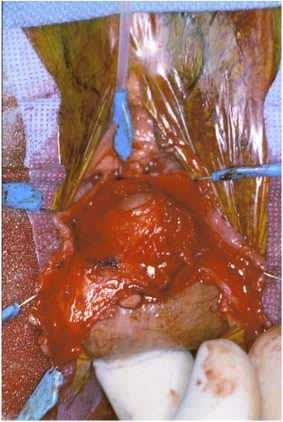
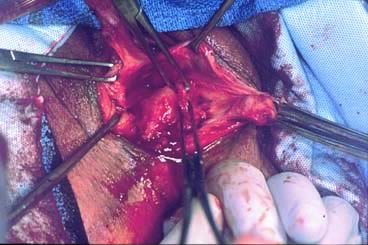
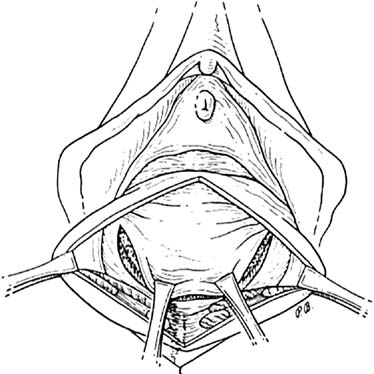
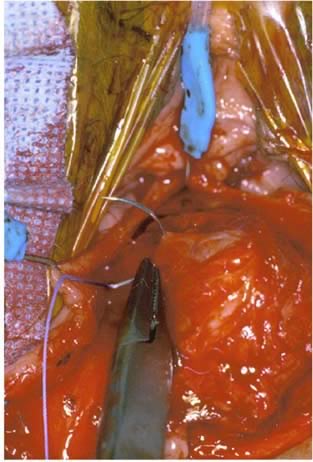
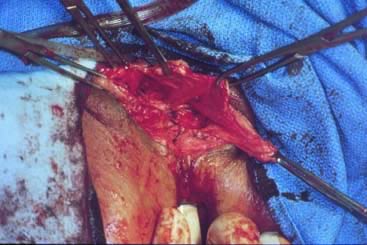
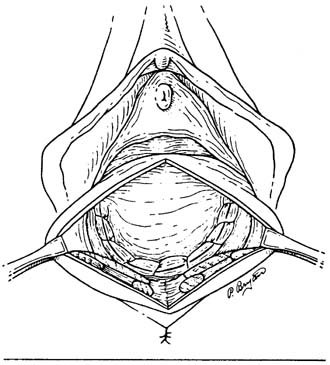
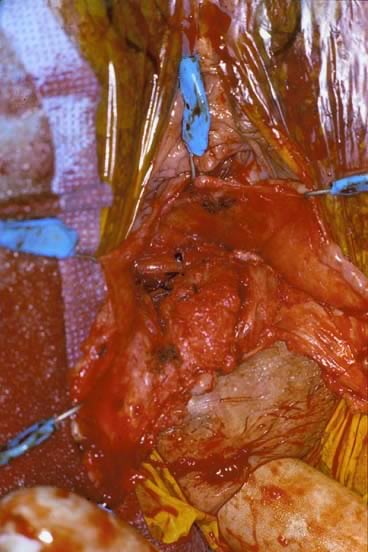
Defecography
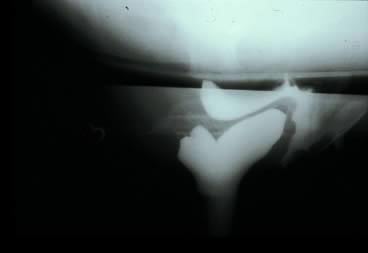
Defecography, first described by Burhenne in 1964,8 increasingly is being used for preoperative evaluation of pelvic organ prolapse. Defecography is believed useful by some because it provides objective outcomes and identifies anatomic abnormalities. The technique involves filling the rectum with a barium paste that is the consistency of stool and opacifying the vagina. Fluoroscopy is used at rest, on contracting the pelvic floor muscles, on straining down, and during defecation (Fig. 1).19 The fluoroscopic examination grades rectoceles during maximal distention as small (<2 cm), moderate (2 to 4 cm), and large (>4 cm).1,10,19 Kelvin and associates19 noted that barium was retained only in moderate to large sized rectoceles. The relevance of the rectocele size compared with symptoms has not been shown24; however, larger rectoceles are associated with fecal trapping.10
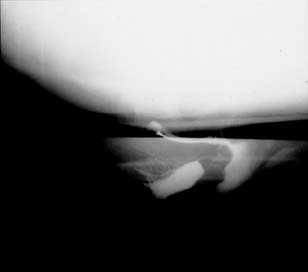
Defecography is a good diagnostic tool to help exclude other defecation disorders that may increase the risk of recurrence of symptoms despite anatomic repair. Radiographically, anismus or paradoxical sphincter response is observed as a decrease or less than 5% increase in the anal rectal angle during straining.17,25 The anterior rectal wall enfolding 6 to 8 cm inside the rectum identifies internal intussusception.19 An enterocele may be suspected if the distance between the vagina and the rectum is 2 cm or greater and the concurrent herniation of small loops of bowel is seen within the rectovaginal space.19 Significant rectoceles tend to trap barium during defecography (Fig. 2). If barium entrapment is not present, some physicians1,22 discourage surgical correction. Although defecography has been shown to be reproducible when clinical data are present,26 the significance of fecal trapping and usefulness of correlating data between fecal trapping and the need for repair are controversial and need to be studied further.
Scintigraphic defecography is another tool to evaluate the physiology of rectal emptying. It not only assesses the rate and completeness of emptying, but also assesses the anorectal angle and pelvic floor descent. Advantages include less radiation exposure and precise quantification. Disadvantages include its inability to evaluate the anatomic detail or view pelvic floor anatomy.17
Ultrasound
The use of endosonography of the anus has proved to be valuable in detecting incontinence disorders by imaging the anatomic integrity of the internal and external anal sphincter. Sandridge and Thorp27 stated anatomic defects are best detected with an endovaginal probe by measuring rectal length and diameter, puborectalis thickness and angle, thickness and integrity of the internal and external anal sphincters, and curvature of the anal canal.
Anal Mammometry
Anal mammometry measures rectal pressures by a transducer or balloon. Its measurement of rectal sensation evaluates first feeling, urge, and discomfort. This information is used to distinguish causes of constipation. When an individual is able to tolerate increased volumes without signs of increased discomfort or the urge to defecate, overflow incontinence may occur. Careful consideration must be made during this evaluation process because individuals with small volumes may have an irritable rectum causing incontinence or urgency. These disorders may mimic rectocele symptoms, such as incontinence or incomplete emptying. If misdiagnosis of a rectocele is made, rectocele repair may exacerbate these disorders by causing worsening of symptoms.17,18,28
Electromyography and Nerve Conduction Studies
Electromyography and nerve conduction studies also have been used to evaluate defecation disorders. Obstetric trauma denervates and causes atrophy of the pelvic floor muscles, which may lead to subsequent pelvic floor weakness. This denervation may be detected by electromyography studies, and pudendal terminal motor latency can be used as a method to detect the causes of pelvic floor weakness.
Colonic Transit Studies
Colonic transit studies are used to assess distal bowel motility and may be indicated when infrequent stooling is reported by a patient with a rectocele. The patient ingests radiopaque markers, which are measured and counted in the right colon, left colon, sigmoid colon, and rectum. Clinically slow transit time is defined as fewer than two bowel movements per week over several years. The utility in individuals with rectoceles is inconclusive, although some have normal transit times, whereas others have prolonged times.25 Individuals with unimproved symptoms after rectocele repair were found preoperatively to have longer transit times.21