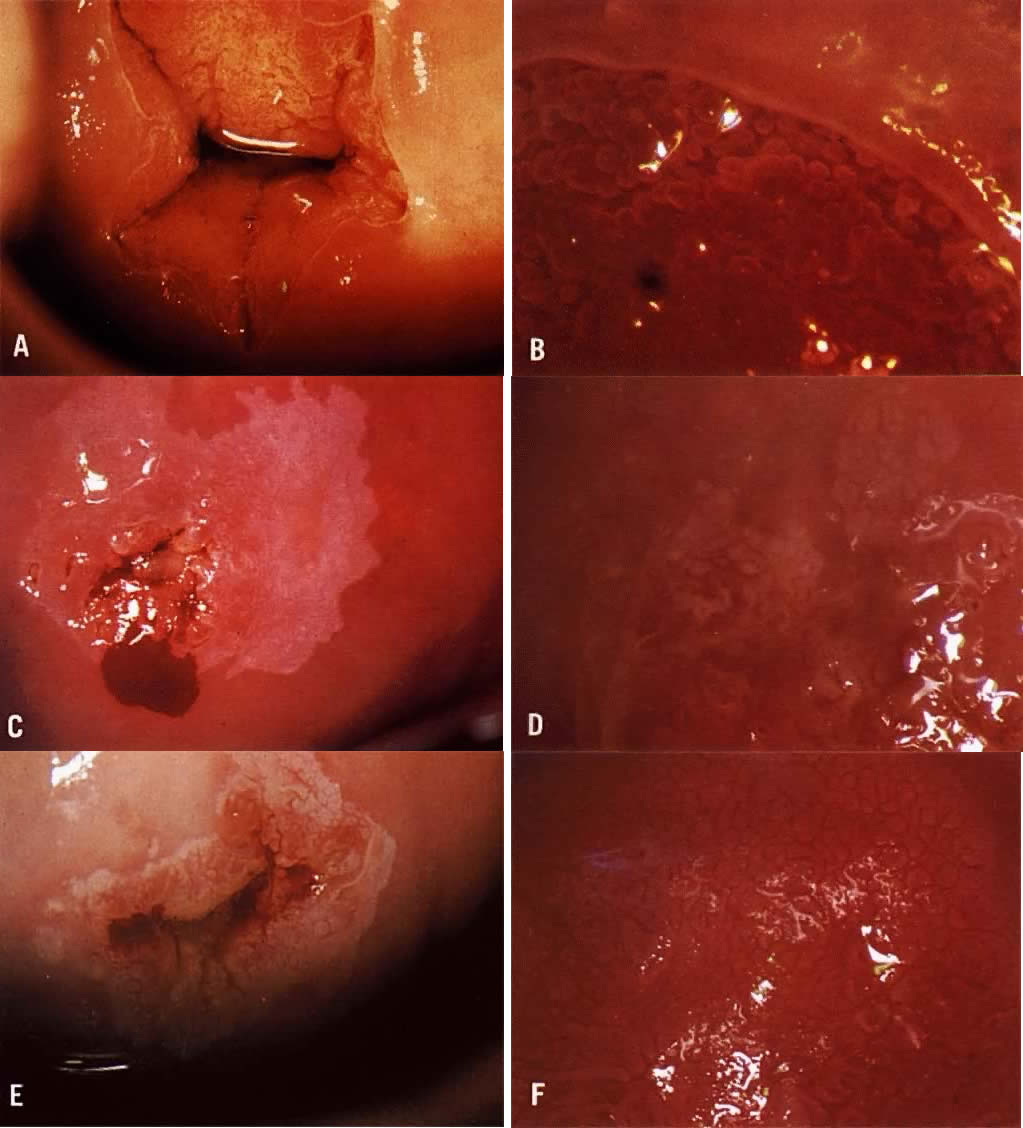
only are generally much whiter with indistinct borders (Color Plate 1C). Areas of HPV may or may not be raised above surrounding tissues. Most
areas of white epithelium have vascular changes (see below). When a white area is observed on the cervix prior to the application of
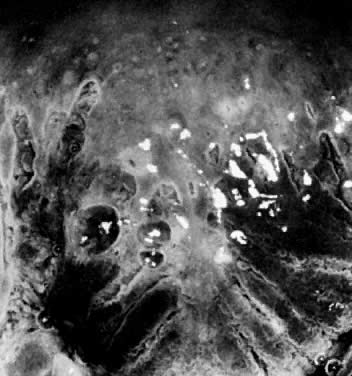
acetic acid, it is referred to as leukoplakia. These areas represent
hyperkeratosis (Fig. 10) and are often caused by infection with the HPV virus. Unfortunately, without
biopsy, it is impossible to know whether the epithelium underneath
the hyperkeratotic area is abnormal. Therefore, it is generally recommended
that at least one representative leukoplakic area be biopsied
during a colposcopic examination. 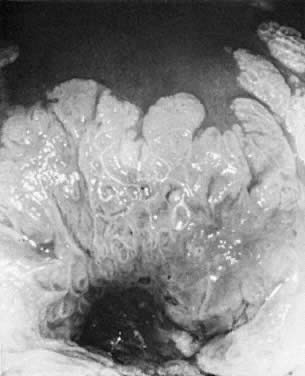 Fig. 10. The cervix prior to application of acetic acid. A very dense hyperkeratotic
pattern is noted surrounding the cervical os. No comment can be made
about the underlying epithelial vascular pattern. Fig. 10. The cervix prior to application of acetic acid. A very dense hyperkeratotic
pattern is noted surrounding the cervical os. No comment can be made
about the underlying epithelial vascular pattern.
|
Vascular Patterns If a neoplastic stimulant (some combination of HPV infection, immune status, and
genetics) is applied to an area of metaplasia, the resultant
neoplastic epithelium causes blood vessels to grow into the epithelium. Some
authors suggest that neoplasia induces neovascularization, whereas
others believe that neoplasia merely influences the capillary vessels
found in columnar epithelium to persist and grow.7 Regardless of the cause, the most common finding when CIN* is present
is a change in the vasculature. With CIN, the vessels are no longer confined
to the area under the transparent squamous epithelium but are found
within the epithelium itself. The increased nuclear/cytoplasmic ratio
in CIN causes the epithelium to lose its transparency after the application
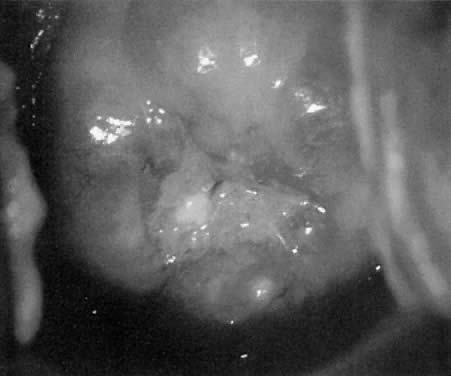
of acetic acid. Sometimes the vessels of CIN remain as distinct capillary loops, but extend
to the surface. In higher grade lesions (CIN III), these vessels
may actually extend a millimeter or two above the surface. This can be
recognized since the tips of these vascular loops often reflect light. When
these loops are the predominant pattern, it is termed punctation. (Fig. 11, Fig. 12, and Fig. 13)  Fig. 11. Punctate vessels as seen in CIN.(Kolstad P, Stafl A: Atlas of Colposcopy. Baltimore, University Park Press, 1972) Fig. 11. Punctate vessels as seen in CIN.(Kolstad P, Stafl A: Atlas of Colposcopy. Baltimore, University Park Press, 1972)
|
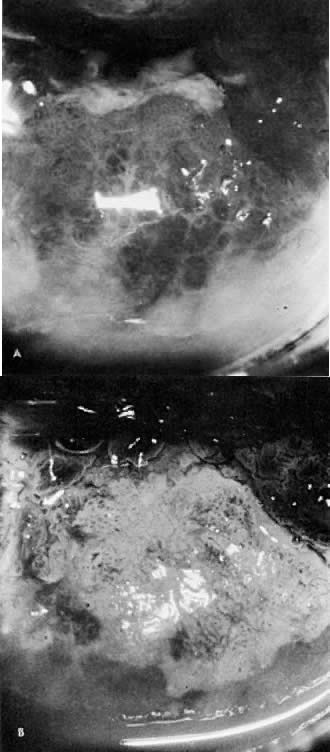 Fig. 12. A. Prior to the application of acetic acid. B. After the application of acetic acid. The posterior lip of the cervix
demonstrates a coarse white epithelium with punctation and a sharply contrasting
border with normal tissue. Biopsy demonstrated CIN III. Fig. 12. A. Prior to the application of acetic acid. B. After the application of acetic acid. The posterior lip of the cervix
demonstrates a coarse white epithelium with punctation and a sharply contrasting
border with normal tissue. Biopsy demonstrated CIN III.
|
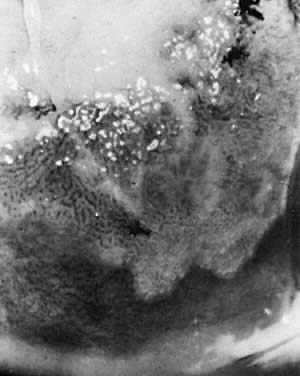 Fig. 13. A wide transformation zone demonstrates white epithelium with coarse punctation. Mucus
obscures the cervical os at upper left. Biopsy showed
CIN III. Fig. 13. A wide transformation zone demonstrates white epithelium with coarse punctation. Mucus
obscures the cervical os at upper left. Biopsy showed
CIN III.
|
In other cases of neoplasia, the intraepithelial vessels do not form simple
loops but communicate with each other, forming a mosaic pattern around
an epithelial core (Fig. 14). This pattern is termed mosaic. It does not appear that punctate patterns
become mosaic patterns, but rather the time at which the neoplastic
insult occurs determines which pattern will be present. Both mosaic
and punctation can be present in lesions of CIN I, CIN II, and CIN III (Fig. 15, Fig. 16, Fig. 17, and Color Plate 1D and Color Plate 1E), that is, neither is more severe than the other. 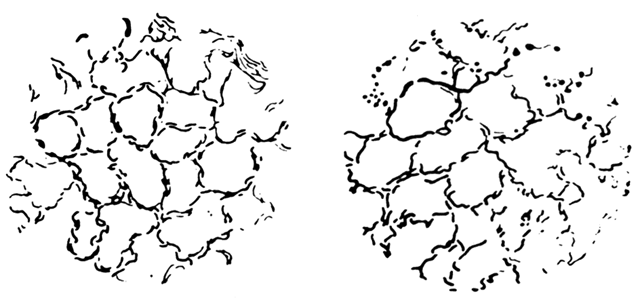 Fig. 14. Mosaic vessels. Common finding in all grades of CIN. A greater distance
between the capillaries suggests higher grade disease.(Kolstad P, Stafl A: Atlas of Colposcopy. Baltimore, University Park Press, 1972) Fig. 14. Mosaic vessels. Common finding in all grades of CIN. A greater distance
between the capillaries suggests higher grade disease.(Kolstad P, Stafl A: Atlas of Colposcopy. Baltimore, University Park Press, 1972)
|
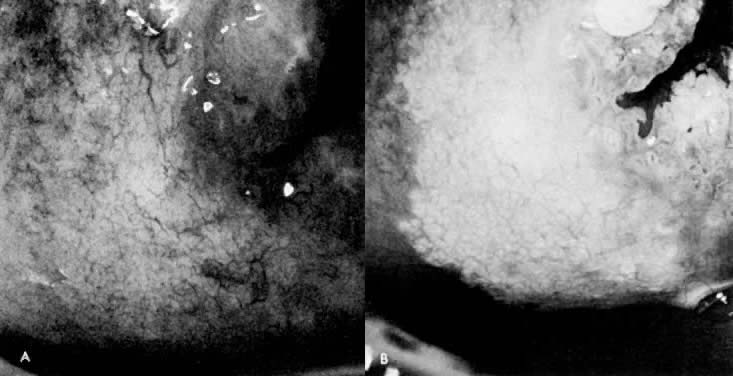 Fig. 15. A. Prior to application of acetic acid. The cervix demonstrates a fine network
capillary pattern. The cervical os is at upper right. B. After application of acetic acid. White epithelium with a fine mosaic
is noted. Biopsy demonstrated CIN I. Fig. 15. A. Prior to application of acetic acid. The cervix demonstrates a fine network
capillary pattern. The cervical os is at upper right. B. After application of acetic acid. White epithelium with a fine mosaic
is noted. Biopsy demonstrated CIN I.
|
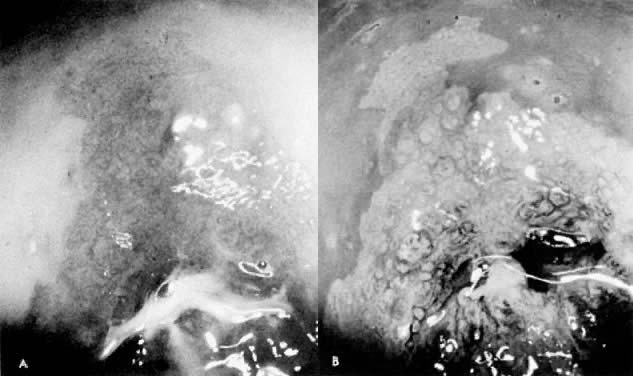 Fig. 16. A. Prior to application of acetic acid. The cervical os is at lower center. B. After application of acetic acid. A sharp-bordered white epithelium with
fine punctation and a coarse mosaic is seen. Biopsy demonstrated CIN
III. Fig. 16. A. Prior to application of acetic acid. The cervical os is at lower center. B. After application of acetic acid. A sharp-bordered white epithelium with
fine punctation and a coarse mosaic is seen. Biopsy demonstrated CIN
III.
|
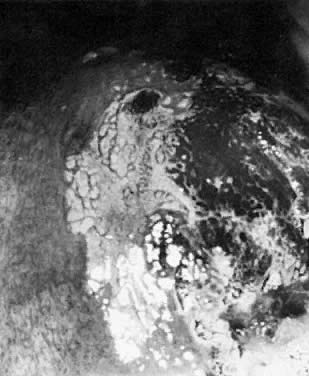 Fig. 17. The cervix reveals a localized area of dense white epithelium at the 9- to 11-o'clock
position. A moderately coarse mosaic is noted, as well
as punctation. The cervical os is at lower right. Biopsy demonstrated
CIN III. Fig. 17. The cervix reveals a localized area of dense white epithelium at the 9- to 11-o'clock
position. A moderately coarse mosaic is noted, as well
as punctation. The cervical os is at lower right. Biopsy demonstrated
CIN III.
|
In general, when the capillary loops of punctation or the tiles of mosaic
produce vessels that are more than 200 microns apart (0.2 mm), this
suggests a more severe lesion. In addition, if the vascular loops or
mosaic tiles vary in size, shape, and density, a more severe lesion is
suspected (Color Plate 1F). The term “atypical vessels” refers only to vasculature that
suggests the presence of invasive cancer. This term should not be used
to describe punctation or mosaic. Atypical vessels are never seen in
normal epithelium, squamous metaplasia, or Cin. These vessels are typically
large, branch in unusual, irregular ways, often run horizontally
through the epithelium, and form bizarre shapes (Fig. 18, Fig. 19, and Color Plate 2A). Because invasive cervix cancer is a rare finding, colposcopists should
have reference materials available to review whenever a bizarre vessel
is seen. If there is any question about the pattern, biopsy or referral
to a colposcopist with more experience is wise. 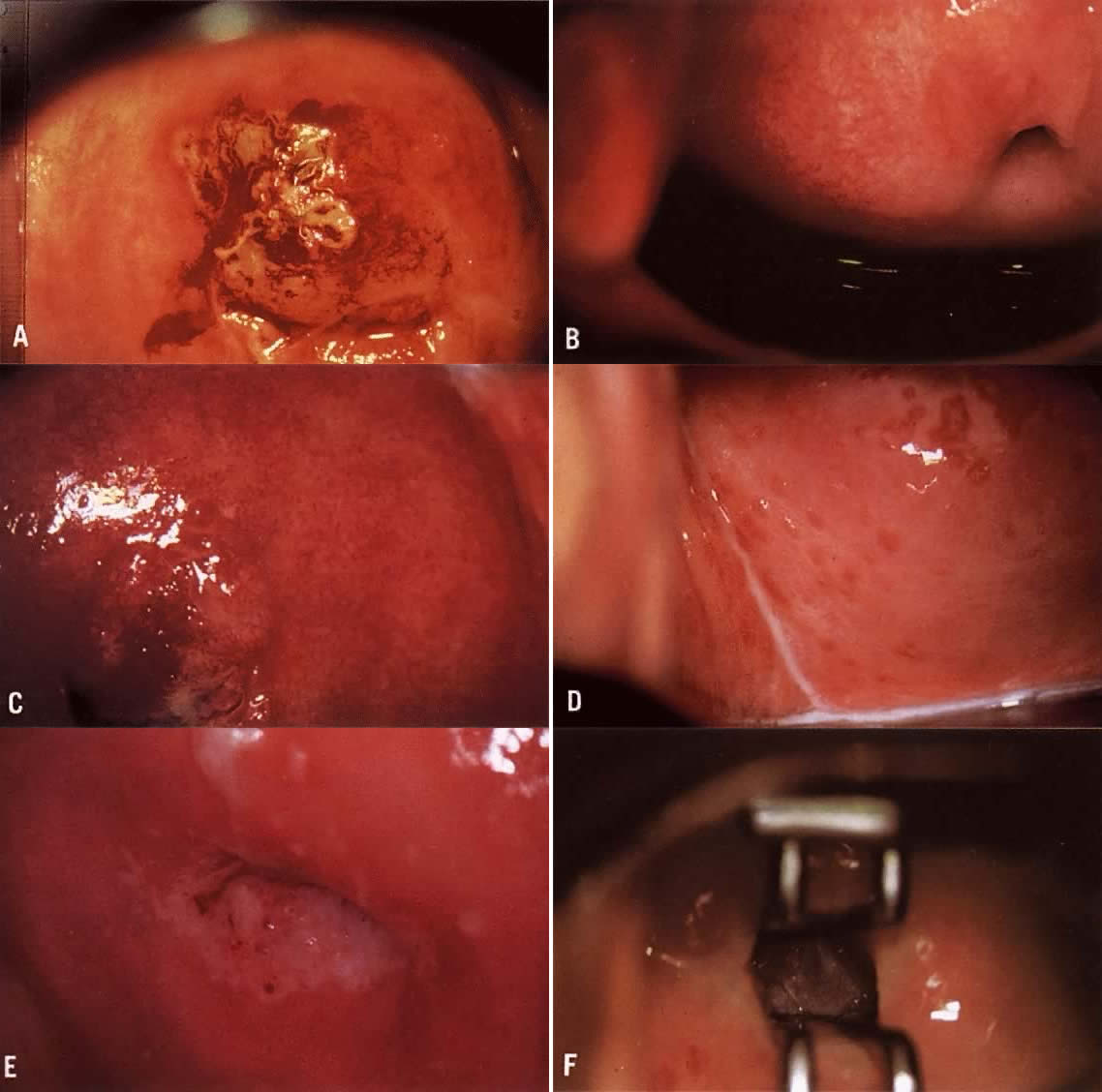 Color Plate 2A. Atypical vessels. The lesion on the anterior lip of the cervix exhibits
large vessels running horizontal to the surface. The border is very
distinct and the edge is raised. The lesion color is more yellow than
white. Biopsy revealed invasive squamous carcinoma to a depth of 4 mm. Conization
was consistent and radical hysterectomy found no evidence
of disease spread. Patient is alive and well 17 years later. B. Normal network of capillaries beneath normal epithelium, which is transparent. Color Plate 2C. Bacterial vaginosis. Note the blurring of the margins of the subepithelial
vessels. The squamous epithelium remains transparent. D. Trichomonas cervicovaginitis. The “strawberry spots” are actually
dilated vessels. They do not exhibit abnormal branching. Color Plate 2E. White epithelium on the posterior lip of the cervix. The examinations
unsatisfactory because the entire transformation zone cannot be see and
the extent of the lesion is not known. F. An endocervical speculum is used to expose the upper extent of the lesion
Although seen entirely, an excisional procedure rather than ablative
is suggested due tot he endocervical extension. Color Plate 2A. Atypical vessels. The lesion on the anterior lip of the cervix exhibits
large vessels running horizontal to the surface. The border is very
distinct and the edge is raised. The lesion color is more yellow than
white. Biopsy revealed invasive squamous carcinoma to a depth of 4 mm. Conization
was consistent and radical hysterectomy found no evidence
of disease spread. Patient is alive and well 17 years later. B. Normal network of capillaries beneath normal epithelium, which is transparent. Color Plate 2C. Bacterial vaginosis. Note the blurring of the margins of the subepithelial
vessels. The squamous epithelium remains transparent. D. Trichomonas cervicovaginitis. The “strawberry spots” are actually
dilated vessels. They do not exhibit abnormal branching. Color Plate 2E. White epithelium on the posterior lip of the cervix. The examinations
unsatisfactory because the entire transformation zone cannot be see and
the extent of the lesion is not known. F. An endocervical speculum is used to expose the upper extent of the lesion
Although seen entirely, an excisional procedure rather than ablative
is suggested due tot he endocervical extension.
|
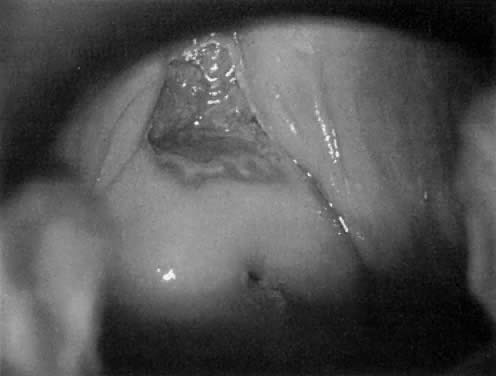
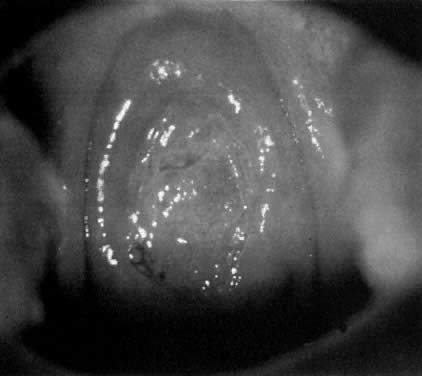
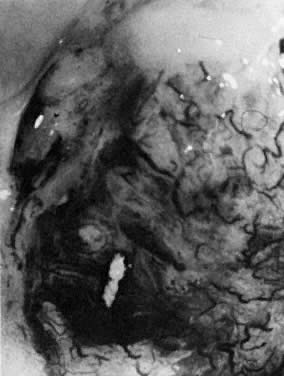 Fig. 18. The external os of the cervix is filled with an irregular-surfaced, sessile
polypoid structure demonstrating grossly distorted, dilated, and
irregular capillaries. Biopsy confirmed invasive squamous cell carcinoma. Fig. 18. The external os of the cervix is filled with an irregular-surfaced, sessile
polypoid structure demonstrating grossly distorted, dilated, and
irregular capillaries. Biopsy confirmed invasive squamous cell carcinoma.
|
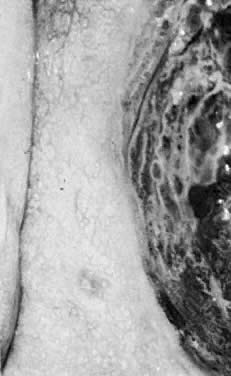
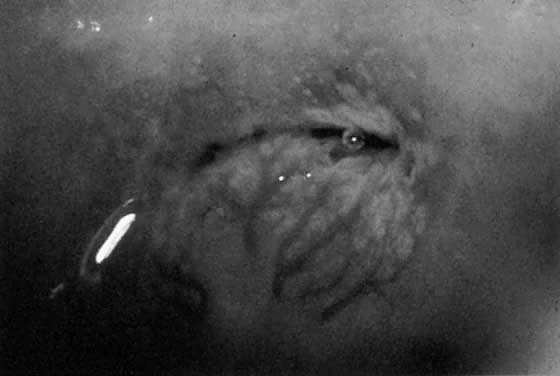 Fig. 19. Normal transformation zone with large vessels overlying nabothian cysts. Note
that these vessels branch normally (arborize). They do not resemble
the vessels in Figure 18 or Color Plate 2A. Fig. 19. Normal transformation zone with large vessels overlying nabothian cysts. Note
that these vessels branch normally (arborize). They do not resemble
the vessels in Figure 18 or Color Plate 2A.
|
Surface Contour A smooth, regular surface contour is normal in mature, squamous epithelium
and squamous metaplasia. When CIN occurs, as it progresses in severity, the
surface may become rougher and more irregular as mosaic tiles
push up between vascular margins. In areas of invasive carcinoma, the
surface contour may be grossly uneven, having a cauliflower-like appearance. Areas
of hyperkeratosis may be grossly irregular in contour, but
these areas lack any of the accompanying vascular aberrations seen
in CIN. Border with Normal Tissue Areas with significant CIN demonstrate a sharp border with the surrounding
pale pink normal tissue. A sharply contrasting border around a geographic
area of white epithelium signifies an area of epithelial abnormality (the
sharper the border, the more marked is the histologic abnormality). Areas
of squamous metaplasia or HPV demonstrate a diffuse, poorly
defined border with normal tissue. The border contrast often can
be enhanced by use of the green filter. Inflammation The presence of infection or inflammation may complicate the colposcopic
examination. When bacterial vaginosis, trichomoniasis, or candidiasis
is present, there is often considerable discharge, as well as some hyperemia
that may make evaluation of the cervix difficult (Fig. 20 and Color Plate 2B and Color Plate 2C). Particularly when dealing with Pap smears suggesting less significant
lesions, it is suggested that cervicovaginitis be cleared before colposcopy
is performed. Trichomoniasis may cause dilated capillary loops (strawberry
spots) that make evaluation of the TZ difficult (Color Plate 2D). 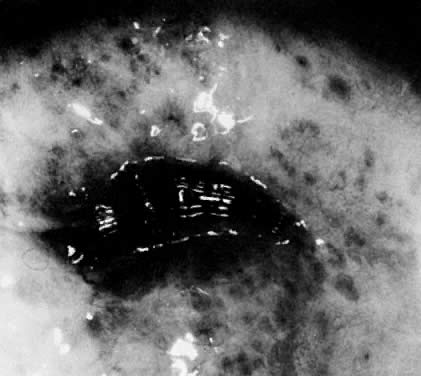 Fig. 20. Typical blotchy appearance of the inflamed cervix. Fig. 20. Typical blotchy appearance of the inflamed cervix.
|
Natural History of CIN Colposcopists must thoroughly understand the natural history of CIN lesions, as
well as the multiple terminologies that have been used to describe
them. Figure 21 shows the relationship of the terminologies used to describe intraepithelial
neoplasia of the cervix. In the United States, the dysplasia/carcinoma
in situ terminology was used for many years. However, it was convincingly
demonstrated that light microscopy could not distinguish between
severe dysplasia and carcinoma in situ (CIS). Therefore, the CIN
terminology, which combines severe dysplasia and CIS, was developed by
Dr. Ralph Richart. The CIN terminology is still most widely used to
describe histopathologic changes. 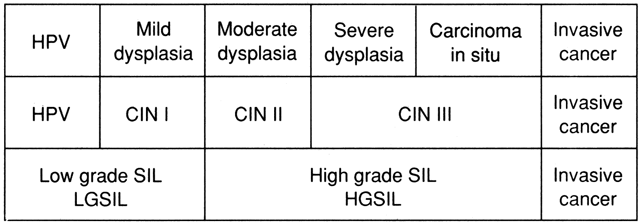 Fig. 21. Dysplasia, CIN, and SIL terminologies for cervical neoplasia. Fig. 21. Dysplasia, CIN, and SIL terminologies for cervical neoplasia.
|
The Bethesda System for Cytology Reporting developed the high grade and
low grade squamous intraepithelial lesion terminology.1 This terminology is also rapidly being adopted for histopathology reports. It
has been demonstrated that cytology using light microscopy cannot
distinguish between an HPV infection and a lesion that has the ability
to progress (mild dysplasia). It now appears that the distinction
also cannot be made on histologic specimens. Indeed, it is not important to distinguish between HPV and mild dysplasia. What
the clinician needs to know is whether an early lesion has the
ability to progress. Unfortunately, light microscopy cannot make this
distinction. At present, there is no technique that can. Figure 22 graphically demonstrates the chance of spontaneous resolution of various
intraepithelial problems. In general, HPV infections are believed to
be totally reversible, though they may also persist indefinitely.8 The body does seem to be able to make antibodies that can successfully
clear the virus in some individuals. However, in many cases the virus
persists, and in a few women some cells may undergo a change that begins
the cascade that can ultimately lead to cervical cancer. This change
cannot be detected by light microscopy, DNA probes, or HPV subtyping
at present. 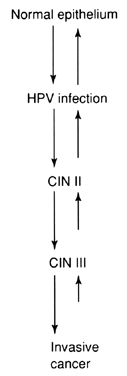 Fig. 22. HPV infections are usually reversible. However, the chance of regression
decreases as the severity increases. Fig. 22. HPV infections are usually reversible. However, the chance of regression
decreases as the severity increases.
|
The body does seem to be able to clear many intraepithelial neoplastic
lesions. However, once the lesion has progressed to a full thickness change (CIN
III/severe dysplasia/CIS), unless the lesion is treated, it
will likely become invasive cancer. The role of cytology and colposcopy, then, is to detect focal intraepithelial
lesions, thus allowing the clinician to treat them before invasive
cancer occurs. In most cases, treatment of CIN is an office-based
procedure. Hysterectomy is not indicated for CIN except in the most unusual
circumstances. Etiology of CIN Since 1842 investigators have searched for the etiology of cervical cancer.9 Initial crude epidemiologic studies discovered that women who never had
sexual intercourse never developed invasive squamous cancer of the cervix. More
sophisticated studies continue to suggest that neoplasia of
the cervix is a sexually transmissible disease. That is, some factor
or factors transmitted during sexual activity eventuates in the development
of cervical cancer. In the 1960s and early 1970s, it appeared that herpes simplex virus-2 (HSV-2) might
be the transmissible factor causing cervical neoplasia.10 Later studies showed that an HSV-2 infection was largely an indicator
of increased risk based on sexual activity rather than the true etiologic
agent of cervical neoplasia. In 1977 Meisels and associates reported that the vast majority of cases
previously diagnosed as mild dysplasia were human papilloma virus infection.11 This remarkable discovery—as with most newly discovered biologic
events—prompted an immediate, far reaching, and overzealous response. It
was observed that koilocytes, squamous cells with a clear halo
surrounding each nucleus, were indicative of HPV infection. It was
later shown that these clear areas often were filled with HPV. Many substances
can cause koilocytosis (though HPV infection is most common). However, when
koilocytes also have wrinkling and clumping of the nuclear
DNA (raisinoid nuclei), the changes are almost always evidence of
HPV infection.12 Considerable time was wasted for nearly a decade trying to decide whether
a patient had “just HPV infection” or “CIN I.” Originally
both entities were treated most often by the newly available
ablative technique, CO2 laser. Not surprisingly, it was eventually reported that ablative techniques
often did not cure a patient of an HPV infection. Clinicians, therefore, began
to treat the two entities differently, that is, patients
with “just” HPV were not treated whereas those with CIN
I were treated. Unfortunately, it has now become clear that light microscopy cannot distinguish
between HPV infection and CIN I. For this reason, TBS places
evidence of both HPV infection and mild dysplasia into the same category, LGSIL. It
is also agreed by most histopathologists that it is impossible
to tell the difference between these two entities on biopsy specimens. Thus, the
clinician cannot distinguish the patient with only evidence
of HPV infection from the patient who has the earliest changes
of neoplasia. HPV is a DNA virus that appears to be specific to humans. There are now
more than 70 subtypes identified; most tend to be localized to certain
areas of the body. For example, one subtype tends to produce plantar
warts, another common warts on the fingers and hands, and several types
tend to be found in the genital area. HPV infections have been found
in the oral cavity, in the esophagus, in the anal region, and many other
sites.13 HPV types 6 and 11 tend to cause common genital warts. These subtypes have
only rarely been identified in invasive cancers of the lower genital
tract except for verrucous carcinoma of the vulva. HPV 6 and 11 are
rarely found in CIN II and III.14 Genital warts usually become apparent to the patient and her physician
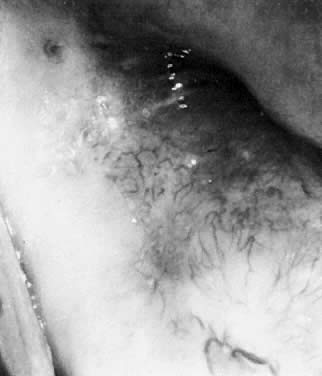
only when present on the vulva (Fig. 23). However, whenever one genital site is involved (vulva, vagina, or cervix), there
is a greater than 80% chance that the entire lower genital
tract harbors the HPV virus. That is, HPV tends to be a field infection
rather than single site. Infections of the cervix usually result in
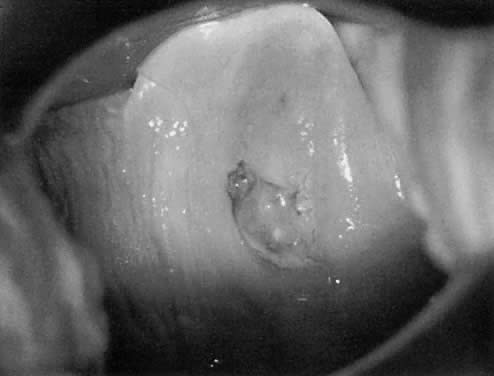
acetowhite changes (Fig. 24). Occasionally, a discrete wart is observed on the cervix (Fig. 25). 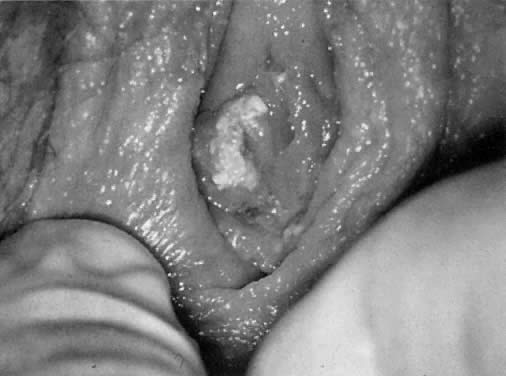 Fig. 23. Typical genital wart. This lesion is adjacent to the urethra. Fig. 23. Typical genital wart. This lesion is adjacent to the urethra.
|
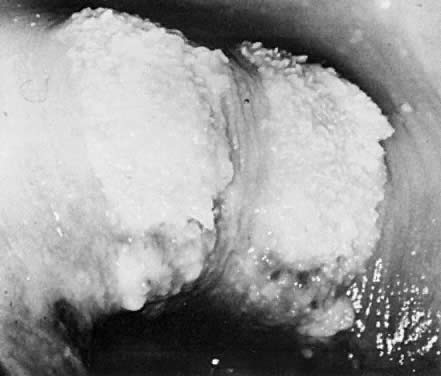 Fig. 24. HPV infection of the anterior lip of the cervix. Fig. 24. HPV infection of the anterior lip of the cervix.
|
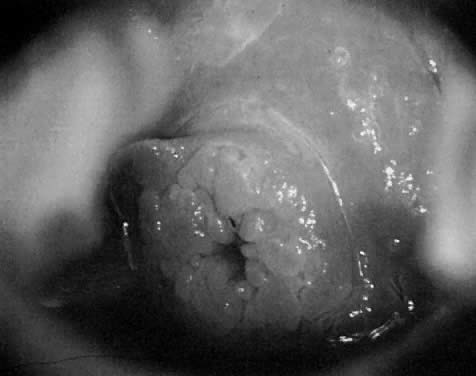
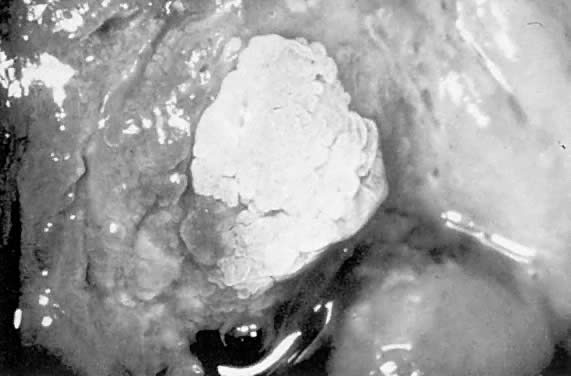 Fig. 25. Cervical wart. A discrete exophytic wart of the cervix is a relatively
uncommon finding. Fig. 25. Cervical wart. A discrete exophytic wart of the cervix is a relatively
uncommon finding.
|
Many HPV types have been isolated from lower genital tract invasive carcinomas. By
far, the most commonly isolated type in squamous lesions is
HPV 16. HPV 18 is also found with some frequency in squamous lesions, and
commonly in adenocarcinomas of the endocervix.15 HPV DNA also can be found incorporated into the cellular DNA of most cases
of CIN III and in many cases of CIN II. In invasive cancers, the
HPV DNA is incorporated directly into the cell genome, and through various
interruptions of the normal cell cycle cause a cell line (clone) to
develop which is immortal. The exact mechanism by which such immortality
is caused by the virus is currently under study. HPV cannot be grown under usual conditions in a microbiology or virology
laboratory. Several sophisticated laboratory systems have been developed
for determining the presence of HPV and assigning the appropriate
subtype. For several years, the “Southern blot” technique
has been the one most often used by research laboratories. More recently, polymerase
chain reaction (PCR) techniques have been able to identify
low levels of viral DNA. Although early PCR reports showed an extremely
high prevalence of the virus in the general population, these reports
are now known to have been caused by contamination of the identification
system. Nonetheless, PCR remains a powerful and important tool
in the investigation of these viruses. The prevalence of HPV among women in the United States is not well understood. Studies
of samples obtained from young, sexually active women
suggest that HPV prevalence might be as high as 30%.16 These populations may or may not be representative of United States women, in
general. Additionally, it has been shown that the prevalence of
HPV identified in any group is a function of the number of times the
group has been studied.17 Although PCR techniques should have the ability to identify latent infections
that are not histologically or clinically apparent, it appears
that any identification technique misses some infections. For example, a
population might demonstrate a 20% prevalence of HPV infection if
sampled once, but the same population may have a total 25% prevalence
if resampled. With the discovery that HPV (particularly subtypes 16, 18, 30, 45, 56, and
several others) can be found in virtually all cases of squamous cancer
of the cervix, research attention was focused on this agent. It now
appears that, in almost all cases of cervical neoplasia, prior infection
with HPV is necessary. When multivariate analyses have been performed, all
other risk factors, except smoking, disappear. That is, early
sexual intercourse, multiple sexual partners, and exposure to HSV-2 are
not important when compared with HPV infection. However, HPV is not a complete carcinogen, nor is HPV infection a sufficient
cause for the development of neoplasia. That is, of the millions
and millions of women who have HPV infection, few ever develop cervical
neoplasia, and almost none ever develop cervix cancer. A combination
of events is probably responsible for this observation. First, the body's
immune system may defeat the HPV virus and/or may attack and
destroy early neoplastic cell clones. In addition, many early intraepithelial
neoplastic lesions are destroyed by office treatment and thus
never lead to invasive cancer of the cervix. Smoking appears to be an independent risk factor for the development of
cervical neoplasia among women with HPV infections.18 Cervical neoplasia is increased approximately twofold, and vulvar neoplasia
is increased approximately fourfold. Some of the carcinogens in
inhaled smoke appear in high concentration in the cervical mucus. In addition, there
is a loss of immune-competent cells in the cervical epithelium
and stroma when biopsies of smokers are compared to nonsmokers. |