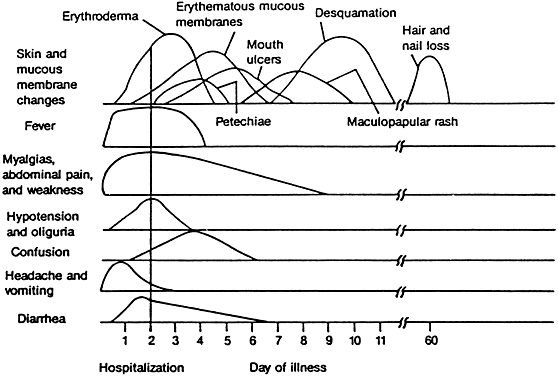
The onset of TSS is usually abrupt, with symptoms of severe disease, including high fever, a sunburn-like diffuse erythema, chills, malaise, headache, sore throat, myalgia, fatigue, vomiting, diarrhea (often with incontinence), abdominal pain, and dizziness or syncope (Fig. 1).1,2 The course of patients with TSS is frequently stormy, marked by dramatic multiorgan dysfunction and possible failure. Altered sensorium with disorientation, somnolence, or agitation is common.28 In some patients, however, onset is gradual, and the characteristic rash is not seen for several days.2 Examination usually reveals a patient with fever, tachycardia, low or unobtainable blood pressure, muscle tenderness (most commonly in the abdominal area), “strawberry” or “beefy” red mucous membranes, and conjunctival hyperemia without a purulent discharge.1,2 Wiesenthal and Todd's “screening” TSS case definition is listed in Table 1.12 In women, erythema and edema may be most prominent in the perineum and inner thighs and can be accompanied by vaginal erythema, edema, and discharge compatible with S. aureus-induced vaginitis in menstrual-associated TSS.2 Menses-associated TSS is frequently associated with signs and symptoms of vaginal inflammation.2
TABLE 1. Diagnostic Criteria for Toxic Shock Syndrome*
Screening TSS Definition
Acute fever (<7 days, 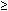 38.5°C or 102°F)
38.5°C or 102°F)
Erythroderma without Nikolsky's sign
Conjunctival hyperemia without purulent exudate
Pharyngeal hyperemia
Case Definition of Toxic Shock Syndrome
Fever (temperature 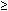 38.9°C or 102°F)
38.9°C or 102°F)
Rash characterized by diffuse macular erythroderma
Desquamation occurring 1–2 weeks after onset of illness
Hypotension (systolic BP 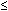 90 mm Hg in adults) or orthostatic syncope
90 mm Hg in adults) or orthostatic syncope
Involvement of three or more of the following:
Gastrointestinal tract (vomiting or diarrhea at onset of illness)
Muscle (myalgia or creatine phosphokinase level twice normal)
Mucous membrane (vaginal, oropharyngeal, or conjunctival hyperemia)
Kidney (BUN or creatinine level twice normal or more, or 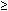 5 WBCs, per high-power field in absence of urinary tract infection)
5 WBCs, per high-power field in absence of urinary tract infection)
Liver (total bilirubin, aspartate transferase [AST], or alanine
transferase [ALT] twice normal level)
Blood (platelets 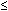 100,000/μl)
100,000/μl)
CNS (disorientation or alterations in consciousness without focal neurologic
signs, without fever and hypotension)
Negative throat and cerebrospinal fluid cultures (a positive blood culture for S. aureus does not exclude infection)
Negative serologic tests for Rocky Mountain spotted fever, leptospirosis, rubeola
BP, blood pressure; BUN, blood urea nitrogen; WBCs, white blood cells; CNS, central nervous system.
Pending evidence of absence of other possible causes.
Wiesenthal AM, Ress-men M, Caston SA: Toxic shock syndrome. I. Clinical exclusion of other syndromes by strict screening definitions. Am J Epidemiol 122:847, 1985.
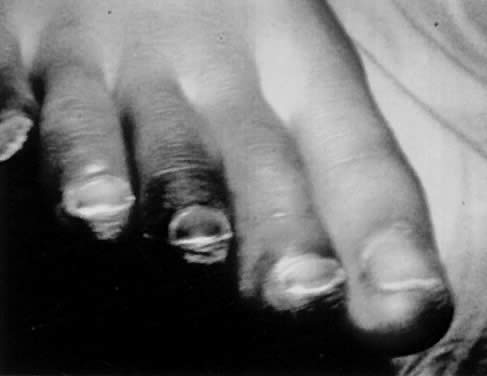
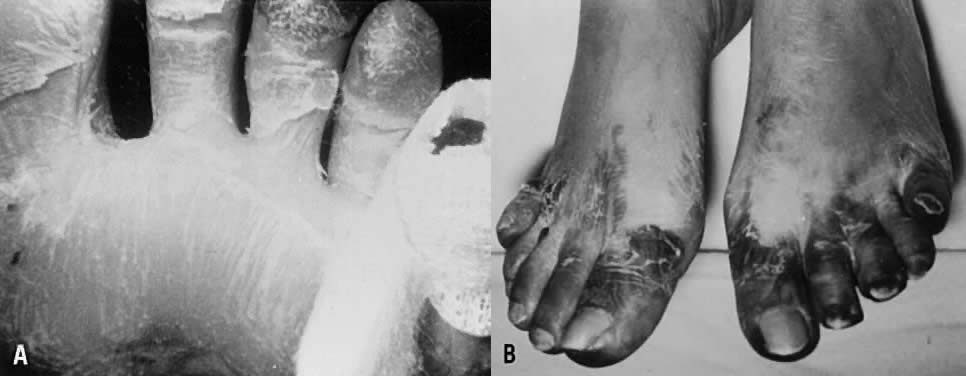
Characteristic delayed manifestations of TSS include oral mucosal ulcerations; a fine, generalized, ashlike desquamation; and peeling of skin at the ends of fingers and toes and on the palms and soles of the hands and feet. These occur in the first and second weeks of illness (Fig. 2). Inadequate perfusion may lead to necrosis and eventual loss of fingers and toes (Fig. 3). The onset and prominence of any of these signs and symptoms may vary (Fig. 1).29
|
|
Mechanisms of death commonly include refractory cardiac arrhythmia, respiratory failure, and disseminated intravascular coagulation. Prolonged sequelae among survivors include chronic renal failure, gangrene of fingers and toes, and loss of hair and nails. Neurologic sequelae, including peripheral neuropathies, impaired memory, and inability to concentrate, have been reported.30
Necropsy findings demonstrate generalized inflammation or necrosis of small vessels, generalized tissue edema, and widespread ulcerated inflamed epithelium, including gastroduodenitis, cholangitis, bronchitis, and bronchiolitis, in addition to acute ulcerative vaginitis in menses-associated cases.31,32 Such cervicovaginal ulcers are not necessarily tampon-induced, because they are also found in women who have never used tampons. In menses-associated cases, paravaginal vasculitis is prominent, suggesting that the vagina is the site of entry for an injurious agent that affects blood vessels and endodermal-derived structures.32
Laboratory test abnormalities reflect multiorgan damage or dysfunction caused by direct toxin effect or secondary organ alterations resulting from vascular changes or hypotension, as well as generalized cytokine release. Most dramatic are the rapid rises in serum creatinine and blood urea nitrogen levels and the yet to be elucidated hypocalcemia and hypophosphatemia. Renal function abnormalities and the presence of casts and sterile pyuria may erroneously suggest pyelonephritis. Other abnormalities include leukocytosis with a left shift, lymphopenia, anemia, thrombocytopenia, and abnormal liver enzyme levels, blood gases, and electrocardiographic findings (Table 2). The differential diagnosis of TSS is extensive (Table 3). The suggested initial evaluation is summarized in Table 4.
TABLE 2. Laboratory Abnormalities in Toxic Shock Syndrome
Abnormality | Patients Affected (%) |
↓ Serum calcium | 83 |
WBCs in urine | 81 |
↓ Total serum protein | 81 |
↓ Serum albumin | 76 |
Leukocytosis with ↑ bands | 72 |
↑ Serum bilirubin | 88 |
↑ AST, ALT | 65 |
↑ Prothrombin time | 64 |
↑ BUN/creatinine | 61 |
↑ Serum creatinine kinase | 53 |
↓ Hemoglobin/hematocrit | 50 |
↓ Serum phosphorus | 50 |
↓ Platelets | 25 |
WBCs, white blood cells; AST, aspartate transferase; ALT, alanine transferase; BUN, blood urea nitrogen.
Adapted from Tofte RW, Williams DN: Clinical and laboratory manifestations of toxic shock syndrome. Ann Intern Med 96:843, 1982.
TABLE 3. Differential Diagnosis of Toxic Shock Syndrome
Exanthemas
Kawasaki's disease (mucocutaneous lymph node syndrome)*
Streptococcal scarlet fever*
Staphylococcal scarlet fever*
Rubeola and rubella
Meningococcemia and other septicemias*
Rocky Mountain spotted fever*
Leptospirosis*
Erythema multiforme
Stevens-Johnson syndrome
Systemic lupus erythematosus
Legionnaire's disease
Gastrointestinal disorders
Gastroenteritis
Staphylococcal food poisoning
Causes of acute abdomen (e.g. appendicitis)
Other disorders
Acute pyelonephritis*
Septic shock*
Hemolytic uremic syndrome
Rhabdomyolysis
Tick typhus
Tularemia
Pelvic inflammatory disease
Acute rheumatic fever
Kawasaki's disease
* Primary differentials.
TABLE 4. Suggested Initial Evaluation and Treatment of Possible Toxic Shock
Syndrome Patients
Evaluation
History and physical examination, including pelvic examination
Remove tampons or other objects from vagina; drain wounds
Strongly consider surgical extrapation of focal abscess or local infection
Laboratory tests
Culture blood, vagina, wound, oropharynx, and cerebrospinal fluid if indicated
Urinalysis
CBC with differential and platelet count
Serum electrolyte, calcium, phosphate, and albumin assessment
Liver enzyme determination
Coagulation tests
Chest radiograph
Blood gases
Electrocardiogram
Store serum for serologic tests
Resuscitation and integrated support of all body systems
Intravenous (IV) fluid administration and monitoring (CVP or Swan-Ganz
catheter, Foley catheter)
Fluid administration (up to 12 L/d)
Antibiotics
β-Lactamase---resistant antibiotic adequate for S. aureus
If sepsis possible, broad-spectrum coverage until blood cultures negative
If Rocky Mountain spotted fever possible, ensure tetracycline treatment
If group A streptococcal infection, consider treatment with penicillin
and/or clindamycin
Measures in selected patients
Corticosteroids (1 g hydrocortisone IV every 6---8 hours for 3 days)
Vasopressor, dopamine
Calcium supplementation
Blood and/or blood components
Respiratory support for ARDS
Renal dialysis
CBC, complete blood count; CVP, central venous pressure; ARDS, adult respiratory distress syndrome.