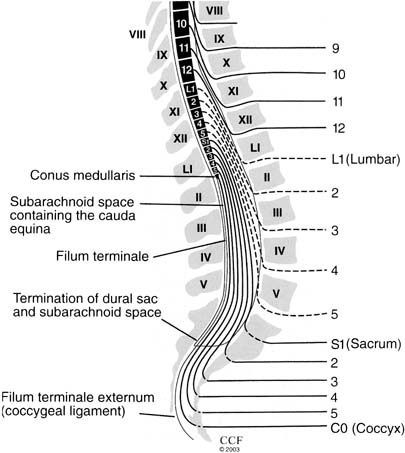
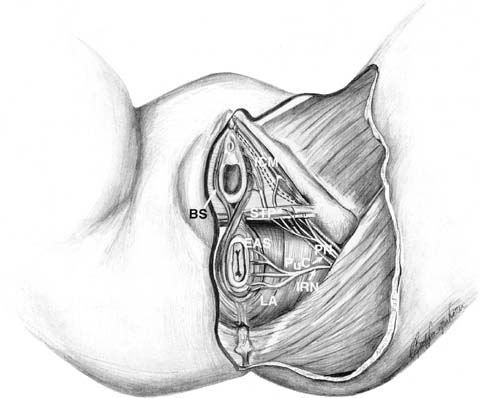
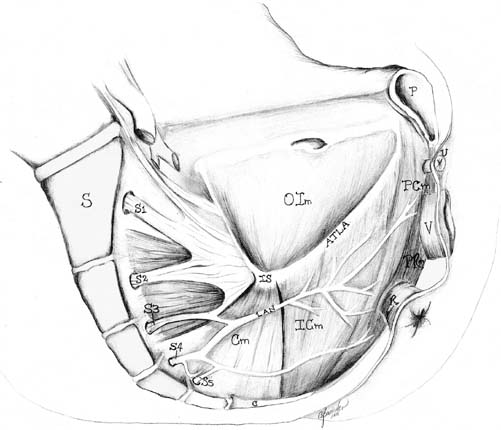
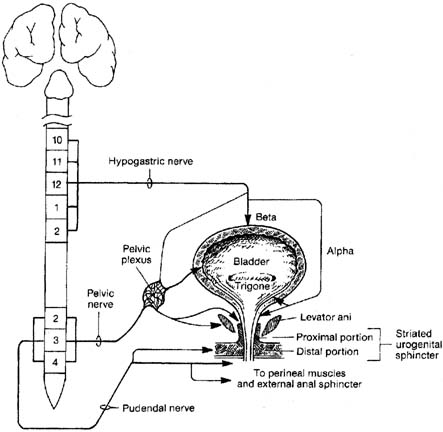
CnEMG is a widely used diagnostic test for evaluating the neuromuscular
integrity of skeletal muscles. It can be used to differentiate normal
muscle from muscle that is neuropathic or myopathic. A concentric needle
electrode consists of a stainless steel outer cannula within that
runs a fine silver, steel, or platinum wire that is insulated except at
its tip. The concentric needle is inserted into a muscle where the inner
wire serves as a recording electrode while the outer cannula serves
as a reference electrode. Bioelectrical potentials are measured as
voltage differences between the two electrodes that are then recorded, displayed, and
analyzed. A CnEMG examination provides information on the insertional activity, spontaneous
activity, motor unit action potentials (MUAPs), and
recruitment pattern (also known as interference pattern) of
a muscle. Insertional activity is the electrical activity that occurs
when the needle electrode is first introduced into a muscle or is moved, is
the result of mechanical stimulation or injury of the muscle
fibers, and usually stops within approximately 2 seconds of movement. After
resolution of the insertional activity, a typical limb muscle is
electrically silent when at rest. Denervated muscle fibers may produce
rhythmic spontaneous electric potentials, such as fibrillation waves
or positive sharp waves. The presence of this spontaneous activity in
a resting muscle is a sign of denervation. In contrast to limb muscles
but similar to the postural muscles of the back, the levator ani muscles, and
the urethral and anal sphincters are tonically active and demonstrate
normal continuously firing action potentials even at rest. It
is important to be able to distinguish abnormal spontaneous activity
from this normal tonic activity when performing CnEMG on these muscles. The motor unit is the smallest functional unit of the motor system and
consists of a motor neuron, its axon, and all of the muscle fibers innervated
by the axon. The motor unit in a normal human limb muscle consists
of several dozen muscle fibers lying within an area of 5 to 10 mm
in diameter. The motor unit action potential (MUAP) is a compound
potential representing the sum of the individual action potentials
generated in the few muscle fibers of the motor unit that are within
the pick-up range of the recording electrode. With slight voluntary
contraction of a muscle, individual MUAPs can be seen and MUAP
analysis can be performed. Analyzing the shape, amplitude, duration, and
number of phases of a MUAP allows a normal motor unit to be distinguished
from one that has been denervated and reinnervated or one that
is myopathic. Increasing force within a muscle is accomplished by an
orderly increase in the firing rate of individual MUAPs and the recruitment
of additional MUAPs. In normal muscle, as the force of voluntary
contraction increases, motor units are recruited in a specific order
that is determined by the thresholds that are inherent to the individual
unit.41 Analyzing this recruitment pattern provides additional information about
the health of a muscle and its innervation. When a muscle is maximally
contracted, the number and firing rate of the individual MUAPs becomes
so great that they can no longer be distinguished and the baseline
is obscured. This is called an interference pattern (IP). Neuropathic
and myopathic muscles each have distinct interference patterns
that can be readily distinguished from that of normal muscle. CnEMG can be performed on an individual muscle, as on the anal sphincter
when evaluating isolated fecal incontinence, or can be systematically
performed on several muscles to determine the level of a neurologic
injury. All muscles that are innervated by one spinal segment (level) are
referred to as a myotome. Using CnEMG to systematically
evaluate several muscles in different myotomes often permits localization
of a lesion to the spinal root, nerve plexus, or an individual peripheral
nerve. In a patient with bowel or bladder dysfunction in which
a neurologic cause is suspected, a thorough evaluation often requires
EMG of the lower extremity as well as pelvic floor musculature. Technique The clinical history and sacral neurologic examination should be carefully
reviewed before beginning the EMG evaluation, because this will help
determine which muscles should be examined. Ideally, the evaluation
should be performed in a shielded room to prevent interference from other
electronic devices or AC power cords. The patient is placed in a
comfortable position that allows access to the pelvic floor musculature, usually
the dorsal lithotomy or lateral decubitus positions. A grounding
surface electrode is applied. Most laboratories do not use local
anesthetics in any form before insertion of the needle electrodes, although
some authors advocate using a topical anesthetic, particularly
for examination of the urethral or anal sphincter.38 After wiping the skin overlying the appropriate muscle with alcohol, the
concentric needle electrode is inserted until the insertional activity
of the muscle is noted, confirming that the electrode is within the
muscle. The EMG investigation consists of analysis of electrical activity
at rest (assessment of spontaneous activity), at slight
voluntary contraction (MUAP analysis), and at strong contraction (interference
pattern analysis). Generally the most painful
portion of the examination is the initial insertion through the
skin, so several sites within the muscle should be sampled by moving
the tip of the needle without removing it from the skin. Depending on
the muscle, more than one skin penetration may be necessary. Both the
visual output on the screen of the EMG machine and the audio output from
the speaker are used to assess the quality of the recording and identify
electrophysiological phenomena. Commonly used settings for CnEMG
evaluation of the pelvic floor muscles are: filter settings, 5 Hz to 10 kHz; horizontal
sweep speed, 10 msec/div; and gain setting, 50 to 500 μV/div.38 Anal Sphincter Both the subcutaneous and deep portions of the external anal sphincter (EAS) are
accessible for CnEMG evaluation. To study the subcutaneous
portion of the EAS, the needle electrode is inserted 1 centimeter
outside the mucocutaneous junction of the anal orifice to a depth
of 3 to 6 mm beneath the skin.42 The deep portion of the EAS is assessed by inserting the needle at the
mucocutaneous junction of the anus, at an angle of approximately 30 degrees
to the anal canal axis, for a depth of 1 to 3 cm.42 Typically the subcutaneous and/or deep portion of the EAS are sampled
in at least four quadrants, approximately divided into the upper and
lower and left and right portions of the sphincter. Ideally, 20 or
more MUAPs should be sampled during the evaluation. Anal mapping has been used in the past to identify the location of a sphincter defect
before an anal sphincteroplasty in the treatment of fecal incontinence. To
identify the precise location of a sphincter defect, more needle
insertions are typically required, usually at 9:00, 10:00, 12:00, 2:00, 3:00, and 6:00. Endo-anal ultrasound has largely replaced
anal mapping with EMG for the identification and localization of sphincter
defects.43,44 Urethral Sphincter The external urethral sphincter can be studied by inserting the electrode
into the sphincter muscle periurethrally or transvaginally. For the
periurethral approach, the needle is inserted approximately 5 mm anterior
to the external urethral meatus (12:00 position) to a depth
of approximately 1 to 2 cm.45 For the transvaginal approach, the posterior vaginal wall is retracted
with a speculum and the needle is inserted approximately 2 cm proximal
to the external urethral meatus, off the midline, and directed laterally
into the urethral sphincter.45 Although slightly more painful, the periurethral approach provides superior
sampling of the urethral sphincter, obtaining as many as twice the
number of MUAPs as the transvaginal approach.45 At least 10 MUAPs should be recorded to adequately evaluate the urethra.46 Bulbocavernosus The bulbocavernosus muscle is a readily accessible perineal muscle that
is innervated by the perineal branch of the pudendal nerve. The bulbocavernosus
muscle can be reached either transmucosally with a needle insertion
medial to the labia minora or through the skin lateral to the
labia majora.38 Levator Ani The iliococcygeus and pubococcygeus portions of the levator ani muscle
complex are most easily investigated using a transvaginal approach. The
muscles are localized by first inserting two fingers into the vagina
and asking the patient to contract. One side of the levator complex is
isolated and the electrode is inserted using the opposite hand in at
least two sites on the muscle. This is then repeated on the opposite
side. Although there is no standardized location for needle insertion
into the levator ani muscles, one technique that has been proposed uses
the ischial spine as a fixed reference point and samples two sites relative
to this easily identifiable landmark.47 The first site is located 2 cm caudal and medial from the ischial spine. The
second site is 2 cm further medial. The puborectalis muscle can
be accessed from a perineal approach. A 75-mm electrode is required
and is inserted approximately 1 centimeter posterior to the anus
in the midline for a depth of 3 to 5 cm. Lower Extremity Bladder and bowel dysfunction are not uncommon when there are lesions involving
the lumbosacral nerve roots and/or lumbosacral plexus. These
lesions typically also affect innervation of the lower extremity. Therefore, whenever
a patient's history and examination suggest lower
extremity involvement as well as bowel and/or bladder dysfunction, the
electrophysiologic evaluation should include the muscles of
the lower extremity and the pelvic floor. A detailed description of the
CnEMG evaluation of the lower extremity musculature is beyond the scope
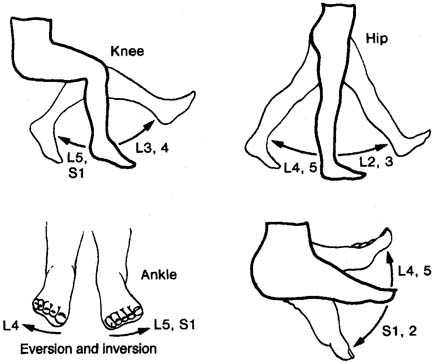
of this text. However, muscles that are often useful in such an evaluation
include: quadriceps (L3, L4; femoral nerve); adductor
longus (L4; obturator nerve); tibialis anterior (L4, L5; deep
peroneal nerve); gastrocnemius (S1, S2; tibial nerve); gluteus
maximus (S1; superior gluteal nerve); gluteus
medius (L5; inferior gluteal nerve); adductor hallucis brevis (S1, S2; tibial
nerve); and the paraspinal muscles from
L3 to S1. Interpretation CnEMG is a valuable tool for evaluating lower motor neuron disease and
muscle disease. Lesions involving the upper motor neurons in isolation
have a normal EMG evaluation. The initial assessment in any CnEMG examination
is an evaluation for insertional activity. The presence of insertional
activity confirms that the electrode has been placed within
the muscle. Absence of insertional activity with an appropriately placed
needle electrode usually means a complete atrophy of the examined muscle. Once
the needle has been properly placed, the patient is asked
to completely relax the muscle being examined and the presence or absence
of spontaneous activity is assessed. In the urethral sphincter, the
anal sphincter, and the levator ani muscles, which all tonically contract
even in the resting state, the only normal spontaneous activity
is normal MUAPs. In limb muscles and the bulbocavernosus muscle, which
do not tonically contract, there should be a complete absence of spontaneous
activity at rest. The pelvic floor muscles achieve complete electrical
silence during voiding and defecation. Spontaneous activity that
is found in pathologic conditions includes fibrillation potentials, positive
sharp waves, complex repetitive discharges, fasciculations, myotonia, myokymia, and
neuromyotonia (Fig. 7). Fibrillation potentials are biphasic muscle fiber potentials that
occur spontaneously and typically have an amplitude of between 20 and 300 μV
and a duration of less than 5 msec.41 They usually fire rhythmically at a rate of 2 to 20 Hz. Fibrillation potentials
develop 2 to 30 days after an injury and can occur with a motor
nerve lesion as well as with primary muscle diseases such as acute
muscle injury, muscular dystrophies, and inflammatory myopathies. Positive
sharp waves are biphasic waves with an amplitude of 20 to 500 μV
and a 1- to 5-msec duration.41 They are more common than fibrillations and have a similar clinical significance. Complex
repetitive discharges (CRD) are spontaneous
high-frequency discharges with a regular firing pattern beginning
and ending abruptly and can be bizarre in appearance. They can
be found after chronic partial denervation, muscular dystrophy, inflammatory
myopathies, and in some metabolic disorders.41 The striated urethral sphincter appears to be particularly likely to develop
CRDs, which have been found in association with urinary retention
in young women (Fowler's syndrome) and even in some
neurologically normal women.48,49 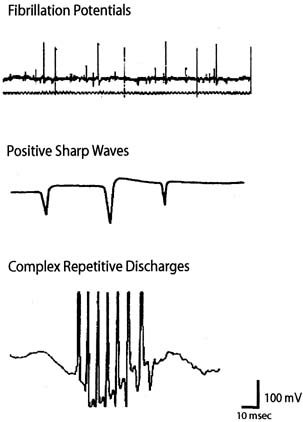 Fig. 7. Examples of abnormal spontaneous activity seen during concentric needle
EMG. Fig. 7. Examples of abnormal spontaneous activity seen during concentric needle
EMG.
|
After a determination about spontaneous activity is made, the patient is
asked to slightly contract the muscle being examined and attention is
turned to MUAP analysis. The shape, amplitude, duration, number of phases, and
stability of each MUAP should be assessed. Most EMG units have
a trigger and delay mechanisms that allow MUAPs to be “frozen” for
more careful analysis. Ideally, 20 or more MUAPs should be
sampled for each muscle. This is often difficult for striated urethral
sphincter, in which analysis of 10 MUAPs is often all that can be achieved
because of the small size of the muscle.46 Muscles that have been denervated and reinnervated and muscles that are
myopathic have distinct MUAP characteristics from those of normal muscle (Fig. 8). The morphology of a MUAP reflects, among other things, the number
and local concentration of muscle fibers comprising a motor unit. After
a neuronal injury, the muscle fibers innervated by that neuron begin
to atrophy. Adjacent nerve fibers attempt to reinnervate these denervated
muscle fibers, resulting in a neuron that now supplies a greater
number of muscle fibers. This creates a MUAP with larger amplitude, longer
duration, and a greater number of phases and turns. Because these
changes depend on reinnervation by an adjacent motor neuron, large
complex polyphasic MUAPs are not typically seen until 3 to 6 months after
a nerve injury. In the setting of an acute nerve injury, reinnervation
has not had time to occur and MUAP morphology is normal. Myopathic
injury results in a loss of muscle fibers and therefore a motor unit
with fewer muscle fibers. This results in a MUAP with shorter duration
and smaller amplitude than normal. 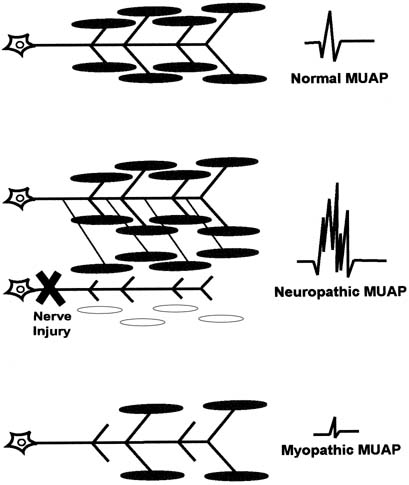 Fig. 8. A normal motor unit (top left) consists of a motor neuron, its
axon, and all of the muscle fibers that it innervates. A normal motor
unit action potential (MUAP) (top right) is characterized
by its amplitude, duration, and number of phases (usually 3 to 4). Denervation of a motor unit (middle) results
in death of some motor fibers and reinnervation of others via collateral
sprouting from an adjacent motor unit axon. This results in a more
complex MUAP with greater amplitude, duration, and number of phases. Myopathy (bottom) results in death of muscle fibers without
subsequent reinnervation, resulting in MUAPs with decreased amplitude. Fig. 8. A normal motor unit (top left) consists of a motor neuron, its
axon, and all of the muscle fibers that it innervates. A normal motor
unit action potential (MUAP) (top right) is characterized
by its amplitude, duration, and number of phases (usually 3 to 4). Denervation of a motor unit (middle) results
in death of some motor fibers and reinnervation of others via collateral
sprouting from an adjacent motor unit axon. This results in a more
complex MUAP with greater amplitude, duration, and number of phases. Myopathy (bottom) results in death of muscle fibers without
subsequent reinnervation, resulting in MUAPs with decreased amplitude.
|
After performing MUAP analysis, the patient is asked to increase her contraction
effort and motor unit recruitment is assessed. In normal motor
unit recruitment, there is a sequential increase in the firing rate
of MUAPs as well as a sequential acquisition of new higher threshold
MUAPs as the force of voluntary contraction increases. An interference
pattern is called full or complete when the number and firing rate of the individual MUAPs becomes so great
that they can no longer be distinguished and the baseline is obscured (Fig. 9). Recruitment is said to be normal when a complete interference pattern
occurs at maximal effort. In neuropathic disorders, there are a
reduced number of motor units so there is decreased recruitment and an
incomplete interference pattern, although the remaining units fire rapidly. In
myopathic disorders, there are reduced muscle fibers, but a
normal number of motor units. A complete interference pattern is seen, but
it occurs at much less than maximal effort and the amplitude to
the MUAPs is reduced. 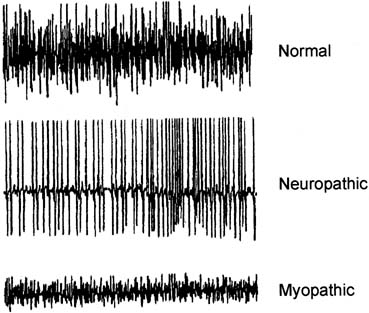 Fig. 9. Normal interference pattern (top); neuropathic interference pattern
characterized increased amplitude, reduced number of MUAPs, and
a rapid rate (middle); myopathic interference pattern characterized
by decreased amplitude and a normal number of MUAPs. Myopathic
interference patterns become full at lesser degrees of muscle contraction. Fig. 9. Normal interference pattern (top); neuropathic interference pattern
characterized increased amplitude, reduced number of MUAPs, and
a rapid rate (middle); myopathic interference pattern characterized
by decreased amplitude and a normal number of MUAPs. Myopathic
interference patterns become full at lesser degrees of muscle contraction.
|
The chronicity of a nerve lesion can be determined by the CnEMG features
present. In an acute nerve lesion, reinnervation has not occurred so
the MUAPs have normal morphology. There are a reduced number of motor
units, however, so recruitment is decreased. Between 2 and 30 days after
an injury, spontaneous activity such as fibrillation potentials and
positive sharp waves become apparent and insertional activity becomes
longer. Three to 6 months after an injury, reinnervation has occurred
so the MUAPs become larger and more complex. The location of a nerve
lesion is determined by the pattern of muscles demonstrating denervation. For
instance, neuropathic CnEMG findings in the external anal sphincter, but
normal findings in the levator ani muscle and bulbocavernosus
muscle suggest an isolated lesion involving the inferior rectal branch
of the pudendal nerve. In contrast, if neuropathic findings were
found in the external anal sphincter, the right levator ani muscle, the
right gluteus maximus, and gluteus medius muscles, but not on the left
side or in the L5, S1 paraspinal muscles, this would suggest a right-side
lumbosacral plexus lesion. Traditionally, CnEMG has depended largely on an examiners auditory and
visual impression for analysis. In an attempt to improve the accuracy, reliability, and
speed with which CnEMG can be performed, quantitative
techniques have been developed. Although the first form of quantitative
EMG (qEMG) was introduced in the 1950s, it did not become
widespread until recently, when newer EMG machines became computerized.50 The technical aspects of performing a qEMG evaluation are identical to
conventional CnEMG described. The difference is in the analysis, in which
computerized digital analysis is performed on the collected electromyographic
data, allowing a much more detailed evaluation of neuromuscular
function in a shorter period of time. Two complementary forms of
qEMG are: (1) analysis of multiple motor unit action potentials (multi-MUAP analysis) and (2) interference
pattern analysis. In several recent studies, qEMG has been applied
to the investigation of the pelvic floor muscles.31,47,51,52,53 In multi-MUAP analysis, the examiner identifies a period of crisp
EMG activity during a slight to moderate muscle contraction. The computer
uses a system of template matching to automatically extract, quantify, and
sort any well-defined MUAPs according to their shape.54 This allows a rapid acquisition of data, such that the 20 to 30 MUAPs
needed to adequately describe a muscle can be obtained in only a few minutes.47 Several MUAP parameters are then quantified, including amplitude, duration, area, number
of phases, and number of turns, increase time, and
spike duration. Thickness (area/amplitude) and size index (2*log [amplitude + area/amplitude]) are
automatically calculated by the computer and assist in differentiating
between normal and myopathic or neuropathic muscles.55 Normative data for the external anal sphincter and levator ani muscles
have been published and are available for comparison.47,51,52 Interference pattern analysis is a broad term used to describe one of several
automatic quantitative techniques for evaluating muscle recruitment. Computerized
analysis of interference patterns is particularly useful
because the electromyographic signal recorded at even a moderate
contraction is too dense to be accurately assessed by visual inspection.56 The most popular technique for interference pattern analysis is turns/amplitudes
cloud analysis (Fig. 10). This method of analysis is even faster than multi-MUAP analysis (2 to 3 minutes) and does not require a standardized
force of contraction, eliminating an important source of variability.54,56 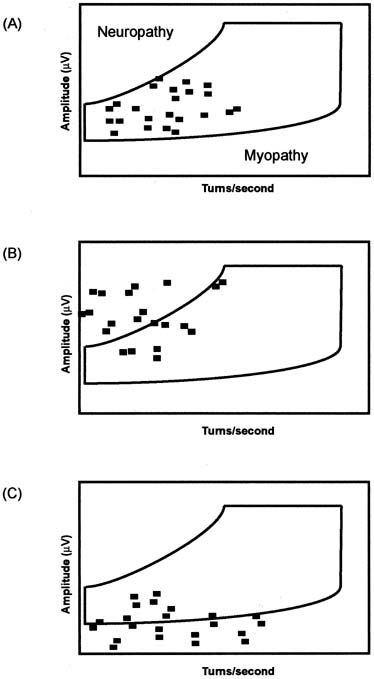 Fig. 10. Turn/amplitude cloud analysis is a quantitative method of analyzing
interference patterns. Normal muscle (top) is characterized
by all points being located within the normal cloud boundaries. Neuropathic
muscle (middle) is characterized by MUAPs with greater
amplitude and decreased turns/second, so points will be located
above and to the left of the normal cloud. Myopathic muscle is characterized
by MUAPs with decreased amplitude so points will be located below
the normal cloud. Fig. 10. Turn/amplitude cloud analysis is a quantitative method of analyzing
interference patterns. Normal muscle (top) is characterized
by all points being located within the normal cloud boundaries. Neuropathic
muscle (middle) is characterized by MUAPs with greater
amplitude and decreased turns/second, so points will be located
above and to the left of the normal cloud. Myopathic muscle is characterized
by MUAPs with decreased amplitude so points will be located below
the normal cloud.
|
Limitations While CnEMG is the most valuable tool for investigating lower motor neuron
damage of the pelvic floor, it has several limitations. The primary
limitation of needle EMG techniques in the evaluation of women with
pelvic floor dysfunction is the lack of experience on the part of most
gynecologists and the limited access to qualified experts with interest
in studies of pelvic floor muscles and sphincters.57 The role of CnEMG in the day-to-day evaluation and management
of patients with urinary and fecal incontinence or other pelvic
floor disorders has yet to be established, and thus far appears to be
limited. Techniques for EMG of the pelvic floor muscles have yet to be
standardized, although some progress has been made towards standardization
of the evaluation of the anal sphincter.42,52,53 The adoption of qEMG techniques has improved the speed, accuracy, and
reliability of conventional CnEMG evaluations, but widespread applicability
remains limited because of the specialized equipment and expertise
needed. The concentric needle electrode is invasive and can be painful, although
it is usually well-tolerated in most patients. |