Symmonds recommends several surgical principles to improve the success
rate of any technique for VVF repair: wide mobilization of the bladder, excision
of all scar tissue at the risk of increasing the size of the
fistula in an attempt to create a “fresh bladder injury, ” a
tension-free layer closure of the bladder and the vagina, nontraumatizing
technique, and good hemostasis with complete bladder drainage
postoperatively.28 Most authors agree that the best chance at closure of the fistula is at
the first attempt. In general, the vaginal approach avoids the potential morbidity associated
with abdominal surgery and is believed to provide a quicker recovery
and a more cosmetic result. Lee and colleagues2 and Tancer9 report vaginal success rates of 98% and 100%, respectively. However, there
are circumstances for which an abdominal approach has
traditionally been favored: larger fistulas; fistulas located high
on the posterior wall; fistulas adjacent to the ureters, where improved
exposure is necessary; to correct concurrent intraabdominal pathology
if it exists; and in cases in which the vaginal vault is small, precluding
a vaginal approach. Involvement of the ureter may require an abdominal
approach to facilitate reimplantation or the placement of stents. Patients
with a small or contracted bladder or a very large fistula
may require augmentation with bowel. A combined vaginal and abdominal
approach can be helpful in certain circumstances. The authors have described
a combined approach to the repair of a large fistula secondary
to a vaginal foreign body that involved the trigone.29 Vaginal Repair Patient positioning is a matter of physician preference. The patient is
examined under anesthesia. The ureteral orifices may be catheterized
cystoscopically if there is concern about the ureters, especially if they
lie at the edge of the fistula or if the fistula is high in the vaginal
vault. Labial retraction sutures and an episiotomy or Schuchardt
incision may be helpful for a small introitus and vaginal vault. Placement
of stay or traction sutures at the margins of the fistula or the
insertion and inflation of a Foley or Fogarty catheter helps to identify
the fistula’s edges and may bring the tract closer to the surgeon. Some
fistula surgeons infiltrate the vaginal mucosa with saline
solution or a dilution of epinephrine (1:200,000) to aid dissection
and decrease oozing. There is no consensus on the need to excise the fistulous tract. Some authors
advocate its total removal, whereas others prefer not to débride
the margins, thereby avoiding an increase in the size of the
defect. Iselin and colleagues advocate the excision of the fistula tract
and vaginal cuff scar, enabling the surgeon to suture viable tissues
in every layer to promote wound healing, obviating the need for interpositional
flaps or grafts.30 They had a 100% cure rate on first attempt. In his series of 65 transvaginal
repairs, Raz did not excise the fistula tract in any patient
and had no apparent adverse effects.31 Cruikshank did not excise the tract in his series of 11 patients and had
a 100% cure rate.32 Zacharin warns that excision of the fistula scar markedly increases the
risk of operative failure.33 Elkins and coworkers34 and Lawson35 also advised against excising the tract in large obstetric fistulas. The two transvaginal techniques commonly performed are the flap-splitting
technique and the Latzko procedure. The flap-splitting technique involves
wide mobilization of the vaginal mucosa from the edge of the fistula. The
bladder is closed in two layers. The first is submucosal with
interrupted Lembert sutures. A second layer is used to close the muscularis
and reduce tension on the first suture line. If the defect is
in the trigonal area, the repair should be in a transverse direction, because
a vertical closure may draw the ureters to the midline and lead
to kinking or obstruction. The authors prefer to close the vagina using
interrupted sutures to preserve vaginal length. The flap-splitting
technique does not foreshorten the vagina and has a success rate equivalent
to that of the Latzko procedure. Martius36 described the use of this technique for small fistulas and Raz37 reported a 100% cure rate in his series of twenty patients. Disadvantages
include the possibility of mobilizing the tissues too much, leading
to avascular necrosis of suture lines or incorporating the ureters
into the closure.
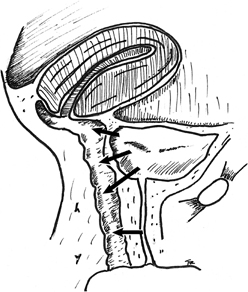
The Latzko partial colpocleisis is championed by many38,39
(Fig. 2). An elliptical
portion of vaginal mucosa is mobilized around the fistula tract, at least
2.5 cm in all directions. The pubovesical fascia and vaginal mucosa are
closed in layers, using interrupted sutures. The vesical edges of the
fistula are not denuded. The posterior vaginal wall becomes the posterior
bladder wall and reepithelializes with urothelium. Because the bladder
musculature is not sutured to itself, there is no tension across the suture
lines. Success rates greater than 89% are reported for the first
attempt. Other advantages include short operating time, minimal blood
loss, and low postoperative morbidity. It is especially effective in patients
with radiation-induced fistulas. Disadvantages include a loss of vaginal
length and possible interference with sexual function.
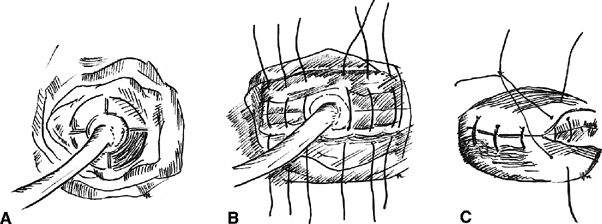 Fig. 2. The Latzko procedure. A. The vaginal epithelium is removed by quadrant. A Foley catheter is placed
within the fistula for traction. B. A deep layer of sutures is placed in the transverse axis. C. A second layer of sutures is placed, imbricating the first. The vaginal
mucosal is later closed over the repair. Fig. 2. The Latzko procedure. A. The vaginal epithelium is removed by quadrant. A Foley catheter is placed
within the fistula for traction. B. A deep layer of sutures is placed in the transverse axis. C. A second layer of sutures is placed, imbricating the first. The vaginal
mucosal is later closed over the repair.
|
Interposition flaps or grafts may be used in larger or recurrent fistulas
or those involving the urethra or bladder neck. Pedicle flaps bring
additional blood supply, improve the lymphatic drainage, and distance
suture lines. In 1928, Martius first described the use of the labial
fat pad as an interposition graft.40 The vascular supply to the graft inferiorly is from the internal pudendal
artery and superiorly is the external pudendal artery (Fig. 3). The key is the appreciation and preservation of one of these vascular
bundles. Birkhoff and associates reported a 100% success rate
in six patients with transvaginal repairs of VVF using the Martius technique.41 Elkins and colleagues reported a success/closure rate of 96% (24 of 25 procedures) in
a series of mostly postobstetric injuries.42 Alternatively, a gracilis muscle flap may be used as first described by
Ingelman-Sundberg,43 and later modified by Hamlin and Nicholson.44 The technique involves dissection of the gracilis muscle and separation
of its attachment to the medial condyle of the femur. The blood supply
to the muscle from the femoral artery is preserved, and the graft may
be tunneled to the introitus and sutured into place at the fistula. 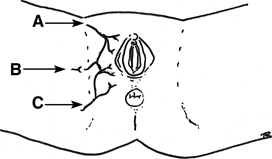 Fig. 3. Martius pedicle blood supply. A. External pudendal artery. B. Branch of the obturator artery. C. Internal pudendal artery. Fig. 3. Martius pedicle blood supply. A. External pudendal artery. B. Branch of the obturator artery. C. Internal pudendal artery.
|
The repair should be watertight and tested with the instillation of methylene
blue or indigo carmine into the bladder. The authors prefer to
leave a pack in the vagina for 24 hours postoperatively and continuously
drain the bladder with a 16 French Silastic suprapubic catheter for 3 weeks
and discontinue the catheter after the patient successfully passes
voiding trials. The patient is maintained on prophylactic antibiotics
while the catheter is in situ.
In general, the repair of urethrovaginal fistulas is often more difficult
than the closure of VVFs and may leave the patient incontinent. It is
generally reported that the success rate of urethrovaginal fistula repair
is 73% to 100%.45,46
The same principles of VVF repair apply to the repair of urethrovaginal
fistulas: wide mobilization of tissue planes, layered closure, and the
use of interposition grafts when appropriate.
Noble originally described urethral reconstruction using bilateral vaginal flaps formed into a tube around a catheter.47 Skin grafted from the labia aided in maintaining cosmesis. Birkhoff and
associates believe that the reestablishment of continence is facilitated
by the use of the Martius interposition flap.41 In addition, Gray found that 50% of his patients were incontinent
postoperatively without this added intervention.45 Moir described using suburethral buttressing sutures to aid with recovery
of continence.48 Symmonds and Hill achieved continence in 37 of 50 patients (74%) with
significant urethral destruction by constructing a neourethra, using
smooth muscle that remains in the urethral “roof” and
the creation of a Martius-type flap to aid with tension-free closure.49 They advocate delaying a retropubic suspension because concomitant suspension
may lead to disruption or attenuation of the suburethral repair
or devascularize the repair. There may also be difficulties associated
in preselecting patients who would go on to require a subsequent suspension. Leach
has described a simultaneous needle suspension for patients
in whom stress incontinence is associated with a urethrovaginal
fistula.50 A pedicle buttock flap has also been described for the repair of larger
urethrovaginal fistulas.51 Fernandes and coworkers have reported the use of an anterior advancement
flap of bladder and turning it into a tube to reconstruct an entire
urethra.52 Patch grafts of bladder mucosa have also been used with satisfactory results
for urethral reconstruction in a series by Omo-Dare.53 Abdominal Repair The patient should be in low lithotomy position with vaginal access. Incision
type is also a matter of physician preference. The advantage to
a low midline incision is that omentum can be mobilized for a graft, and
it can be extended easily, although access to the omentum can be achieved
with Maylard or Cherney incisions. Simple fistulas can be repaired
transvesically (extraperitoneal), but an intraperitoneal approach
is preferable for more complicated fistulas.
In the transvesical technique, the bladder is opened at the dome, the
fistula is excised, and the bladder muscularis is mobilized off the vagina.
The defects are then closed in layers. Exposure may be difficult, and
because of this, many surgeons prefer the intraperitoneal approach. Much
of the early work with this technique was pioneered by O’Conor
and associates54,55,56
(Fig. 4). The
bladder is bisected vertically down to the fistula tract. All scar is
excised, and the bladder is widely mobilized off the vagina. The bladder
and vagina are closed in layers. Whereas the O’Conor technique
requires extensive dissection, Ostad and colleagues described an abdominal
approach to repair with a free bladder mucosal graft in a series of six
patients.57 There is reduced need for extensive
dissection, and it is useful for repairs close to the ureters and in large
or recurrent defects.
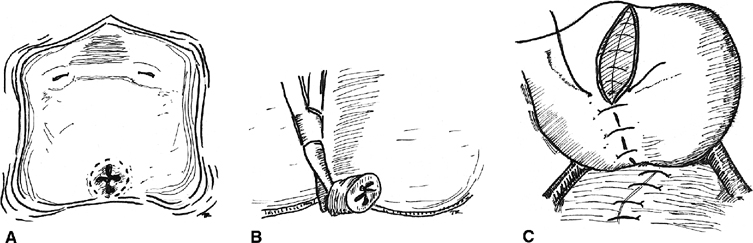 Fig. 4. Abdominal repair (after O’Conor). A. The bladder is bivalved, thus exposing the fistula, B. The fistula tract is excised, including the vaginal epithelium. C. The bladder and vagina are closed in layers. At this point. an omental
flap may be interposed. Fig. 4. Abdominal repair (after O’Conor). A. The bladder is bivalved, thus exposing the fistula, B. The fistula tract is excised, including the vaginal epithelium. C. The bladder and vagina are closed in layers. At this point. an omental
flap may be interposed.
|
The use of vascularized tissue grafts can be helpful in ensuring a successful
repair. One of the more popular graft sources is the omentum (Fig. 5). It has a dual blood supply, and some surgeons recommend dividing the
splenic vessels on the left to help with mobilization. Others prefer
to base the pedicle on the gastroduodenal vessels of the right side. Rectus
muscle has also been used as graft for abdominal repairs. In a recent
review, the use of interposition flaps was evaluated for VVFs of
benign and malignant etiologies.58 All repairs with interposition grafts were successful without regard to
etiology, whereas only 63% and 67% of repairs were successful
for benign and malignant fistulas, respectively. The authors recommended
that transabdominal VVF repairs be performed with an interposition
flap regardless of the appearance of healthy surrounding tissues
and etiology. 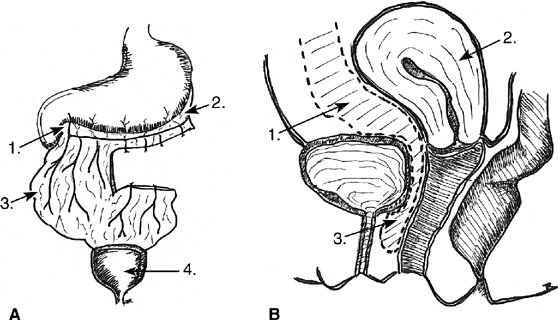 Fig. 5. Omental graft. A. Mobilization of an omental graft is demonstrated. 1. Right gastroepiploic
artery. 2. Left gastroepiploic artery. 3. The omental pedicle. 4. Bladder. B. Interposition of the graft. 1. Omental flap. 2. Uterus. 3. Omental flap
sutured between the bladder and the vagina. Fig. 5. Omental graft. A. Mobilization of an omental graft is demonstrated. 1. Right gastroepiploic
artery. 2. Left gastroepiploic artery. 3. The omental pedicle. 4. Bladder. B. Interposition of the graft. 1. Omental flap. 2. Uterus. 3. Omental flap
sutured between the bladder and the vagina.
|
Minimally Invasive Repair Several minimally invasive approaches have been reported to be successful
in closing VVFs smaller than 8 mm. Fibrin occlusion of a VVF was first
reported by Pettersson and coworkers in 1979.59 To protect the fibrin plug, an indwelling urethral catheter was left in situ for 8 weeks, and the patient was give tranexamic acid to block fibrinolytic
activity for 8 weeks. In a recent case report, Morita and Tokue
describe a successful endoscopic closure of a 5-mm radiation-induced VVF
with fibrin glue and bovine collagen.60 This required electrofulguration of the tract. Stovsky and associates
retrospectively reviewed a series of 15 patients who were treated with
electrocoagulation for VVF of a few millimeters or less.61 Fulguration was successful as the sole treatment modality in 9 of 12 patients (75%). Falk
and Orkin reported on 10 patients treated by
fulguration with a Bugbee electrode followed by 10 days of catheter
drainage.62 All patients with fistulas of 3 mm or less in size were cured, whereas
fulguration in 2 patients with 6-mm fistulas failed. Novel laparoscopic suturing techniques led McKay to try transurethral suture
repairs of VVFs.63 By transurethally inserting a 5-mm laparoscopic sleeve alongside a 17 French
cystourethroscope with a 30-degree lens and utilizing an extracorporeal
knot tying technique, he was successful in both patients in his
series who had failed a previous procedure. Miklos and colleagues described
the interposition of an omental flap utilizing a laparoscopic
approach for a recurrent VVF that had persisted despite two operations
using the Latzko colpocleisis and prolonged catheterization.64 Dogra and Nabi described a case of a small VVF following abdominal hysterectomy
in which closure was achieved using endoscopic neodymium:yttrium-aluminum-garnet (Nd-YAG) laser fulguration.65 Aycinena reported the use of an ordinary metal screw forced through the
fistula from the vagina into the bladder as a means of denuding the epithelium
from the fistulous tract and promoting healing.24 Seven patients with small fistulas were successfully managed with this
technique. Timing of Repair
The timing of repair remains controversial. The traditional belief is
to wait a minimum of 3 months after hysterectomy or the last attempt at
repair, because inflammatory or necrotic fistula margins have been judged
responsible for surgical failure. Others propose that early intervention
is safe. Collins and coworkers, in a series of 38 patients all of whom
had a transvaginal repair within 60 days after diagnosis, reported a 28%
failure rate.66 Persky and associates have
also been advocates for early repair, and they reported a series of seven
patients who underwent successful fistula repair within 7 to 10 days of
the antecedent surgery.67 Cruikshank also
had success in 10 of 11 fistulas in his series repaired between 10 to
35 days after a hysterectomy.32 The average
time to fistula repair in Iselin’s series was 16 weeks with 100%
cure rate.30 Wein has attributed surgical
failures to an inadequate delay until repair.7,10
Lee and colleagues recommended delaying surgery to increase the success
rate of the first attempt at repair.2
The optimum time for repair involves resolution of necrotic tissue and
inflammatory changes and stabilization of the size of the fistula. Higher
failure rates are associated with repairs on tissue that is acutely
inflamed, edematous, ischemic, or necrotic. Scar excision may allow
earlier repair because the inflamed cuff margins may be cut back to healthy
tissue. Some fistulous communications recognized immediately postoperatively
or after trauma with minimal inflammatory changes may lend
themselves to early repair. In irradiated tissues, the interval to repair
should be longer. Most agree that treatment should primarily be tailored to the individual
patient. If the fistula is recognized within the first 48 hours postoperatively, the
tissue should be more mobile, have less inflammation, and
be amenable to early repair. Fistulas arising several days after
surgery are frequently accompanied by significant edema, inflammation, and
induration, making early repair less successful. An interval of 3 months
from injury to repair in obstetric and surgical fistulas will
allow the inflammatory reaction to subside before proceeding with repair. Up
to 1 year may be required for improvement in the tissues in radiation-induced
fistulas prior to repair. Prolonged catheterization may
also lead to spontaneous closure in small fistulas, but the outcome is
unpredictable. Large fistulas may be easier to repair once the tract
is allowed to scar. Conversely, delay of closure may have a negative
impact on the quality of life of the patients. |