The anatomy and physiology of the anal canal have been well studied. Current literature expands upon Shafik's original work on the triple loop theory.12,13,14,15,16 The anal canal measures 2.5 to 4 cm in length and extends from the mucocutaneous junction caudally through the dentate line, above which columnar colonic mucosa is found. Here the anal mucosa is composed of cuboidal cells formed into 8 to 14 folds called the rectal columns of Morgagni, which overlie an internal hemorrhoidal plexus. The arterial blood supply originates from the superior hemorrhoidal artery (the terminal branch of the inferior mesenteric artery) and from the middle and inferior hemorrhoidal arteries, which arise from the anterior division of the internal iliac artery. The venous drainage returns to the portal system by way of the superior hemorrhoidal and inferior mesenteric veins. The middle and inferior hemorrhoidal veins return to the internal iliac system. The boundaries of the anal canal are the coccyx posteriorly, with interposed adipose and muscular tissues; and the ischiorectal fossa, with Alcock's canal laterally.
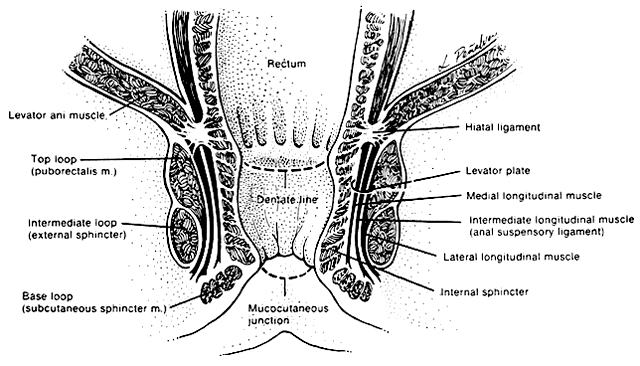
The anal canal is surrounded by two major muscle groups, the involuntary internal and the voluntary external sphincter systems (Fig. 1) These two systems provide a fine balance between continence and controlled intermittent evacuations of fecal material. The capacity to distinguish between flatus and variable stool consistency is achieved through a complicated integration of pelvic musculature.
|
The involuntary internal anal sphincter is composed of autonomically innervated circular smooth muscle fibers that lie within the wall of the anal canal. These fibers remain in a steady state of contracture, yielding tone to the anal canal and maintaining it in a collapsed position when nonfunctioning. As the rectal ampulla fills and distends, the sensory stimulus for evacuation begins the process of defecation. The contribution of the internal sphincter to anal continence has been studied with magnetic resonance imaging.6 In this study, the internal sphincter was found to contribute 54% of the anterior thickness of the sphincter in nulliparous women. Application of this knowledge has important implications in the repair of third- and fourth-degree perineal lacerations and contrasts with the traditional understanding portrayed in the medical literature.
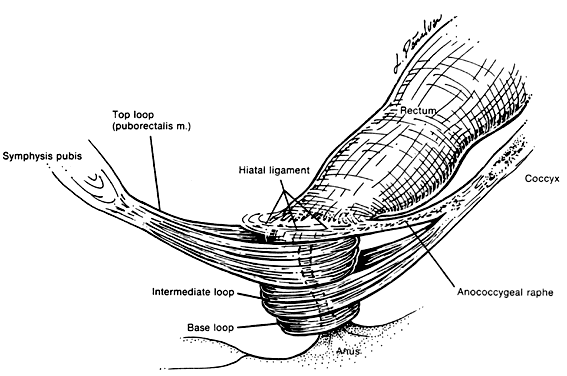
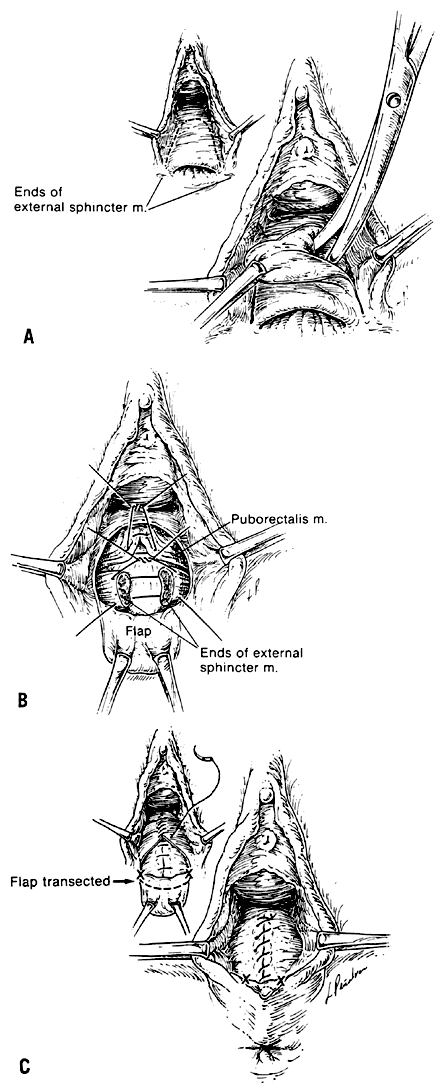
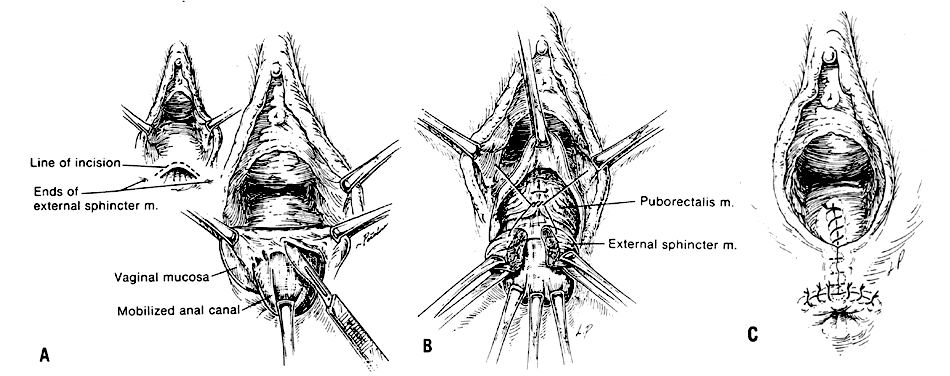
The voluntary external anal sphincter (Fig. 2,) composed of multiple interrelated skeletal muscle loops (separated by their fascial coverings), lies in close approximation to the levator ani and the muscles of the urogenital diaphragm. This sphincter system contains three muscular loops that surround the anal canal. The top loop is the deepest and fuses with the puborectalis muscle of the levator ani group. It attaches to the pubic symphysis and encircles the colon posteriorly at the anorectal verge. Innervation is provided by the inferior hemorrhoidal branch of the pudendal nerve. This superior loop functions to angulate the anal canal forward in order to maintain continence. The intermediate loop portion of the external sphincter is found anteriorly around the midportion of the anal canal as a fleshy muscle. These fibers terminate posteriorly in an interdigitating fashion and attach to the tip of the coccyx in a fibrous tendon. On voluntary stimulation by the perineal branch of S4, contraction of this intermediate sphincter retracts the anal canal posteriorly to align with the rectum. The third and most superficial muscle of this complex sphincter system is the base loop. The smallest of the three loops, it is composed of skeletal muscle fibers that completely encircle the anal canal distally and attach to the mucocutaneous junction. It is innervated by the inferior hemorrhoidal nerve. These three muscle groups function in concert to provide the capacity for both continence and defecation (Fig. 3.)
|
|
Just above the top loop and puborectalis muscles is the pubococcygeus muscle of the levator ani group. Its circular skeletal muscle fibers extend from the pubic bone to the coccyx, where they fuse as decussating tendons called the anococcygeal raphe. The fleshy, muscular portion of the pubococcygeus encircles the hiatal space, through which pass the pelvic viscera. The pubococcygeus muscle adheres to the colonic wall by dense fascial fibers called the hiatal ligament. Muscular traction on the hiatal ligament effects posterior circumferential distention and elevation of the anal canal.
Between the internal sphincter and the three circular muscle loops of the external sphincter lie the longitudinal fibers of the levator plate. These muscle fibers anchor the U-shaped external sphincter loops to the anal wall and perianal skin. Longitudinal bundles of muscle fibers descend from the pubococcygeus muscle between the internal sphincter and the three loops of the external sphincter system. The most medial smooth-muscle group, the medial longitudinal muscle, attaches the levator plate to the anal canal wall, actually fusing with the wall. The intermediate longitudinal muscle group, also called the suspensory ligament, inserts as the central tendon that attaches above the external anal sphincter base loop and the perianal skin. The lateral longitudinal muscle bundle is a condensation from the top loop of the external anal sphincter, which attaches to the medial surface of the intermediate loop, inserting just above the base loop. These three longitudinal muscle bundles provide continuity and attachments between the internal and external sphincter systems.
Interspersed among these muscle fibers are six perirectal spaces. Four intersphincteric spaces lie among the three longitudinal muscle layers and separate their dense fascial coverings. Between the skin and the base loop is the subcutaneous space, which is continuous with the perirectal spaces. The central space is low around the anal canal and communicates with each of the surrounding spaces. It separates the base loop from the intermediate loop and is an insertion site for the longitudinal muscle tendon bundles. These potential spaces play an important role in the development and repair of perirectal abscesses and rectocutaneous fistulae.
Physiology of the Sphincter Systems
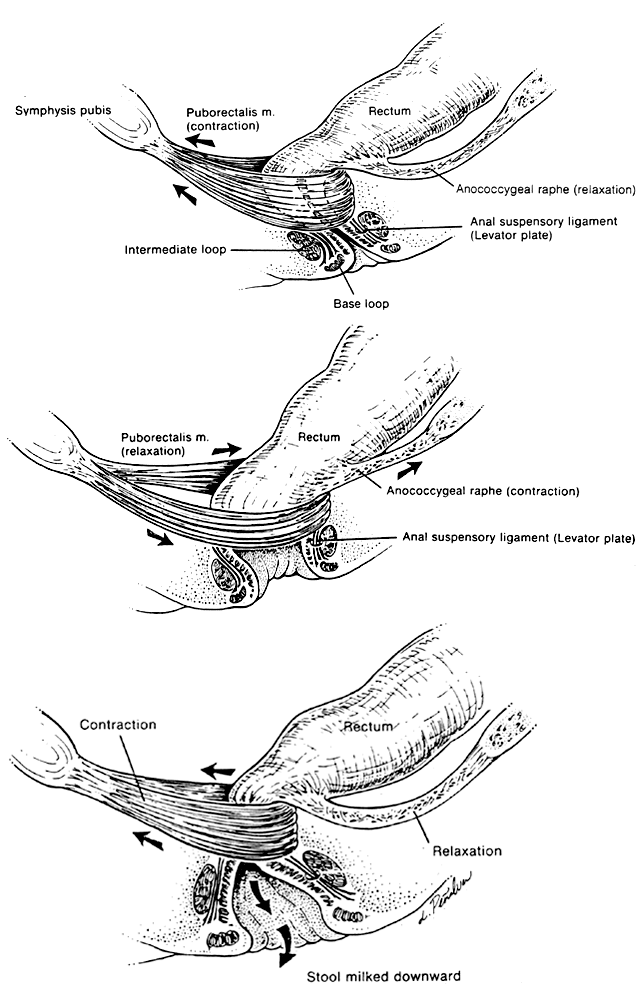
Much investigation has centered on the mechanism of defecation.17,18,19,20 Electromyography, manometric pressure testing, and radiography have been used to explore the function of these muscle systems. When the rectal ampulla fills and distends, the signal for defecation is triggered (see Fig. 3.) Defecation is initiated by Valsalva's maneuver, which increases intra-abdominal pressure. In a reflex manner, the internal anal sphincter must relax to accommodate the bolus. Simultaneous voluntary relaxation of the top loop of the external anal sphincter allows the anal canal to relax posteriorly. Voluntary contraction of the levator plate and the longitudinal muscle bundles acts to widen the anal inlet by means of traction on the hiatal ligament. This action simultaneously shortens the canal length, flares the base loop of the external sphincter outward, and opens the anal orifice. The passage of fecal material distally is completed by intermittent vermicular contractions of the intermediate and base loops, which compress the flow outward. Once emptying is complete, the external loops, anococcygeal raphe, and levator plate all relax, returning to their normal positions, while the puborectalis muscle and the top loop contract, repositioning the canal forward. The process is completed with reflex contraction of the internal sphincter system to recollapse the anal canal, thereby maintaining continence.
Maintenance of a minimal baseline anal pressure is necessary for fecal continence. The internal sphincter contributes approximately 50% of the force necessary to keep the anus closed when empty, and the external sphincter 25% to 30%. Variable filling of the internal hemorrhoidal plexuses provides the remainder of the pressure required to seal the anus by conforming around the sphincters.21 As the anus is distended, the contribution of the internal sphincter declines progressively, while stretching of passive canal wall elements other than the sphincters limits capacity and provides the force to maintain continence. The importance of the involuntary internal sphincter and the role of autonomic dysfunction in fecal incontinence can therefore be understood. Manometric evaluation reveals a distinctive pressure profile of the anal canal resulting from the combined effects of the different muscles that constitute the anal sphincters.22 In the upper anal canal, radial pressure measurements indicate a lower pressure anteriorly, corresponding to the posterolateral location of the puborectalis muscle. In the distal anus, the pressure is less posteriorly because of the orientation of the intermediate loop.
MEASUREMENT OF ANORECTAL PRESSURE.
Simple manometric pressure measurements in some incontinent patients do not always distinguish true deficiencies. More sophisticated techniques involving measurement of anorectal pressure gradients or three-dimensional manometry (vector manometry) may prove more accurate, but are not widely available.23,24
Biofeedback techniques using visual manometry have been successful in the treatment of some patients with fecal incontinence.25 Of motivated patients, 50% to 80% experience improvement in symptoms. An increase in both resting and squeezing pressures can be demonstrated.26 The efficacy persists for at least 5 years in successfully treated patients.27
Of women with idiopathic fecal incontinence, 75% to 80% have evidence of nerve injury to the pelvic floor musculature.28 Such patients have slowing of nerve conduction, which is measured as pudendal nerve terminal motor latency (PNTML) on electromyographic tests.29 Approximately 60% of patients with sphincter injury have concomitant pudendal neuropathy.28 The contributing role of vaginal delivery is underscored by the finding that 42% of women demonstrate slowed conduction in the pudendal nerve (prolonged PNTML) immediately after delivery, of whom 60% recover by 2 months.30
Fiber density, an electromyographic index of the number of muscle fibers innervated by a single nerve axon, is increased in anal incontinence. This effect is believed to result from injury, causing denervation with subsequent reinnervation. Increases in PNTML and fiber density are present in incontinent patients and are associated with aging, multiparity (if delivered vaginally), increased birth weight, and a long duration of the second stage of labor.30 Increases are not found after episiotomy or first- and second-degree perineal lacerations, but do occur after third- or fourth-degree obstetric perineal tears. Patients with anal incontinence have decreased sphincter length, lower maximum resting pressure, and decreased maximum voluntary contraction pressure. Such patients also have decreased sensitivity to electrical stimulation and temperature sensation in the anus.31,32
In normal, continent patients, sensations of temperature and pressure from stool or flatus in the anal canal are better appreciated and evoke an increase in sphincter tone.33 Differences in sensation are postulated to contribute to the difficulty that incontinent patients typically experience in differentiating flatus from solid stool. Pudendal neuropathy is most accurately assessed by measurement of PNTML; however this test is not widely available and may be inaccurate unless performed by an experienced clinician. Manometry is not thought to be useful in the determination of pudendal nerve malfunction.34 One preliminary prospective, controlled trial demonstrates that a component of routine manometry, the rectoanal excitatory reflex, may prove useful in this diagnosis.35 The recognition of pudendal nerve injury and the distinction of this disorder from sphincter injury is vital.
Defecography (evacuation proctography), a radiographic technique to evaluate defecation, can demonstrate rectocele, enterocele, or rectal intussusception, but does not aid in the evaluation of anal incontinence.36,37
Anal endosonography has recently been applied to the evaluation of fecal incontinence. The normal anatomy of the external and internal sphincters as imaged by ultrasound has been described.38 Comparison of anal ultrasonography with anal manometry in normal volunteers demonstrates no correlation of sphincter muscle thickness with squeeze pressure.39
However, defects in the external and/or internal anal sphincters demonstrated ultrasonographically correlate with manometric findings.40,41 In addition, electromyographic mapping of sphincter abnormalities confirms anal endosonographic results.41,42,43 A prospective study of 127 women compared anal manometry and anal ultrasonography before and after vaginal delivery.44 Sphincter defects can be demonstrated by anal ultrasound in 25% of incontinent women believed to have intact sphincters on the basis of a digital examination. This discrepancy may be related, in part, to difficulty in distinguishing scar tissue during the examination. Ultrasound characteristics of scar tissue differ from those of normal muscle.40,46 Incontinent patients with normal manometry may also have sphincter abnormalities seen on ultrasound examination.46 Finally, a small number of patients with idiopathic incontinence resulting from pudendal neuropathy and confirmed by abnormal PNTML will have coexistent sphincter defects.41,43,46 Thus, anal endosonography can provide information that may not be obtained by digital examination or traditional testing, and this information is valuable for those making treatment decisions.