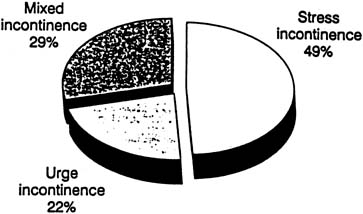
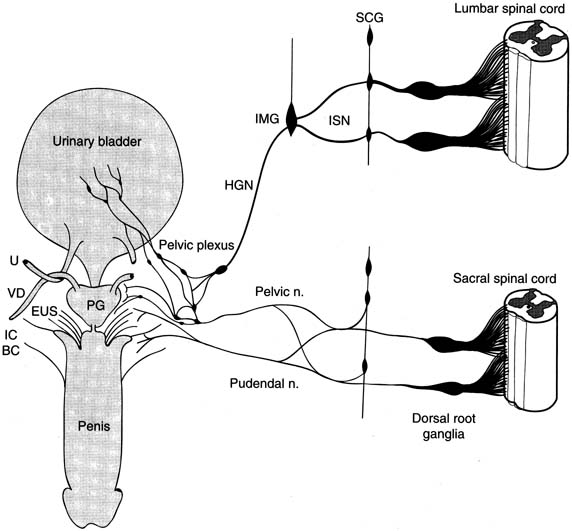
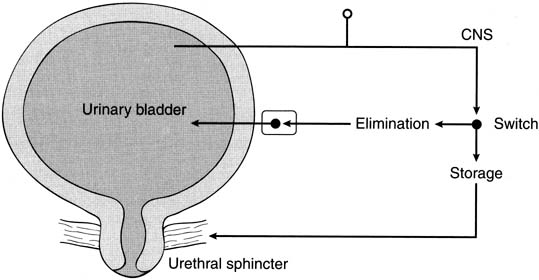
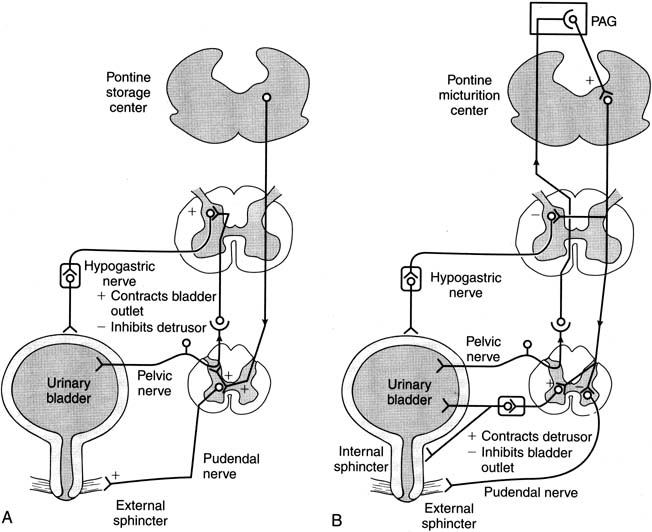
Therapy of OAB has recently become a popular topic both nationally and
internationally for two reasons. As previously described, the number of
persons affected is only now being recognized, and the economic impact
is staggering. As the current population continues to age, the number
of patients promises to increase in the future. New medical therapies
with fewer side effects have been developed, making realistic therapy
easier for the patient. Current therapies for OAB include a variety
of behavioral, pharmacologic, and surgical methods, as shown in Table 7. This section concentrates on those pharmacologic therapies that are the
most commonly used (Table 8). Table 7. Overactive Bladder Treatment Modalities in Common Use
| Therapy Type |
Specific Therapy |
| Behavioral |
Bladder drill |
| |
Timed/prompted voiding |
| Biofeedback |
Various probes and patches |
| Electrical Stimulation |
Vaginal or anal electrical stimulation |
| |
Transcutaneous or percutaneous stimulation |
| |
Peripheral nerve and pelvic floor nerve stimulation |
| |
Sacral nerve neuromodulation |
| Pharmacological |
Table 8 |
| Surgical |
Autoaugmentation |
| |
Enterocystoplasty |
| |
Urinary diversion |
(Yoshimura N, Chancellor MB: Current and future pharmacological treatment
for overactive bladder. J Urology 16 8:1897–1913, 2002)
Table 8. Drugs With Bladder Action
| Classification |
Examples |
Pharmacologic Action |
| Anticholinergic agents |
Atropine, glycopyrrolate
oxybutynin, propantheline, tolterodine |
Inhibit muscarinic receptors, thus, decreasing the
response to cholinergic stimulation. Used to reduce pressure during
bladder filling and treat unstable bladder contractions. |
| Smooth muscle relaxants |
Dicyclomine, flavoxata |
Direct smooth muscle relaxation decreases intravesical
pressure during filling as well as the severity and presence of unstable
bladder contractions. Most agents have some degree of anticholinergic
action. |
| Ca antagonists |
Diltiazem, nifedipine, verapamil |
Used for treating unstable bladder contractions to
decrease the magnitude of spikes by reducing the entrance of calcium
during an action potential. |
| Calcium channel openers |
Cromakalim, pinacidil |
Acts to increase membrane potential and, thus, decrease
myogenic initiation of unstable bladder contractions. |
| Prostaglandin synthesis inhibitors |
Flurbiprofen |
Prostaglandins have been implicated in increased smooth
muscle tone and the induction of spontaneous activity. Inhibition
of prostaglandin synthesis could promote bladder relaxation during
filling and decrease spontaneous bladder activity. |
| β-Adrenergic agonists |
Isoproterenol, terbutaline |
Stimulation of β-receptors induces relaxation
of the bladder body, resulting in decreased intravesical pressure
during filling. |
| Tricyclic antidepressants |
Amitriptyline, imipraminee |
These agents have anticholinergic, direct smooth muscle
relaxant and norepinephrine re-uptake inhibition properties. |
| α-Adrenergic agonists |
Ephedrine, phenylpropanalamine, midodrin, pseudoephedrine |
Increase urethral tone and closure pressure by direct
stimulation of α-adrenergic receptors. |
| Afferent nerve inhibitors |
Dimethyl sulfoxide, capsaicin, resiniferatoxin |
Decrease sensory input from the bladder, thereby, increasing
bladder capacity and reducing bladder hyperreflexia. |
| Botulinum toxin |
Botulinum toxins A & B |
Direct injection into the urethral and bladder skeletal
and smooth muscle to treat detrusor-sphincter dyssynergia and overactive
bladder. Botulinum toxin has been demonstrated to relax the neuromuscular
junction of skeletal and smooth muscle. |
(Yoshimura N, Chancellor MB: Current and future pharmacological treatment
for overactive bladder. J Urology 168:1897–1913, 2002)
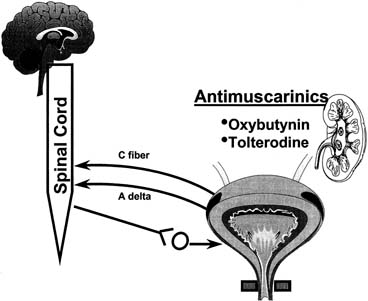 Fig. 7. Most commonly used drugs today to treat overactive bladder are anticholinergics. Despite
all components of lower urinary tract and nervous system
that control it, only anticholinergic drugs have been used in past 30 years
to treat overactive bladder. (From
Yoshimura N, Chancellor MB: Current and future pharmacological treatment
for overactive bladder. J Urol
168:1897–1913, 2002). Fig. 7. Most commonly used drugs today to treat overactive bladder are anticholinergics. Despite
all components of lower urinary tract and nervous system
that control it, only anticholinergic drugs have been used in past 30 years
to treat overactive bladder. (From
Yoshimura N, Chancellor MB: Current and future pharmacological treatment
for overactive bladder. J Urol
168:1897–1913, 2002).
|
Pharmacologic Treatment ANTICHOLINERGIC AGENTS Anticholinergics are competitive inhibitors of acetylcholine that block
muscarinic receptors and thereby inhibit involuntary bladder contractions (Fig. 7). All drugs of this class have similar side effects that, to some
degree, limit their usefulness. These include dry mouth, dry eyes, constipation, and
drowsiness. The prominence of these side effects is shown
in Table 9. General contraindications include closed angle glaucoma and obstructive
uropathy. Table 9. Pharmaceuticals for Overactive Bladder and Their Side Effects
| Drug |
Dosage |
Side Effect |
| Dicyclomine |
20 mg tid |
+++ |
| Flavoxate |
100–200 mg tid–qid |
++ |
| Hyoscyamine |
0.125 mg tid–qid |
++ |
| Imipramine |
10–50 mg bid |
+++ |
| Oxybutynin |
2.5–5.0 mg tid |
++++ |
| Oxybutynin XL |
5–20 mg qd |
+ |
| Propantheline |
15–30 mg tid–qid |
++++ |
| Tolterodine |
1–2 mg bid |
+ |
| Tolterodine LA |
4 mg qd |
+ |
From Cannon TW, Chancellor MB: Pharmacotherapy of the overactive bladder
and advances in drug delivery. Clin Obstet Gynecol 45:205–217, 2002
OXYBUTYNIN Oxybutynin was first approved in 1972 and is an antimuscarine with muscle
relaxant and local anesthetic activity acting mainly on (M3) subtype
receptors, which are responsible for the contractile properties
of the bladder. Efficacy is good (more than 50% symptomatic
improvement), reaching 61% to 86% at a dose
of 15 milligrams per day.67 Common side effects include dry mouth (salivary glands share an M3-type
receptor) and are directly proportional to the dose
administered. A once-daily, controlled-release oxybutynin
extended-release (XL) medicine has been released since
this topic was last reviewed.68 This drug uses a push–pull osmotic system of drug release that avoids the peaks of the immediate-release
formulation. Comparison studies with standard immediate-release
medication showed similar efficacy but reduced side effects.69 The decrease in side effects of the XL product may be explained by absorption
in the large intestine rather than stomach and small intestine, which
limits the formation of oxybutynin metabolites that may be responsible
for the side effects.70,71,72 TOLTERODINE Tolterodine was approved by the FDA in 1998. This drug is more bladder-selective, which
limits some side effects such as dry mouth. This
is desirable, because dry mouth was responsible for the approximately 50% drug
discontinuance rate in the past. In a meta-analysis
of trials involving 1120 patients, moderate to severe dry mouth
was reported in 6% of placebo, 4% of the 2 milligram per
day tolterodine, 17% of 4 milligram per day tolterodine, and 60% of 15 milligram per day tolterodine.73 In 2001, the long-acting formation of tolterodine was approved
by the FDA. This formulation has fewer side effects and similar or slightly
better efficacy than the twice-per-day formulation.74 Oxybutynin Versus Tolterodine Appell75 reported a study of 378 patients randomized to receive either 10-milligram
oxybutynin XL or tolterodine immediate-release (IR) twice
daily. Oxybutynin XL decreased the number of weekly episodes
of urge incontinence from 25.6 to 6.1. IR tolterodine decreased
the number of episodes from 24.1 to 7.8. Oxybutynin XL had better efficacy (p = .03) than IR tolterodine. There were no differences in the
side-effect profiles. The Antimuscarine Clinical Effectiveness
Trial (ACET) was recently reported by Sussman and Garely.76 This study consisted of two trials. Patients with overactive bladder were
randomized to treatment with either 2 milligram or 4 milligram of
tolterodine extended-release (TER) or oxybutynin extended-release (OER). A total of 1289 patients were enrolled. After 3 weeks
of therapy, 70% of patients in the TER 4-milligram
group improved compared with 60% in the OER 10-milligram
group. Response to therapy was greater in patients who
perceived their bladder condition as moderate to severe. Dry mouth was
dose-dependent with both drugs. Patients treated with TER 4 milligram
had a significantly lower severity of dry mouth compared with
OER 10-milligram users. Most recently, Diokno and colleagues77 reported results of the Overactive Bladder: Performance of Extended Release
Agents (OPERA) trial. The trial was a randomized, double-blind, active-control study at 71 United States centers
from November 2000 to October 2001. An extended-release formulation
of oxybutynin 10-milligram per day and extended-release
tolterodine 4-milligram per day were administered for 2 weeks
to 790 women with 21 to 60 urge incontinence episodes per week
and more than 10 voids per 24 hours. Women treated with both drugs showed
improvement in weekly urge incontinence episodes. Oxybutynin was more
effective at reducing frequency (p = .003) and 23% of the oxybutynin group reported no
episodes of incontinence versus 16.8% of the tolterodine group (p = .03). However, dry mouth was more common with oxybutynin (p = .02). Propantheline Propantheline bromide was an early drug with antimuscarinic activity. The
usual adult dose was 7.5 to 30 milligrams, three to four times daily. The
side effect profile makes this drug of minimal current use. Future Directions in Pharmacologic Therapy Darifenacin is a new drug currently undergoing phase 3 FDA trials. This
drug has an 11-fold higher affinity for M3 than for M2 receptors. It
was similar to atropine in blocking acetylcholine-induced
contractions in the guinea pig bladder and had a five-fold lower
affinity for parotid gland M3 receptors.78 Other Agents TRICYCLIC ANTIDEPRESSANTS These drugs classically act centrally and peripherally to block uptake
of serotonin and norepinephrine. While the exact mode of action is unknown, tricyclics
appear to have both anticholinergic and musculotropic
effects. This combination of effects makes this class of drugs especially
useful in patients with OAB and incontinence. Imipramine is the most common agent in this class of drugs and is usually
started at 25 milligrams per day. Dosing is increased by 25 milligrams
per week until efficacy is achieved or side effects are not tolerated. Cardiac
toxicity is the most important potential side effect. Additional
side effects include dry mucous membranes, sleep disturbances, personality
changes, weakness, and fatigue. Tricyclics should not be
stopped abruptly but tapered to avoid rebound depression. DESMOPRESSIN Desmopressin is a synthetic vasopressin analogue with strong antidiuretic
effects commonly used in children with enuresis. Desmopressin may decrease
urine output for approximately 6 hours, allowing the patient more
prolonged sleep.79,80 Fluid overload, hyponatremia, and subsequent seizures are possible severe
side effects. Currently, research is focused on a variety of other agents that may act
on the bladder via adrenergic, purinergic various neuropeptides and
tachykinins. While some of these agents show promise, they are considered
research tools at the present time. For a detailed discussion of current
knowledge of these agents, the interested reader is referred to
a very complete review by Yashimura and Chancellor.81 Intravesical, Transdermal, and New Alternative Drugs Several studies have established the efficacy of intravesical administration
of anticholinergics as an alternative to oral therapy. However, practicality
is not high, secondary to the need for intermittent self-catheterization. Agents so far studied include emperonium bromide, lidocaine, oxybutynin, and verapamil. Intravesical oxybutynin has
been successful in patients who have not been helped by oral therapy.82 Symptomatic improvement was 55% to 90% and side effects
were uncommon. As previously mentioned, transdermal and intravesical therapy
may produce significantly fewer side effects than oral therapy
secondary to lower blood levels of the oxybutynin metabolite desethyloxybutynin
produced by first pass in the liver and P450 metabolism in the
proximal gut.83 Transdermal oxybutynin is an exciting possibility for a new delivery system. Davila84 reported the use of transdermal versus IR oxybutynin in a randomized trial. The
authors observed similar efficacy with fewer side effects of
dry mouth compared with oral therapy. Intravesical Pump A recent novel delivery system to allow continued high intravesical levels
of drug without repeated instrumentation is the intravesical pump (Fig. 8). The reservoir must obviously not be too small to be voided but
not large enough to cause irritation or obstruction. The reservoir is
designed to release drug at a constant rate and, when empty, to be retrieved
by a flexible cystoscope.  Fig. 8. Bladder pump is currently under development to deliver constant dose of
oxybutynin intravesically for up to 30 days. Bladder drug delivery device
is inserted using catheter-based applicator and removed using
flexible cystoscope and grasper. (From
Yoshimura N, Chancellor MB: Current and future pharmacological treatment
for overactive bladder. J Urol
168:1897–1913, 2002). Fig. 8. Bladder pump is currently under development to deliver constant dose of
oxybutynin intravesically for up to 30 days. Bladder drug delivery device
is inserted using catheter-based applicator and removed using
flexible cystoscope and grasper. (From
Yoshimura N, Chancellor MB: Current and future pharmacological treatment
for overactive bladder. J Urol
168:1897–1913, 2002).
|
Intravesical Peppers The vanilloids such as capsaicin and resiniferatoxin activate nociceptive
sensory nerve fibers through an ion channel known as vanilloid receptor
subtype 1 (VR1).85 Intravesical capsaicin has been used recently with limited success in
patients with multiple sclerosis or spinal cord injury. Chancellor and
deGroat86 performed a meta-analysis of six series including 131 patients. Bladder
capacity improved and symptomatic improvement occurred in 72%. Resiniferatoxin
is approximately 1000-times more potent
than capsaicin.87 Both agents are vanilloid receptor agonists that result in desensitization; however, resiniferatoxin causes less discomfort. Botulinum Toxin Botulinum toxin was first isolated in 1897.88 The toxin acts by inhibiting acetylcholine at the presynaptic cholinergic
junction, resulting in decreased muscle contractility and muscle atrophy. This
appears to be reversible with axon regrowth in 3 to 6 months. Recently, botulinum
toxin has gained wide popularity in cosmetic
surgery. Dykstra89 and others reported on use of botulinum toxin to treat patients who have
detrusor dyssynergia secondary to spinal cord injury. Schurch90 reported increased bladder capacity and decreased mean maximum detrusor
voiding pressure in patients treated with toxin. Phelan91 recently reported a series of patients with voiding dysfunction who were
using indwelling or intermittent catheterizations. Improvement occurred
in 19 of 21 patients who were able to discontinue use of catheters. Behavior Modification and Pelvic Floor Exercises Kegel92 first described the use of pelvic floor muscle (PFM) exercises
to treat urinary incontinence in 1948. He reported a high success rate
of 84% in his study. This early study did not differentiate
between urge, stress, or other forms of incontinence; however, later
studies have demonstrated that PFM exercises may be effective in treatment
of symptoms of overactive bladder. The basis of such exercises is
the observation that electrical stimulation of PFM appears to inhibit
detrusor contractions. An early study by Godec93 demonstrated decreased bladder hyperactivity and increase in bladder capacity
after mild electrical stimulation, and deGroat26 noted increased sympathetic firing during bladder filling, representing guarding reflexes to promote continence. Bladder drills, bladder training, and multicomponent behavioral training are current extensions of Kegel's original work. The purpose of all
these treatments is to diminish symptoms and improve bladder control
through systematic changes in the patient's behavior. These therapies
have been recognized as efficacious and are recommended as first-line
therapy for incontinence in adults by the Agency for Health
Care Policy and Research.94 The bladder drill was an intensive procedure that required patients to
increase intervals between voidings. This was usually performed on an
inpatient basis. Studies in the 1970s and 1980s combined anticholinergics
and sedation with bladder training and reported a success rate of 82% to 86%.95 Similar success has been reported with randomized trials conducted as
an outpatient procedure.96 A modification of the bladder drill, termed bladder training is performed more gradually on an outpatient basis. The rationale is that
frequency and urgency are not only a result but also an initiating
factor for uninhibited detrusor contractions. Increased voiding frequency
leads to decreased bladder capacity and detrusor instability. Bladder
training attempts to break the cycle by requiring the patient to
resist urgency and postpone voiding until scheduled intervals. Pelvic Floor Exercises and Biofeedback Nygaard and associates97 recently reported use of pelvic floor exercises in the treatment of OAB. They
report a significant decrease in mean number of incontinent episodes
per day in a group of 14 women studied over 3 months. Fifty percent
of the patients described excellent or good results. It is important
that the patient be properly educated regarding pelvic floor anatomy
and assessed by a trained examiner for performance of exercises. The
typical protocol calls for 50 contractions per day in two or three divided
sessions. Each contraction is sustained for five seconds followed
by 10 seconds of relaxation. In some patients, biofeedback may be added to aid in identification of
appropriate muscle contractions. An intravaginal EMG probe is used to
sense pelvic musculature activity and is converted to a visual signal
for the patient on a computer screen or to an audio source. Multicomponent behavioral training uses biofeedback and other techniques
using the PFM in an attempt to inhibit bladder contraction. Combined
feedback of bladder pressure and PFM activity allows patients to visualize
detrusor contractions and respond with appropriate pelvic floor
muscle contractions that increase urethral pressure to prevent urine loss. Burgio98 conducted the first randomized trial comparing biofeedback-assisted
behavioral training with standard drug therapy consisting of immediate-release
oxybutynin chloride. Patients age 55 to 92 were randomized
to 8 weeks of behavioral treatment, drug therapy, or placebo. Drug
therapy improved 68.5% of patients and behavioral therapy
improved 80.7%, which was statistically significant. In this
study, 96.5% of the patients treated with behavioral therapy stated
they would be willing to continue therapy indefinitely, while only 54.7% of
those treated with drug therapy would continue indefinitely. Seventy-five percent of those using drug therapy stated
they would like another form of therapy compared with only 14% of
those on behavioral therapy. Although both behavioral and drug therapy are useful for reducing urgency, few
patients are cured by these therapies. Burgio98 showed only 23% of patients were dry after oxybutynin and 30% were
dry after a trial of behavioral training. Burgio99 reported a study that demonstrated combining drug and behavioral therapy
may be more effective than either therapy alone. A crossover design
allowing those patients not satisfied with the initial assignment to
use additional therapy was initiated. Of those initially assigned to behavioral
therapy, only 8 of 65 elected to add drug to their regimen. These
patients improved from 57% to 89% reduction in incontinence
after the addition of oxybutynin. However, 21 of 67 agreed to
add behavioral therapy after drug therapy. Of this group, 84% had
reduction with combined therapy compared with 72% with drug
therapy alone. In summary, research has shown that behavioral training is effective in
reducing urge-type incontinence. Addition of drug therapy to behavior
modification may increase the success further; however, few patients
are completely cured by these techniques. Neuromodulation for Refractory Overactive Bladder The scope of OAB will probably increase with the growing number of older
persons in the future. Included in this number will inevitably be a
group that will be refractory to usual therapy. For that group, neuromodulation
techniques may offer some relief. Neuromodulation techniques
are based on our current understanding of afferent and efferent neurological
pathways in bladder function. The bladder and pelvic floor receive
this innervation from S2, S3, and S4. Sacral parasympathetics provide
excitatory pathways balanced by thoracolumbar sympathetic inhibition. Current neuromodulation techniques are based on early experimental treatment
of urinary incontinence by Caldwell, with electrical stimulation
and subsequent experiments by Schmidt and Tanagho.100 Two devices are currently FDA-approved for peripheral sacral neurostimulation. The
Stoller Afferent Nerve Stimulator (SANS) device
was approved for use in 1999. The device uses a monopolar generator
to stimulate the posterior tibial nerve via an acupuncture needle
placed at the ankle. This area has been recognized as the bladder center by Chinese acupuncture and has nerve projections to S3. Govier101 reported a study of 53 patients treated on a weekly basis with this device
for 12 weeks. Based on urodynamic follow-up, voiding diaries, and
quality of life surveys, 71% of patients were classified
as successful. A 25% and 21% reduction in daytime and
nighttime frequency were noted, and urge incontinence was reduced by 35% in
previously refractory OAB patients. Klingler102 reported 18 patients with refractory urge frequency or pelvic pain syndrome. At 10 months, pain
was significantly reduced in most patients, OAB
was eliminated in 77% of patients, and bladder capacity was
increased from a mean of 197 to 252 milliliters. In addition, daytime
voids were reduced from 16.1 to 8.3 and nighttime voids were reduced
from 4.4 to 1.4. Complications are rare; however, the technique does require
multiple patient visits. The Interstim Sacral Nerve Stimulation System (Medtronics, Minneapolis, MN) was
approved by the FDA for urgency/frequency and nonobstructive
urinary retention in 1999. The device consists of an implantable
neurostimulator attached to electrodes directed through the S3 foramen
to stimulate sensory afferents. Patients initially undergo either
bilateral needle test stimulation or unilateral staged implant before
permanent placement of the device. Jankneqt103 reported a group of 96 refractory patients treated with the device in 2001. The
treated patients demonstrated a significant reduction in number
of urge incontinence occurrences and number of pads used. Siegel104 has reported one of the largest multicenter trials of the device in 260 patients
from a second group of 581 with urge incontinence, urgency/frequency, and
urinary retention. At 18 months, 76% of patients
with urge incontinence were deemed successful while urgency/frequency
responded in 63% of patients. Patients with urinary retention
responded successfully in 71% at 18 months, defined as
elimination or more than 50% reduction in need of catheterization. Kohli and Rosenblatt105 in a recent article have described a good step-by-step treatment
algorithm that uses a complete history and physical examination, appropriate
office testing, and treatment of patients with OAB (Fig. 9). For a complete summary of current neuromodulation techniques, the
reader is referred to Kohli and Rosenblatt.105 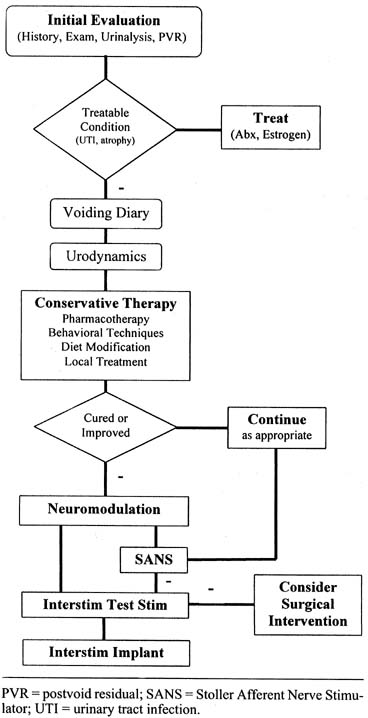 Fig. 9. Treatment algorithm for overactive bladder syndrome. PVR, postvoid residual; SANS, stoller
afferent nerve stimulator; UTI, urinary tract infection. (From
Kohli N, Rosenblatt PL: Neuromodulation techniques for the treatment of
the overactive bladder. Clin Obstet Gynecol
45:218–132, 2002). Fig. 9. Treatment algorithm for overactive bladder syndrome. PVR, postvoid residual; SANS, stoller
afferent nerve stimulator; UTI, urinary tract infection. (From
Kohli N, Rosenblatt PL: Neuromodulation techniques for the treatment of
the overactive bladder. Clin Obstet Gynecol
45:218–132, 2002).
|
In summary, neuromodulation techniques hold promise as an alternative therapy
for OAB cases that do not respond adequately to conventional pharmacotherapy
or behavioral techniques. Future research should continue
to further elucidate our understanding of the mechanisms of action and
how electrical stimulation may modify somatic afferent inhibition of
bladder stimulation. |