Many physicians attempt to establish the etiology of urinary incontinence
based on a brief history and physical examination. This is inadequate
for most incontinent women. To aid the clinician in establishing an
accurate diagnosis before initiating treatment, a number of clinical
tools have been developed. Urogynecologic History The urogynecologic history is an essential part of the evaluation of every
incontinent woman. Although a history alone is inadequate to explain
the patient's symptoms, it does define them. The history acts
as an outline to establish what must be explained by any evaluation. To
help clarify patients' understanding of the problem and to facilitate
their evaluation, patients at the Evanston Continence Center are sent
programmed questionnaires to fill out at home before coming to the office. This
not only speeds up their evaluation, but it enables them to
confirm questionable areas at home and adds to the accuracy of their
responses. Most authors have found history to be inadequate in establishing an accurate
diagnosis,10,26–31 but some have suggested that patients with isolated symptoms may be diagnosed
on the basis of history alone.32,33 Farrar and coworkers32 found history to be quite adequate for diagnosis. Of 56 women who reported
having only symptoms of stress incontinence, 54 (96.4%) were found
to have stable bladders on cystometry; of 100 women who reported having
only urge continence, 89% were found to have detrusor instability. Hastie
and Moisey33 concluded that a history of only stress incontinence was 100% accurate
in establishing genuine stress incontinence as the underlying diagnosis. Our data are quite divergent from these findings. In a subgroup of 43 women
with symptoms isolated to stress incontinence, 34.9% (16) were found
to have involuntary bladder contractions on cystometry (Table 1). The continence history was found to be a poor predictor of the underlying
conditions causing urinary leakage in 218 consecutive women studied. Similarly, Cardozo
and Stanton27 and Webster and associates28 found the continence history to be inaccurate, even in patients whose
reported symptoms were isolated to stress incontinence or urge incontinence. Numerous
studies have shown that more than a history is necessary
to evaluate the cause of a patient's symptoms. Jenson and colleagues31 reviewed the English literature from 1975 to 1987 and found 29 studies
comparing history with diagnosis. Nineteen of these were acceptable for
comparison. The data on 3092 patients evaluated for genuine stress
incontinence showed the symptom of stress incontinence to have a sensitivity
of 90.6% and a specificity of only 51.1%. A history of urge incontinence
in 2950 patients had a sensitivity of 73.5% and a specificity
of only 55.2%. Therefore, a basic clinical evaluation including a physical
examination with a lumbosacral neurologic evaluation, urine culture, measurement
of residual urine, assessment of urethral mobility, demonstration
of urinary leakage, and cystometry is invaluable. This basic
evaluation is an excellent, cost-effective screening method for all
patients and is diagnostic is most cases. A good general medical history
with a record of medication and drug usage as well as a history
of prior surgeries also should be obtained. Prior anti-incontinence surgery
may alter the usual prevalence of various etiologies of incontinence.34 Women who have had a prior unsuccessful anti-incontinence operation have
a higher incidence of detrusor instability, uninhibited urethral relaxation, and
fistulae. TABLE 1. Comparison of Urinary Symptoms and Conditions in 218 Consecutive
Patients Undergoing Multichannel Urodynamics
Symptoms (N) | GSI | DI | GSI & DI | Continent |
Stress incontinence (43) | 25 | 7 | 8 | 3 |
Urge incontinence (13) | 0 | 10 | 0 | 3 |
Stress and urge incontinence (132) | 89 | 13 | 30 | 0 |
Continent (30) | 0 | 0 | 0 | 30 |
Total (218) | 114 | 30 | 38 | 36 |
DI, detrusor instability; GSI, genuine stress incontinence.
The programmed questionnaire may be reviewed rapidly before the patient
is examined and should be reconfirmed with the patient to augment the
examiner's understanding of her problem. Women should be questioned
about urinary incontinence during intercourse, which occurs in as
many as 24% of patients and may adversely affect one half of all patients' sexual
relations.35 This is also a good time to question the patient about anal incontinence
of feces or flatus. In one study,36 anal incontinence was found to affect 51.1% of women reporting urinary
incontinence. Anal incontinence of flatus, liquid stool, and solid waste
affects many women with urinary leakage and should be looked for in
all incontinent patients. Patients are also asked to complete a 24- to 48-hour voiding diary before
coming to our center for their appointment. Patients are asked to record
when they void, the volume voided, when they leak urine, and the
volume they drink. Review of this diary helps the examiner to confirm
the patient's symptoms; however, the diary often does not correlate
well with the symptoms. Although many patients with detrusor overactivity
void often and in small volumes, clinical overlap with patients
who do not have detrusor overactivity prevents the voiding diary from
being used as a diagnostic test.37 It is, however, a very accurate predictor of the degree of a patient's
physical and social dysfunction, and it also serves as a useful
quantitative measure of improvement during therapy for all storage phase
disorders.38 Physical Examination A careful physical examination is performed on all incontinent women and
should focus on the pelvic examination and neurologic evaluation of
the lumbosacral nerve roots. These nerve roots are primarily responsible
for the autonomic control of bladder storage and emptying. Assessment
to rule out systemic diseases (e.g. cardiac, thyroid, adrenal, pancreatic, renal) that may play a role in
incontinence is crucial. PELVIC EXAMINATION. On pelvic examination, the physician should look for signs of genital
tract inflammation, infection, and atrophy that may affect the urethra
and cause an increase in afferent sensation. This may lead to irritative
voiding symptoms, such as urgency, urinary frequency, dysuria, and
possibly urge incontinence. In addition, heavy vaginal discharge or cervical
mucus may be confused by some patients as urinary incontinence. Oral
phenazopyridine (Pyridium), which colors the urine orange, can
be a useful means for such patients to distinguish between vaginal fluids
and urine. Because the urethra and trigone are estrogen-dependent
tissues, estrogen deficiency can contribute to urinary incontinence and
dysfunction.37 Most authors37,39–45 agree that estrogen replacement therapy will improve irritative symptoms
in many patients, but improvement in urinary incontinence has been
inconsistent in clinical trials. In controlled trials, there may43 or may not be improvement in urge incontinence episodes with estrogen
replacement compared with placebo. Uncontrolled trials show improvement
in stress incontinence,41,42,45 but no beneficial effect of estrogen has been found in controlled trials.45,46 Some investigators have shown significant changes in urethral closure
pressure and functional length,39,40,42 whereas others have not.43–46 Meta-analysis of 23 trials from 1969 to 1992 showed subjective improvement (p < 0.01) and an increase in closure pressure with estrogen replacement
therapy. Nearly all these studies have demonstrated improvement in
pressure-transmission ratios that measure the increase in urethral pressure
compared with bladder pressure during coughing.39–42,44 Estrogen replacement therapy should be given when signs of estrogen deficit
are noted before further urodynamic workup is performed. The vaginal
route of administration is preferred initially because of the excellent
absorption of estrogen by the thin, atrophic tissues of the vagina.47 After these observations are made, the examiner should look for any signs
of urethral deformity, such as diverticula or fistulae. Vesicovaginal fistulae usually are found just anterior to the vaginal cuff after hysterectomy
and usually leak urine continuously. Ureteral fistulae may also cause continuous leakage of urine and may exist concurrently
with a vesicovaginal fistula. Ureteral fistulae should be evaluated by
an intravenous pyelogram. When ureteral fistulae are an isolated injury, dye (indigo
carmine or methylene blue) placed by catheter into the
bladder will not stain a vaginal tampon or pack. Urethral fistulae cause
leakage only when urine enters the urethra during episodes of stress
incontinence, unless the fistulae have totally destroyed the functional
upper urethra; there is continuous leakage of urine in such cases. If
a urethral sac is observed, it should be palpated to see whether
it is tender and massaged to see whether it empties urine or pus. If the
diverticulum is distal, such discharge may be found at the urethral
meatus; if it is in the proximal urethra, the discharge can be seen on
urethroscopic examination. Observation of the anterior vaginal wall, posterior wall, and vaginal apex
may be accomplished with the Sims' speculum or posterior half of the
bivalve speculum to identify associated genital prolapse. Genital prolapse
can have profound effects on lower urinary tract function, often
resulting in urinary retention or masking urinary incontinence. Posterior
rotational descent of the anterior vaginal wall results in the
formation of a cystocele, which can represent either a paravaginal detachment of the anterior lateral
vaginal wall from the arcus tendineus fascia or a central defect
due to weakness of the endopelvic connective tissue. Lateral defects
are often associated with preservation of the vaginal rugae and can be
analyzed with the use of a ring forceps or similar device to resupport
the anterior lateral vaginal fornices to the level of the arcus tendinous
fascia bilaterally. We prefer to use the separated blades of a Grave's
speculum inserted laterally into the vagina. This method prevents
overcorrection with anterior displacement of anterior lateral
vaginal fornices. Regardless of the etiology, descent of the anterior
vaginal wall can result in the urethra or bladder neck folding on itself. This
process results in mechanical obstruction or kinking of the urethra, which
can be identified during urethroscopy and on urethral closure
pressure profiles. Potential genuine stress incontinence has been found in 60% of women with
cystoceles protruding to the vaginal introitus or beyond who do not
leak urine with the prolapse unreduced.48 Myers and colleagues also demonstrated that unreduced rectoceles may mask
incontinence.48a Stress testing with reduction of the prolapse using a pessary, Sims' speculum, or
large cotton swabs in the standing position reveals qualitative
information on patients who have potential genuine stress incontinence. Urethral
closure pressure profiles can be used at rest, during
Valsalva's maneuver, and during repetitive coughing to provide quantitative
information about the underlying pathophysiologic effect of
the prolapse.49–51 Some of these women may have urinary retention secondary to this mechanical
obstruction. Reduction of the prolapse during voiding will also
enable the examiner to discover whether retention is merely the result
of mechanical obstruction or of some other pathologic process. In some
women, an underactive detrusor may develop if the bladder is forced
to contract against a closed bladder neck chronically. However, initially
the detrusor muscle will hypertrophy and may cause high-pressure overactive
contractions with risk of upper tract damage, as discussed previously. All
patients with prolapse to the introitus should be examined
with a full bladder after reduction of the prolapse to look for leakage
and to assess for retention. Those patients with residuals greater
than 50 mL should undergo complete evaluation with voiding pressure
studies after reduction of the prolapse. A careful digital examination should be done to assess transvaginal urethral
or bladder tenderness and to rule out pelvic pathology. Urethral
or bladder base tenderness or a strong sense of urgency usually is consistent
with inflammation on endoscopic evaluation, or it may be found
in patients with detrusor overactivity. Much attention is often given
to uterine leiomyomas causing urinary symptoms, but this is infrequently
a cause of incontinence or irritative symptoms. Rectovaginal examination
is an important part of this evaluation. Evaluation of possible
enterocele dissection along the rectovaginal septum may be appreciated, but
often may be missed until surgery. The pelvic examination may
be aided in some patients by defacography, but this is an expensive and
difficult test for patients to endure.52 Anal sphincter tone also should be assessed, but results correlate poorly
with manometric assessment of anal sphincter function. Rectal prolapse
and subtle rectoceles may be appreciated during this examination, but full evaluation often
requires dynamic radiographic evaluation. NEUROLOGIC EXAMINATION. Urinary control in women involves a complex interaction of various reflexes
interacting with the autonomic nervous system, which primarily controls
bladder and urethral function. These are both modulated by cortical
and brain stem centers. Neurologic damage in these areas can cause
significant symptomatology.53 Cerebrovascular disease can cause detrusor hyperreflexia. Peripheral lesions
can lead to an acontractile bladder and overflow incontinence. Spinal
cord lesions can cause detrusor-sphincter dyssynergia with intermittent
voiding patterns and urinary retention resulting from lack of
urethral relaxation during bladder contraction. Peripheral control is primarily modulated by the pelvic (parasympathetic) and hypogastric (sympathetic) nerves.54 The parasympathetic nervous system has axons that originate from sacral
segments S2 to S4 that stimulate release of acetylcholine from postganglionic
neurons in the bladder wall to affect bladder contractions. The
sympathetic ganglia originate in the thoracolumbar spine (T10-L2) and
course through the hypogastric nerve to terminate on the parasympathetic
ganglia of the bladder wall and in the bladder and urethra, where
there are receptors for both alpha and beta fibers.49 The alpha fibers primarily cause contraction of the urethra and bladder
neck, whereas the beta receptors cause detrusor relaxation but probably
only at low volumes by releasing norepinephrine.55 In addition, the sympathetic fibers ending on postganglionic parasympathetic
neurons modulate and depress parasympathetic transmission. This
helps to explain why storage of urine is a passive process. This sympathetic
control may also be the basis for the pharmacologic success sometimes
seen with alpha-adrenergic treatment of detrusor instability. The neurologic examination of incontinent women should start with an assessment
of mental status, cranial nerve integrity, and cerebellar control. Deep
tendon reflexes and muscle strength should be assessed in the
lower extremities to evaluate the anterior lumbosacral spinal cord. The
bulbocavernosus and clitoral reflexes help to demonstrate the integrity
of the sacral nerve roots (Fig. 2). Sensory function may be quickly evaluated from dermatomes L2 through
S2 by testing for sensation of pinprick around the knee. 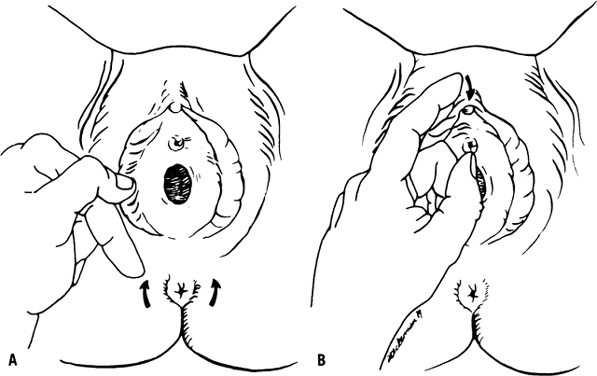 Fig. 2. Bulbocavernosus ( A) and clitoral ( B) sacral reflexes.(Ostergard DR, Bent AE [eds]: Urogynecology and Urodynamics: Theory
and Practice, 3rd ed. Baltimore, Williams & Wilkins, 1991) Fig. 2. Bulbocavernosus ( A) and clitoral ( B) sacral reflexes.(Ostergard DR, Bent AE [eds]: Urogynecology and Urodynamics: Theory
and Practice, 3rd ed. Baltimore, Williams & Wilkins, 1991)
|
If a neurologic deficit is identified or suspected during a simple screening
examination, electromyography should be performed to evaluate (1) skeletal
muscle function and innervation or (2) abnormalities of the
autonomic control of the lower urinary tract. Neurologic consultation
can be helpful in such situations. Evaluation of Urethral Mobility Because part of the underlying pathophysiology of genuine stress incontinence
is believed to be poor transmission of abdominal pressure to the
urethra secondary to urethral hypermobility, an assessment of urethral
mobility should be made. Such assessment also allows us to identify
patients with stress incontinence in the presence of good anatomic support
who do not have genuine stress incontinence. This assessment may
be made in several ways, the simplest of which is the visual assessment
of distal anterior vaginal wall mobility. THE Q-TIP TEST. The Q-tip test, as first described by Crystle and coworkers,56 is an excellent means of quantitatively documenting the presence of urethral
hypermobility. It is a simple, inexpensive way to assess urethral
hypermobility quantitatively without exposing the patient to radiation. Although
the determination of urethral hypermobility on Q-tip testing
is a poor predictor of the etiology of a patient's urinary incontinence,57–60 it remains an accurate and valuable measurement of urethral support. It
is clear that many women develop urethral hypermobility early in their
third and fourth decades but remain continent if their intrinsic sphincteric
mechanism is capable of compensating for the negative pressure
transmission to the urethra created by urethral hypermobility.61 This is why there is a tremendous overlap between stress-incontinent and
continent patients in many,58,59 but not all,60–62 studies. The cotton-tipped applicator soaked in 2% lidocaine jelly is
placed at the bladder neck; then the resting and straining angles are
measured several times. Once inserted, the Q-tip should be pulled snugly
back to the bladder neck so that the bladder wall will not limit its
motion. The test is arbitrarily said to be positive for urethral hypermobility
when the straining angle is greater than 30 to 35 degrees.57,58,63 As described by McGuire,64 patients with stress incontinence who do not have urethral hypermobility
have intrinsic sphincteric deficiency (type III incontinence, discussed
previously), in which stress incontinence is present despite adequate
urethral support. These patients usually are managed with periurethral
injections, obstructive sling procedures, or placement of artificial
sphincters. Straining cystograms and ultrasound studies also can
be used to measure urethral hypermobility, but they are far more expensive
than simple Q-tip testing. SPONTANEOUS UROFLOWMETRY. Spontaneous uroflowmetry is used as a screening test to detect abnormalities
of micturition. It is accomplished by having the patient sit on
a commode and void onto a load cell or a spinning disk, which records
an instantaneous velocity curve of micturition. Measurement of the maximum
flow rate is more useful in men than in women because of its ability
to detect physical obstruction of the urethra, a condition that is
rare in women. Uroflowmetry is primarily a measure of voiding velocity
and is measured in milliliters per second. Because there is little
concern of physical urethral obstruction in women, the pattern of the
uroflow curve is more important than the quantitative measurement of voiding
velocity. Figure 3 A shows a normal uroflowmetry curve. Qualitative assessment of the bell-shaped
uninterrupted curve readily identifies it as a normal study. Figure 3 B shows an abnormal uroflowmetry study with an interrupted intermittent
flow pattern, which may suggest voiding dysfunction. These graphic tracings
are made by electronic uroflowmeters. An average flow rate can be
measured in a much simpler manner by timing the duration of urine flow
and then measuring the volume voided. Simple calculation reveals the
mean flow rate, and the patient can be catheterized to measure the residual
urine. Residual urine volumes greater than 50 mL should be further
investigated. These women should undergo further evaluation of their
voiding mechanism with multichannel voiding-pressure studies. 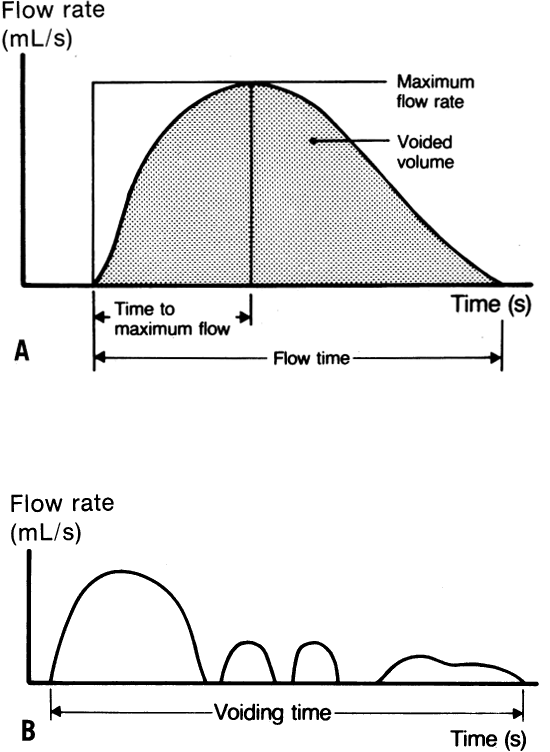 Fig. 3. Normal uroflowmetry study demonstrating a normal bell-shaped pattern ( A) and an abnormal screening uroflowmetry study ( B) in a Valsalva voider with an interrupted, intermittent flow pattern. Fig. 3. Normal uroflowmetry study demonstrating a normal bell-shaped pattern ( A) and an abnormal screening uroflowmetry study ( B) in a Valsalva voider with an interrupted, intermittent flow pattern.
|
Fantl and associates65 showed that although flow rates did not vary significantly with age, parity, weight, height, or
menstrual cycle phase, they were directly affected
by bladder volume. Like Starling's law for the heart, bladder
volume, within normal ranges, directly affects the velocity and efficiency
of bladder emptying.65,66 This makes it difficult to establish normal values for uroflowmetry without
using a nomogram. Based on research by Fantl and colleagues,65 the peak flow rate should be equal to or greater than 15 mL/second for
volumes voided in excess of 200 mL. At this volume, these researchers
found an average peak flow rate in women of 22.6 mL/second, with a standard
deviation of 7.5 mL/second. In women, the flow pattern is more important than the peak flow rate. A
normal asymptomatic woman usually voids with continuous flow until voiding
is completed. Intermittent flow patterns may occur occasionally
in typically asymptomatic women (see Fig. 3 B) but are more common in patients with voiding dysfunction.65 Abnormal uroflowmetry studies should be repeated because patients having
to void in an unfamiliar setting can exhibit falsely abnormal voiding
patterns. Intermittent flow patterns and decreased flow rates are most often explained
by functional obstruction with a failure of pelvic floor relaxation
due to urethral atrophy, inflammation, pain, or fear.66 Genital prolapse with mechanical urethral obstruction is another common
cause of abnormal uroflowmetry in women. One of the most common causes
of intermittent flow patterns in women is Valsalva voiding, which may
be prevalent in women with genuine stress incontinence and decreased
urethral resistance. Valsalva voiding allows these women to empty their
bladders rapidly, with normal to high peak flow rates. After corrective
surgery, however, Valsalva voiding yields inefficient or absent
voiding because of restored normal pressure transmission to the urethra, and
therefore can be disastrous. Detrusor sphincter dyssynergia (a neurologic loss of coordination of the
urethra and bladder) also can cause intermittent flow patterns and retention. Less
commonly, underactive and acontractile bladders cause decreased
flow rates, intermittent flow, and elevated residual urine. Medications (e.g. alpha-adrenergics, anticholinergics) also can have adverse affects on
voiding function, and these effects can be detected on spontaneous uroflowmetry. Uroflowmetry
is a simple, noninvasive test that can screen
for abnormal voiding function. If studies are abnormal or voiding dysfunction
is suspected, then electromyography and voiding pressure studies
should be performed. ENDOSCOPIC EVALUATION. The Robertson urethroscope, with its 0-degree lens and closed sheath, can
be used to assess the urethra and trigone in a retrograde fashion
and to observe their function. Using the cystoscope to evaluate the urethra
is inadequate because it is important to visualize the urethra on
entry, before its normal appearance is distorted by insertion of the
scope. Urethroscopy may be done with the use of carbon dioxide, water, or
saline as the distending media. Although carbon dioxide is less messy
and can offer a better view of the urethra than water or saline, it
forms carbonic acid in the bladder, which can irritate the bladder
and cause artificial detrusor contractions during filling.67 The urethra is inspected in a retrograde fashion, and urethral color, inflammation, and
the presence of the normal urethral folds and gland openings
may be observed. Pathologic findings such as polyps, cysts, inflammatory
fronds, increased exudate, condylomata, and diverticula all
may be noted in the urethra. These findings often correlate to irritative
symptoms such as dysuria, urgency, and frequency of micturition. On
entry into the bladder, the trigone and ureteral orifices should be
inspected. The ureteral orifices should be examined for their appearance
and function; normal ejaculation of clear urine and good retraction
of the ureteral orifices should be noted. After examination of the urethra
and trigone, the examiner can use the urethroscope to observe the
dynamic function of the urethra during filling and with various maneuvers. Dynamic urethroscopy was first described by Robertson.68 The urethroscope is used to observe the bladder neck during filling to
diagnose detrusor overactivity, and during Valsalva's maneuver and
coughing to identify passive opening of the bladder neck, as seen in
genuine stress incontinence. Sand and coworkers69 and Scotti and associates,70 however, have shown that dynamic urethroscopy, although fairly specific, is
an insensitive screening test for detrusor instability and genuine
stress incontinence. Cystoscopy also can be used during the routine evaluation of incontinent women, but
it is not advocated in the absence of irritative voiding symptoms or
hematuria. Cystoscopic examination in symptomatic patients occasionally
reveals signs of chronic cystitis, cystitis cystica or glandularis, interstitial
cystitis, polyps, hemangiomas, or neoplasms that might
not be identified with the urethroscope. These conditions can produce
increased afferent sensation leading to sensory urge incontinence and
therefore are important to rule out in women reporting symptoms of urgency, frequency, and
urge incontinence. In women with irritative daytime symptoms, nocturia, and suprapubic pain
or discomfort before voiding, the diagnosis of interstitial cystitis
is likely. Punctate suburothelial hemorrhages with redistention of the
bladder during cystoscopy helps establish this diagnosis. We usually
can observe these cystoscopic findings in the office without anesthesia. However, cystoscopy
under anesthesia (general or regional) is needed
to definitively rule out interstitial cystitis. A potassium test using
KC1 bladder instillation has been described by Parsons as another
way to help differentiate these patients with a defective glucosaminoglycans
layer who will experience severe bladder pain with instillation
of this solution when compared with saline.70a This test is limited by its relatively poor (70%) sensitivity. CYSTOMETRY. Cystometry is an important diagnostic test used to measure the change
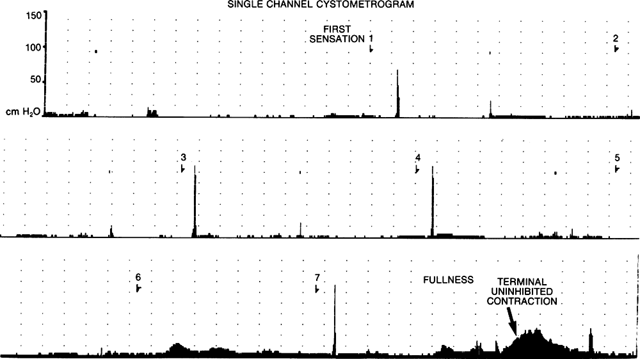
in bladder pressure during bladder filling. Pressure measurement during
bladder filling allows for the evaluation of bladder sensation, compliance, capacity, and
detrusor inhibition during the storage phase. In
a normal cystometry study, the bladder is compliant enough to allow for
filling to capacity without any significant increase in bladder pressure, whereas
the overactive detrusor will produce involuntary phasic
contractions during filling, even at low volumes. Cystometry may be performed
in many different ways and may be affected by many different
variables (Table 2). TABLE 2. Factors Influencing Cystometry
Filling media H2O
Saline
Urine
CO2
Radiographic contrast
Media temperature Room temperature
Iced infusions
Body temperature
Filling method Orthograde
Orthograde with diuresis
Retrograde
Slow fill (1---10 mL/min)
Medium fill (10---100 mL/min)
Rapid fill (>100 mL/min)
Infusion mode Continuous
Intermittent
Position Supine
Sitting
Standing
Ambulatory
Provocative maneuvers Cough
Heel bounce
Position change
Hand washing
Valsalva
Running water
Rectal distention
One of the simplest and least expensive ways to observe bladder filling
is eyeball cystometry. This is a qualitative examination that lends itself well to examination
at the bedside in the nonambulatory patient. A self-retaining catheter
is placed transurethrally, and the bladder is progressively filled
by pouring sterile water intermittently into an irrigation syringe attached
to the catheter, 50 mL at a time. The syringe is held approximately 15 cm
above the patient's pubic bone. The patient's first
sensation to void and maximum cystometric capacity (the volume at which
the patient notes pain or piloerection) are recorded, and involuntary
bladder contractions are suggested by a rising rather than falling
meniscus, associated with a strong urge to urinate or leakage around
the catheter. A rising meniscus may be a reflection of increased abdominal
pressure that may be aborted by asking the patient to relax or inspire. Abdominal
relaxation may be confirmed by abdominal palpation. Ouslander
and colleagues71 found eyeball cystometry to have a sensitivity of 75%, a specificity of 79%, and
a positive predictive value of 85% in diagnosing detrusor overactivity
compared with multichannel electronic cystometry in geriatric
patients. This method also allows for the measurement of the residual
urine with the initial catheterization. Although not a perfect screening
test, its simplicity allows it to be used in situations in which
testing otherwise would not be possible. Simple quantitative assessment during bladder filling can be achieved by retrograde incremental cystometry, with the patient standing. This can be done with commercially prepared
devices (Fig. 4), or a water cystometer can be constructed. First, the bladder is filled
with a small amount of fluid and the baseline bladder pressure recorded. The
bladder is then filled in a retrograde fashion by gravity, 50 mL
at a time, stopping approximately every minute to measure the bladder
pressure. The patient's first desire to void and maximum cystometric
capacity are recorded with the corresponding bladder pressure
at these volumes. Bladder pressure is recorded by reading the meniscus
every 50 mL at rest and after provocation with coughing, heel bouncing, or
hand washing. The cystometrogram is considered positive for detrusor
overactivity if the bladder pressure rises by more than 15 cm H2O during filling. This is further confirmed by the presence of involuntary
leakage with removal of the catheter at maximum cystometric capacity. Stable
bladders usually have pressure increases equal to or less than 10 cm
H2O during filling, with equivocal studies showing pressure increases of 10 to 15 cm
H2O. Repeating these equivocal studies has been shown to improve diagnostic
accuracy.72 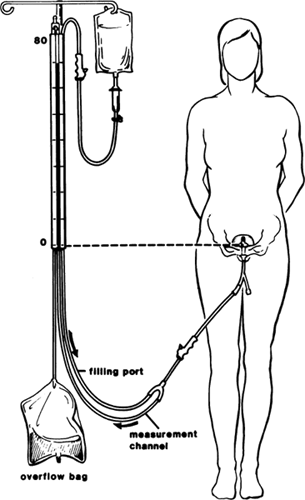 Fig. 4. A simple manometric cytometry unit that can be used to perform incremental, retrograde
cystometry.(Sand PK, Brubaker LT, Novak: Simple standing incremental cystometry as
a screening method for detrusor instability. Obstet Gynecol 77:453–457, 1991) Fig. 4. A simple manometric cytometry unit that can be used to perform incremental, retrograde
cystometry.(Sand PK, Brubaker LT, Novak: Simple standing incremental cystometry as
a screening method for detrusor instability. Obstet Gynecol 77:453–457, 1991)
|
Simple, incremental, retrograde studies are limited in their sensitivity
because they miss small increases in bladder pressure that would create
urinary leakage in the absence of the obstructing catheter. This can
be avoided in some cases by the use of small (4-Fr) catheters without
retention balloons that are taped to the patient's leg. These
studies are limited in their specificity mainly by the misinterpretation
of increased intra-abdominal pressure as increased detrusor pressure. This
artifact can be avoided by the use of subtracted cystometry. In a prospective trial, Sand and coworkers73 found standing retrograde incremental cystometry results to be reproducible
in 84% of patients, with a sensitivity of 84.3% and specificity
of 69.4% with one cystometrogram. The performance of two standing retrograde
incremental cystometrograms on two occasions increased the sensitivity
to 92.3% in 100 consecutive incontinent women when compared with
multichannel urethrocystometry. These simple, inexpensive retrograde
cystometers can be used quite accurately in screening incontinent women.73–75 Subtracted cystometry can be used to improve diagnostic accuracy in the
diagnosis of detrusor overactivity. Specificity is improved when both
abdominal and bladder pressure are measured, allowing the urodynamicist
to distinguish between abdominal pressure effects and those changes
intrinsic to the bladder. Abdominal pressure can be measured through
the vagina, the rectum, or suprapubically. Instantaneous electronic subtraction of bladder pressure from abdominal
pressure allows for continuous recording of the true detrusor pressure. A
low-compliance bladder may be diagnosed when detrusor pressure gradually
rises to 15 cm H2O or higher during filling. Phasic pressure increases associated with symptoms
of urgency or urge incontinence are indicative of detrusor overactivity. Provocative
maneuvers can be used more easily and with less
confusion during subtracted cystometry. This also improves the diagnostic
sensitivity of the test. The easiest way to perform subtracted cystometry
is to use an electronic cystometer that records two or three
channels of activity. These electronic cystometers typically cost $5,000 to $20,000. When more accuracy or information is needed regarding the bladder's
ability to store urine, examiners can use urethrocystometry combined
with electromyography. Monitoring urethral pressure responses and the
electrical activity of the periurethral and intrinsic urethral skeletal
muscle further augments our ability to understand the detrusor's
reaction to bladder filling. Measurement of urethral pressure also
enhances our ability to detect detrusor overactivity, which usually is
preceded by urethral relaxation. Sudden urethral pressure decline or fluctuation suggests impending involuntary
detrusor contractions, signaling the urodynamicist to try to push
the patient a little further. Ambulatory urodynamics are studies that allow for the performance of cystometry during normal
orthograde filling from renal urine production, which can be used in
the patient's own environment, rather than in the clinic setting. These
are the most physiologic and sensitive studies currently available.76–78 McInterney and associates76 found ambulatory urodynamics to be far more sensitive in women with urge
incontinence. However, van Waalwijk van Doorn and colleagues77 showed that one third of asymptomatic volunteers had involuntary detrusor
contractions during a 5-hour ambulatory study. This may profoundly
limit the diagnostic accuracy of ambulatory urodynamics. These studies
also are very expensive and not readily available in most centers.78 The patient's urine is the most physiologic medium, but it is very
difficult to use during retrograde cystometry; therefore most investigators
use carbon dioxide, water, saline, or radiographic contrast media. Although
carbon dioxide has been used widely in the past, most investigators
have replaced it with liquid media.67,78,79 Carbon dioxide is easy to use compared with liquid media, and it allows
for rapid flow rates that are more provocative. It also allows cystometry
to be repeated rapidly; however, as mentioned previously, it is
an irritating medium that forms carbonic acid when mixed with urine.67 Water and saline are used by many investigators interchangeably, but saline
is probably more physiologic, especially when instilled at body
temperature. Little has been described regarding the accuracy of radiographic
solutions as media for cystometry, but most of these solutions
are nonirritating and probably comparable to saline. A contrast medium
such as 35% diodone, however, is quite irritating and is more likely
to produce involuntary bladder contractions.78 Contrast media should be used for cystometry when combined with videocystourethrography
to allow visualization of the bladder during filling. Cystometry can be performed with the patient in a number of positions, but
standing studies are the most provocative and sensitive. In elderly
or infirm patients, standing may be difficult; birthing chairs or commodes
can be used for such patients in studies that allow the patient
to sit upright. Movement to a standing position with assistance may be
provocative in some of these patients.80 Various provocative maneuvers can be used during cystometry (see Table 2) to help elicit involuntary bladder contractions. Although some maneuvers
may not be physiologic, it should be recognized that stationery cystometry
can miss involuntary detrusor contractions in up to 50% of patients
reporting urge incontinence.78 Therefore, artificial provocation may be necessary to compensate for the
inherent insensitivity of these laboratory urodynamic studies. A recent
study of provocative maneuvers to elicit detrusor instability by
Mayer and coworkers81 showed hand washing to be the most provocative. We commonly have the patient
cough and heel-bounce after every 50 to 100 mL of fluid infused
and reserve hand washing and other forms of provocation until the end
of the study if involuntary detrusor contractions have not been recorded. Some involuntary detrusor contractions can be missed even with the use
of electronic, continuous, multichannel, subtracted cystometric evaluations
with provocation. Low-pressure detrusor contractions can be mistaken
for episodes of stress incontinence in these patients unless the
isometric force of these contractions can be measured. The use of stop
tests in these ambiguous situations can identify isometric contractions
that might otherwise go unnoticed.82 Voluntary interruption of the stream of urine, mechanical obstruction
of the bladder neck, transurethrally with a catheter or manually through
the vagina, will close the bladder neck and allow for measurement of
the true isometric bladder pressure. These stop tests help to improve
the sensitivity of cystometry and also can be useful during instrumented
voiding studies. Bladder compliance also may be assessed during cystometry. This
represents the change in volume associated with a change
in pressure (Δv/Δp). Because the bladder is composed of
both active elements (smooth muscle) and passive elements (collagen and
elastin), the evaluation of the filling phase also must include the
distensibility of viscoelastic properties of the bladder.83 In the absence of phasic contractions, changes in these properties suggest
the possibility of bladder injury due to inflammatory disease, radiation, or
surgical trauma. In these patients, cystometry may reveal
a low-compliance bladder with a slow, gradual rise in true detrusor pressure
during filling. STRESS TESTING. Stress testing is a simple clinical test to demonstrate urinary leakage. This
test is accomplished by having a patient cough, perform Valsalva's
maneuver, or do whatever it normally takes to increase intra-abdominal
pressure to cause her to reproduce her stress incontinence. It
is most provocative with a full bladder and with the patient standing, as
opposed to lying supine. Urinary leakage coincident with an increase
in intra-abdominal pressure is regarded as a positive sign of stress
incontinence; however, continuous urinary leakage after a sudden
increase in intra-abdominal pressure may represent stress-induced detrusor
contractions after provocation.81 PAD TESTING. Pad testing is a method of documenting and quantifying urinary leakage
objectively. Various methods of pad testing have been described in the
literature. They are all based on the use a preweighed sanitary napkin
or collecting device to measure urine loss over a period of time during
planned or random activities. Twenty-minute, 40-minute, 1-hour, 2-hour, 12-hour, and 24-hour tests have all been used to document and quantify
urinary incontinence. The pad test can be a useful adjunct to
urodynamic evaluation when urinary leakage is not exhibited in the artificial
testing environment of a physician's office or clinic. Jorgensen and associates84 found that the 1-hour pad test endorsed by the International Continence
Society detected nearly twice the number of incontinent women as stress
testing and voiding cystourethrography. Because of the variable diuresis
with an oral fluid load, this is consistent with the findings of
some investigators85,86 but inconsistent with those of others.87,88 It is preferable to use a fixed-volume 20-minute pad test in clinical
trials. This test incorporates all the activities of the standard 1-hour
test into a simpler, faster, and more reproducible format.89,90 With rare exceptions (excessive perspiration or vaginal discharge), urine
loss equal to or greater than 1 g/hr indicates urinary leakage on
pad testing. In healthy subjects, mean pad weight increases are less than 1 g/hr.91,92 Home pad tests spanning extended periods of time are best able to reproduce
the magnitude of the patient's incontinence.93 Although the correlation between 24-hour and 48-hour pad tests with the
standard 1-hour test is low (r = 0.35), the reproducibility of a 24-hour pad test is quite good (r = 0.96).93 Extended testing correlates better with a patient's symptoms.93 These urodynamic tests allow physicians to demonstrate urinary leakage
and to understand its etiology in most patients. Because of the artificial
nature of the office environment and the inherent lack of sensitivity
of some of these tests, not all patients will demonstrate urinary
leakage during the screening examination. When the screening evaluation
fails to explain the patient's symptoms, she should undergo more
complex multichannel urodynamic and radiographic studies. Multichannel Urodynamics Initial evaluation with the simple methods described may not be sufficient
to define the etiology of an incontinent woman's problem. If
some aspect of the patient's symptoms remains unexplained, more sophisticated
testing in a urodynamics laboratory is indicated. Other indications
include previously failed anti-incontinence operations; age
older than 60 years; continuous or insensible urine loss, or both; suspicion
of neuropathic dysfunction; mixed incontinence symptoms; urinary
retention; and women with presumed intrinsic sphincteric deficiency
in the absence of urethral hypermobility.94,95 Complex multichannel urodynamic and radiographic studies require more
expensive equipment that may not always be available to the clinician. In
these instances, decisions regarding referral can be difficult. URETHROCYSTOMETRY. The performance of urethrocystometry involves the use of pressure-measuring
catheters and transducers that are placed in the urethra, bladder, and
rectum or vagina to measure intra-abdominal pressure.96–98 Analog or digital urodynamic monitors are used to measure these three
pressures and the true detrusor pressure from instantaneous subtraction
of abdominal from bladder pressure and urethral closure pressure that
is calculated by subtraction of bladder pressure from urethral pressure. Measurement
of these five pressures with or without electromyography (EMG) can
be accomplished during filling to create an urethrocystometry
study (Fig. 5).90 Urethral and bladder pressures can be measured by the same microtransducer
catheter. This also can be used to fill the bladder through a small
infusion channel in the center of the catheter (Fig. 6). Patients are positioned on birthing chairs, x-ray tables, or urodynamic
commode chairs, and a single transducer catheter is placed in either
the vagina or rectum to measure the intra-abdominal pressure. The dual
transducer catheter is placed in the urethra and positioned so that
the proximal microtransducer, which is 6 cm from the catheter tip and
distal transducer, is placed at the point of maximal urethral pressure
and oriented laterally. Filling is then begun in a retrograde fashion
either through the lumen of the urethral catheter or via a separate
small catheter or pediatric feeding tube.96 Cystometric parameters and changes are noted as described previously with
subtracted cystometry, but urethrocystometry offers the added advantage
of being able to monitor urethral pressure. 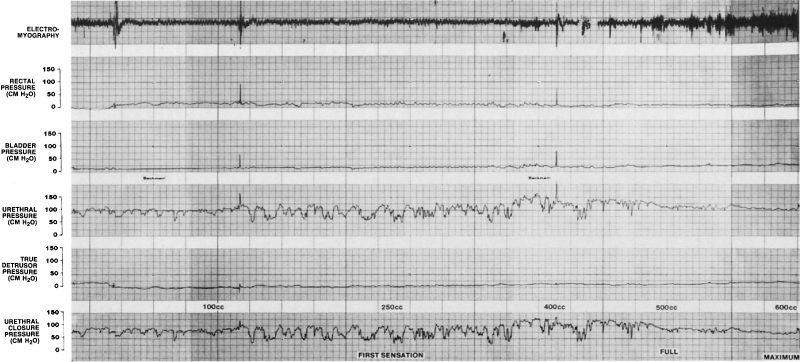 Fig. 5. A urethrocystometry study in a 32-year-old woman with urinary urgency and
frequency, demonstrating urethral instability (marked variation in
urethral pressure) without any detrusor instability. Fig. 5. A urethrocystometry study in a 32-year-old woman with urinary urgency and
frequency, demonstrating urethral instability (marked variation in
urethral pressure) without any detrusor instability.
|
 Fig. 6. Dual-channel microtransducer urodynamic catheters, with and without a central
filling port.(Sand PK, Ostergard DR [eds]: Urodynamics and the Evaluation
of Female Incontinence. London, Springer-Verlag, 1995) Fig. 6. Dual-channel microtransducer urodynamic catheters, with and without a central
filling port.(Sand PK, Ostergard DR [eds]: Urodynamics and the Evaluation
of Female Incontinence. London, Springer-Verlag, 1995)
|
Monitoring urethral pressure during cystometry is advantageous because
it allows the detection of urethral pressure decreases that normally occur
before a bladder contraction, whether voluntary or involuntary, and
such variations usually are associated with corresponding EMG changes. Alternatively, one
may observe marked variations in urethral pressure
without detrusor contractions. This urethral instability may be a
forerunner of detrusor instability (Fig. 7) and often signifies an impending bladder contraction.99–101 These pressure variations may be associated independently with symptoms
of urgency and sensory urge incontinence.99,100 Some investigators question, however, whether these pressure fluctuations
have any significance at all.102 Exaggerated urethral pressure fluctuations may be directly associated
with complete urethral relaxation and may cause urinary incontinence from
uninhibited urethral relaxation.11 Care must be taken to ensure that these urethral pressure variations are
not artificial (i.e. due to patient movement, abdominal pressure changes, voluntary contraction
of the levator ani muscles). Alpha blockers may relieve symptoms
in some patients with urethral instability. Urethral pressure decreases
preceding detrusor instability can be used as a marker for biofeedback
therapy to train patients to inhibit involuntary detrusor contractions. In
healthy women, filling the bladder will lead to a rise in urethral
pressure secondary to increased skeletal muscle activity associated
with a gradual increase in skeletal muscle EMG activity (augmentation). This may be triggered by stretch receptors in the bladder trigone.103 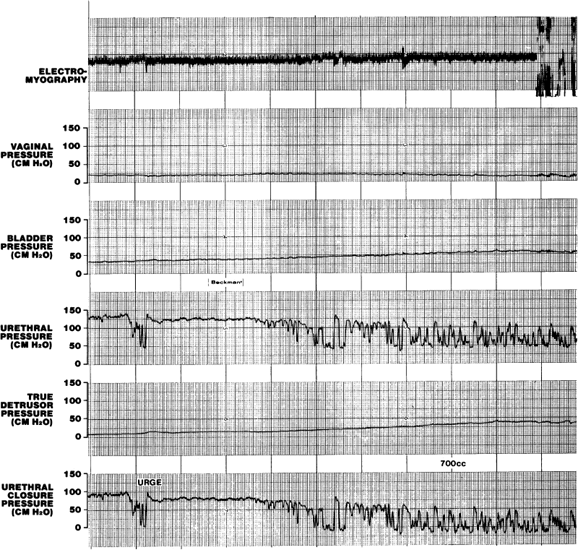 Fig. 7. An episode of urethral instability associated with urgency is a precursor
of further intermittent urethral relaxation associated with an involuntary
detrusor contraction at a maximum cystometric capacity of 700 mL. Fig. 7. An episode of urethral instability associated with urgency is a precursor
of further intermittent urethral relaxation associated with an involuntary
detrusor contraction at a maximum cystometric capacity of 700 mL.
|
Healthy volunteers have been found to have an average maximum cystometric
capacity of 594 mL with the first sensation to void noted at 32% of
capacity.104 Ninety-five percent of the control subjects noted first desire to void
before filling to 300 mL of H2O. URETHRAL CLOSURE PRESSURE PROFILES. Recording urethral closure pressure profiles involves measurement of the
urethral pressure all along the length of the urethra. By slowly withdrawing
a dual microtransducer catheter through the urethra with an
automated withdrawal arm, one can measure the urethral pressure all along
the urethra. This generates a pressure-time plot called a urethral closure pressure profile (UCPP) (Fig. 8). The “pressure” in the urethra measured with this system
is actually force that is generated by urethral smooth muscle, periurethral
vasculature, skeletal muscle in and around the urethra, and inert
collagen and elastic tissue in the urethra.105–109 Damage or attenuation of any of these contributing factors may dramatically
alter the UCPP.105–109 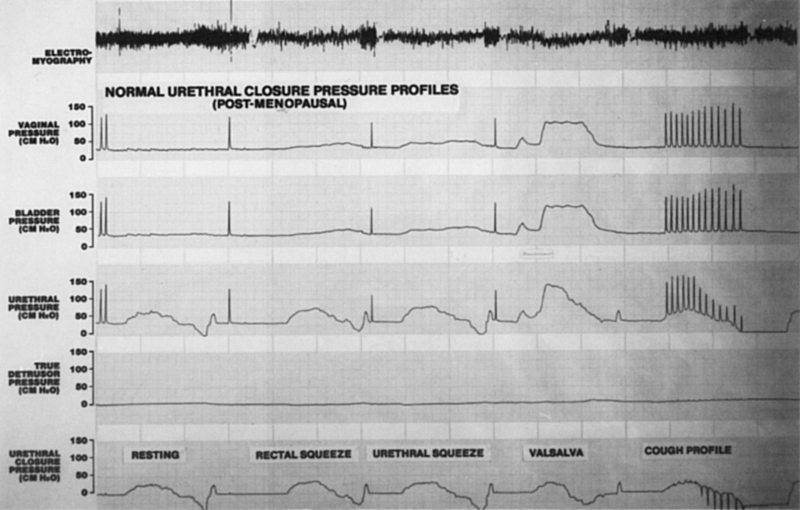 Fig. 8. Normal resting and dynamic urethral closure pressure profiles during augmenting
maneuvers (rectal and urethral squeeze), Valsalva's maneuver, and
repetitive coughing in a postmenopausal woman. (Sand PK, Ostergard DR [eds]: Urodymamics and the Evaluation
of Female Incontinence. London, Springer-Verlag, 1995) Fig. 8. Normal resting and dynamic urethral closure pressure profiles during augmenting
maneuvers (rectal and urethral squeeze), Valsalva's maneuver, and
repetitive coughing in a postmenopausal woman. (Sand PK, Ostergard DR [eds]: Urodymamics and the Evaluation
of Female Incontinence. London, Springer-Verlag, 1995)
|
The UCPP begins as the proximal or urethral pressure transducer enters
the proximal urethra. At this point, the urethral pressure usually is
greater than bladder pressure, resulting in an increase in closure pressure. The
UCPP reaches its peak in the proximal to mid urethra, and then
the closure pressure decreases until in the distal urethra, where
it drops below zero because distal urethral pressure is less than bladder
pressure. The profile ends when the urethral transducer reaches the
urethral meatus, where the urethral pressure reaches zero (atmospheric
pressure), to which it was originally calibrated. The resting profile
can be adversely affected by age, surgery, vaginal delivery, and other
forms of trauma.19,61,102,110 The patient's position,111 the degree of patient relaxation,106 the microtransducer catheter orientation, and the bladder volume107 may all affect the resting UCPP. All these factors should be standardized
when resting profiles are done. Although continent women generally have higher closure pressures and functional
lengths than patients with genuine stress incontinence, there
is tremendous overlap between these two populations.112–114 This makes the resting UCPP a poor diagnostic test for determining who
has genuine stress incontinence.110–115 Dynamic UCPP during Valsalva's maneuver and coughing may be more
discriminant. These tests were designed to study the effects of increased
intra-abdominal pressure on the sphincteric unit. Although many argue
that there is no usefulness in measuring resting urethral closure
pressure, this measurement allows for a graphic illustration of the functional
capability of the sphincter and its internal integrity.116,117 We have shown that women who have urethral closure pressure less than
or equal to 20 cm H2O are at increased risk of failing routine anti-incontinence surgery. This
finding of a low-pressure urethra appears to be the most significant
risk factor for failing anti-incontinence surgery. In these women with
deficient intrinsic sphincteric mechanisms, resupporting the urethra
with retropubic urethropexies and needle suspensions to improve urethral
pressure transmission does not appear to be enough. Although they
are anatomically corrected 97% of the time by these operations, less
than 50% of these women are objectively cured of their genuine stress
incontinence.116 These patients with low-pressure urethras also appear to have decreased
success with nonoperative therapies. Dynamic UCPPs offer the urodynamicist more information about the function
of the sphincter urethrae under stress. These profiles may be done
at any bladder volume and in any position, but standing with a full bladder
is probably the most stressful and sensitive position for this test.114,118 Valsalva profiles are done by withdrawing the urethral pressure catheter
through the urethra after Valsalva's maneuver is initiated. During
these profiles, the ability of Valsalva's maneuver to produce
leakage of urine in the absence of a bladder contraction and with zero
closure pressure and functional length establishes the diagnosis of
genuine stress incontinence. Valsalva's maneuver appears to generate
a more consistent stress compared with repetitive coughing, but cough
profiles appear to be more sensitive and reproducible.119 Hilton and Stanton110 performed cough UCPPs in 120 stress-incontinent women and 20 asymptomatic
women and found that despite bladder-neck opening in 25% of the controls, they
all had pressure transmission ratios equal to or greater
than 100% (i.e. the ratio of increase in urethral pressure with coughing to increase in
bladder pressure with coughing × 100%) (Fig. 9). The stress-incontinent patients all had deficient pressure transmission
ratios,110 as determined by cough UCPPs. Profiles are considered positive for genuine
stress incontinence if urinary leakage occurs with pressure equalization. This
implies not only that there is negative pressure transmission
but also that each negative deflection in closure pressure with
coughing descends to or below the zero closure pressure level. 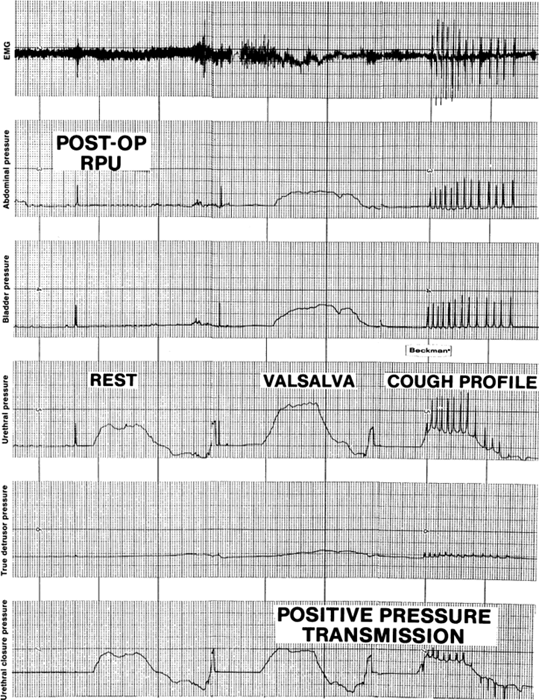 Fig. 9. Valsalva's maneuver and cough urethral closure pressure profiles obtained
after retropubic urethropexy demonstrate positive pressure transmission
compared with the resting profile. The pressure increases more
in the urethral lumen than in the bladder with coughs and Valsalva's
maneuver in the proximal 2 cm of the urethra, resulting in positive
pressure spikes in the urethral closure pressure tracings. Fig. 9. Valsalva's maneuver and cough urethral closure pressure profiles obtained
after retropubic urethropexy demonstrate positive pressure transmission
compared with the resting profile. The pressure increases more
in the urethral lumen than in the bladder with coughs and Valsalva's
maneuver in the proximal 2 cm of the urethra, resulting in positive
pressure spikes in the urethral closure pressure tracings.
|
LEAK POINT PRESSURE. Recently, a new urodynamic test has been described to quantify defective
sphincteric function in patients with genuine stress incontinence.120 The leak point pressure is an indirect measure of urethral resistance
to the outflow of urine. It is a simple measurement of the intra-abdominal
pressure necessary to cause urinary leakage due to genuine stress
incontinence and therefore is a more discriminating measure than clinical
grading of the patient's symptoms. The Valsalva leak point pressure is measured by a vaginal or anal pressure
transducer catheter. Alternatively, a leak point pressure can be measured
with a bladder pressure catheter. The patient is asked to perform
Valsalva's maneuver with gradually increasing force. The minimum
abdominal or bladder pressure that causes urinary leakage is the leak
point pressure. This test has been found quite reproducible and simple
to perform and is useful in monitoring therapy for genuine stress
incontinence. VOIDING PRESSURE STUDIES. The same equipment used to analyze urethrocystometry can be used to analyze
micturition. By using a dual microtransducer catheter or external
transducer-catheter system, urethral and bladder pressure can be measured
by placing the proximal transducer at the point of maximum urethral
pressure. The abdominal pressure can be measured vaginally or rectally; EMG
activity can be measured with needle, wire, patch, or ring electrodes
at the anus or urethra. A load cell and funnel are placed under
the patient to measure urine flow. The patient's voiding mechanism
and function can be analyzed easily with this equipment. Simple
uroflowmetry offers information only about voiding speed, pattern, and
completeness. Men void normally only in one way: by relaxing the urethra
and having a bladder contraction. Women void completely in five different
ways. Like men, they may void by urethral relaxation and detrusor
contraction, or by urethral relaxation alone. Either of these patterns
may be augmented by the Valsalva maneuver. They may also void by
Valsalva alone. Although Valsalva voiding is normal and more common in
women with genuine stress incontinence,121–123 it can cause prolonged urinary retention after surgery for genuine stress
incontinence. In women with Aldrige-type suburethral sling procedures (slings
connected to rectus fascia anteriorly), permanent retention
can occur if Valsalva voiding persists.121 Rud and colleagues121 have shown that normal continent women usually void by urethral relaxation, followed
by a detrusor contraction 3 seconds later. These two events
have the cumulative effect of decreasing urethral closure pressure
with the onset of urine flow 6 seconds after initial urethral relaxation. Urinary
retention can be explained by (1) failure of urethral relaxation
during a detrusor contraction, (2) mechanical obstruction of
the urethra, (3) underactive detrusor contractions (inadequate in time
or magnitude), or (4) an acontractile detrusor. All these will predispose
patients to overflow incontinence. Voiding pressure studies may be
used not only to assess voiding dysfunction but also preoperatively
to analyze borderline voiding mechanisms that may be decompensated by
surgery. These studies allow for the prediction of voiding dysfunction
and retention after anti-incontinence surgery. Retropubic colposuspensions
and needle suspension procedures may increase urethral resistance
significantly and in some patients may cause prolonged paruresis.124 Bhatia and Bergman125 have shown that women with low pressure (below 15 cm H2O) or absent detrusor contractions who use Valsalva's maneuver to
void are at a 12-fold increased risk of requiring prolonged (more than 7 days) postoperative
catheterization. Klutke and coworkers126 have shown that retropubic urethropexies, like the Burch procedure, are
only successful if they increase urethral resistance during voiding. They
looked at pressure flow studies in 178 women after Burch procedures
and found that urethral resistance (detrusor pressure at maximum flow
divided by the square of the maximum flow rate) increased in successful
cases from 0.051 to 0.099, but there was no change from a baseline
urethral resistance of 0.041 in those who were unsuccessful. However, Belair
and associates127 have shown that these operations, which do increase urethral resistance, are
not obstructive, and instead, when compared with nomogram data, return
urethral resistance to normal levels. RADIOGRAPHIC STUDIES. The combination of cystometry and radiographic techniques has made it
possible to study the pressure changes in the lower urinary tract while
monitoring its appearance with fluoroscopy or ultrasound. This type
of study is called videocystourethrography. Although some find that fluoroscopy adds little to the evaluation,123 many find it useful. It is rarely used, however, because of its expense
and the need for dedicated space to house the fluoroscopic and urodynamic
equipment. Anatomic assessment and documentation with corresponding
functional analysis of urethral, detrusor, and abdominal pressures
clearly make this an optimal system to analyze urinary tract pathology. The
alternative is to evaluate the anatomic changes with endoscopy, pelvic
examination, and Q-tip testing (described previously) separate
from the multichannel urodynamic assessment and to integrate the information
mentally. The major disadvantages of videocystourethrography are
the radiation exposure (1 rad), cost, and need for radiology personnel
and space. Fluoroscopic evaluation of the bladder may help to clarify
whether low-pressure, involuntary detrusor contractions are symptomatic
or whether a patient's bladder neck funnels with increases
in intra-abdominal pressure. Bladder neck funneling at rest may define
an incompetent bladder neck in the patient with intrinsic sphincteric
deficiency or type III incontinence that should be treated with periurethral
injections of glutaraldehyde cross-linked (GAX)-collagen, autologous
fat or carbon-coated beads. Postvoiding films of the bladder can be used to analyze urinary residuals
and to look for urethral diverticula. Urethrograms with Tratner catheters
are more sensitive at detecting these diverticula, which can cause
postvoiding dribbling if located distally or sensory urge incontinence
if located proximally. Voiding cystourethrograms can be used to diagnose
ureterovesical reflux in patients with retention. Cystograms can be useful in cases of suspected fistulae (see Fig. 10) and bladder tumors. Figure 10 depicts a hysterosalpingogram in a woman with a vesicouterine fistula. Cystograms
also can be used to evaluate bladder neoplasms, but cystoscopy
is preferable. 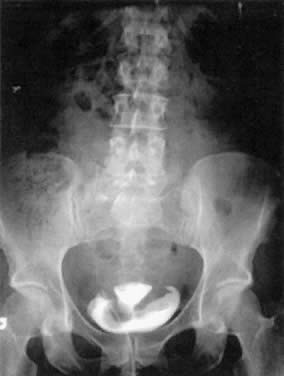 Fig. 10. A hysterosalpingogram demonstrating a vesicouterine fistula after a vaginal
birth. This patient previously had a cesarean section. Fig. 10. A hysterosalpingogram demonstrating a vesicouterine fistula after a vaginal
birth. This patient previously had a cesarean section.
|
|