Functional Ovarian Processes FOLLICULAR MATURATION. As noted earlier, in addition to the delineation of specific ovarian masses, follicular
maturation may be monitored by serial transabdominal
or transvaginal ultrasonography.69 Preovulatory follicular development is seen clearly with progression of
follicle diameter to the 18 to 22 mm range at midcycle. Follicular dominance
and progression are monitored easily by real-time ultrasonography
in correlation with serum estradiol concentration to induce ovulatory
ovarian function70–75 (Figs. 32 and 33). Transvaginal-directed oocyte retrieval for assisted reproductive techniques
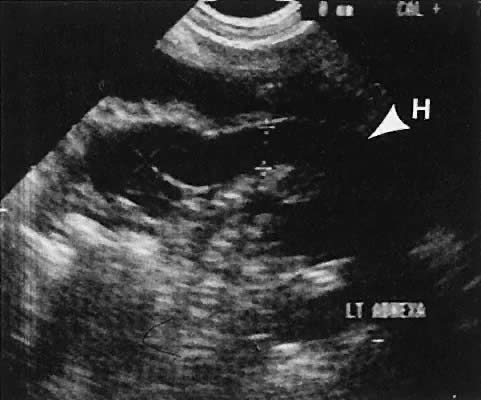
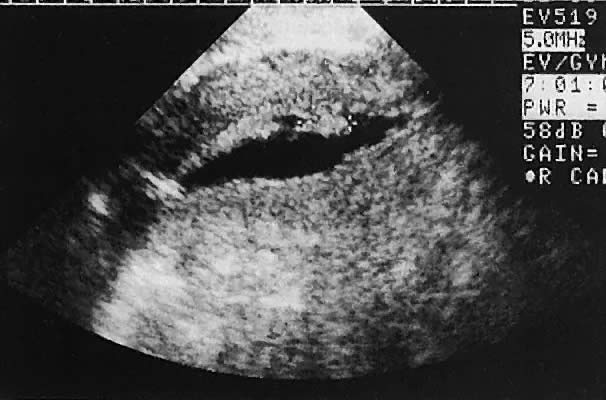
is the preferred method of acquiring oocytes. 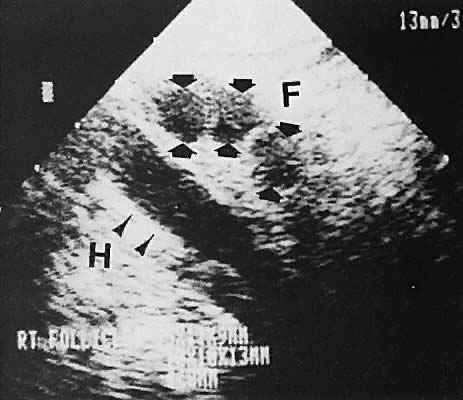 Fig. 32. Preovulatory follicular maturation, clomiphene stimulation. Transvaginal
scan depicts three follicles (F) delineated by wide arrowheads; internal
iliac vein (H) is noted by thin arrowheads. Fig. 32. Preovulatory follicular maturation, clomiphene stimulation. Transvaginal
scan depicts three follicles (F) delineated by wide arrowheads; internal
iliac vein (H) is noted by thin arrowheads.
|
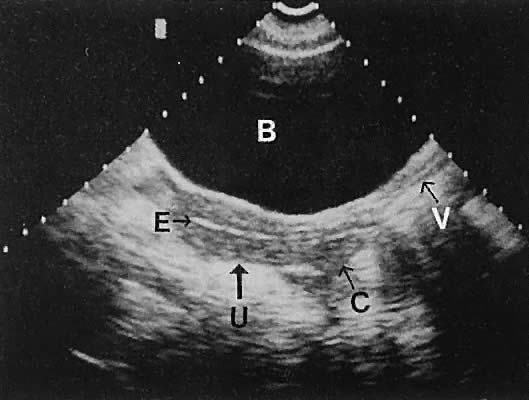
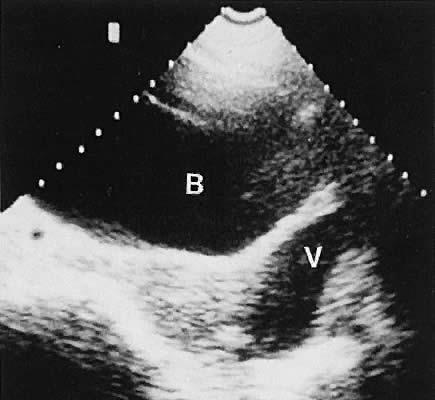
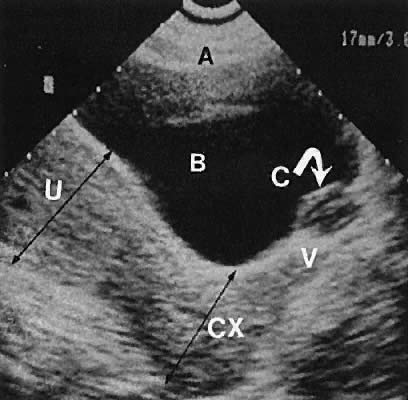
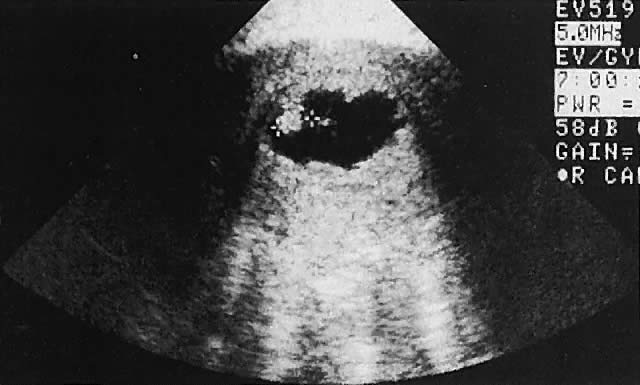
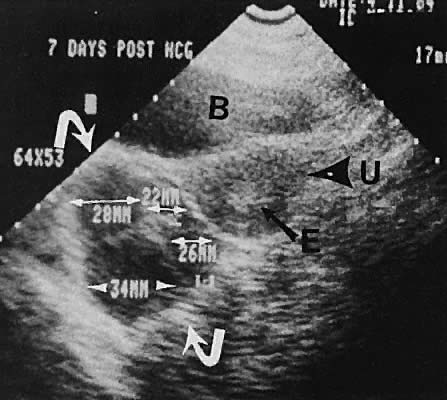 Fig. 33. Multiple follicles stimulated by gonadotropin therapy. Transabdominal scan, transverse
plane, depicts bladder (B), uterus (U), and endometrium (E). Curved
white arrows delineate the ovary, with smaller arrows showing
four follicles in the 22- to 23-mm range. Fig. 33. Multiple follicles stimulated by gonadotropin therapy. Transabdominal scan, transverse
plane, depicts bladder (B), uterus (U), and endometrium (E). Curved
white arrows delineate the ovary, with smaller arrows showing
four follicles in the 22- to 23-mm range.
|
POLYCYSTIC OVARY SYNDROME. Polycystic ovary syndrome produces a wide spectrum of menstrual disturbances
and manifestations of androgen excess. The ovary also may exhibit
a variety of appearances, ranging from multiple cystic follicles (up
to 2.5 cm) of varying sizes to only a few follicle cysts (larger than 2.5 cm)76–82 (Fig. 34). In cases of hyperthecosis, in which fewer follicles are developed, the
ovary would be expected to exhibit a more solid echo pattern. Monitoring
of the changes in ovarian volume with suppressive treatment with
gonadotropin-releasing hormone analogs has been described.83 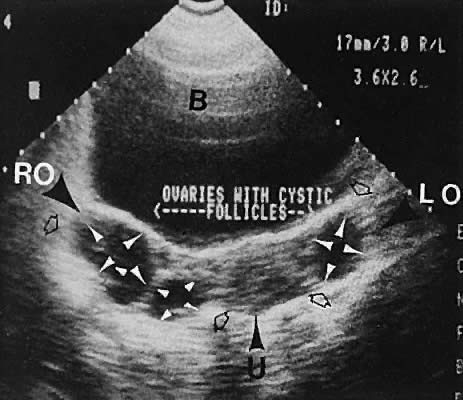 Fig. 34. Polycystic ovary syndrome, with cystic follicles noted in both ovaries. Transabdominal
scan depicts bladder (B), uterus (U), right ovary (RO), and
left ovary (LO). Ovarian size is shown by open arrowheads. Follicle
cysts are shown by small white arrowheads. Fig. 34. Polycystic ovary syndrome, with cystic follicles noted in both ovaries. Transabdominal
scan depicts bladder (B), uterus (U), right ovary (RO), and
left ovary (LO). Ovarian size is shown by open arrowheads. Follicle
cysts are shown by small white arrowheads.
|
OVARIAN MASSES. The ovary has the capacity to develop many different cystic and solid
masses. Transvaginal and transabdominal ultrasonography can categorize
adnexal masses into various echo densities and morphologic types, thereby
narrowing the differential diagnosis. The reliability of ovarian
size assessment has been documented to be high, with a correlation coefficient
of 0.960 between observers documented by Higgins and coworkers.84 Ovarian Cysts. Unilocular cystic masses with few intracystic echoes are most likely serous-type
cysts of the ovary (cystic follicles or follicular cysts) or
serous cystadenomas (Fig. 35). Cystic masses with complex echogenic intracystic echoes are more commonly
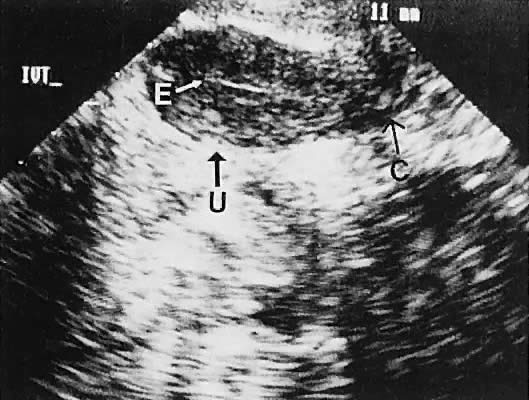
hemorrhagic in origin (hemorrhagic corpus luteum cysts or endometriomata).85 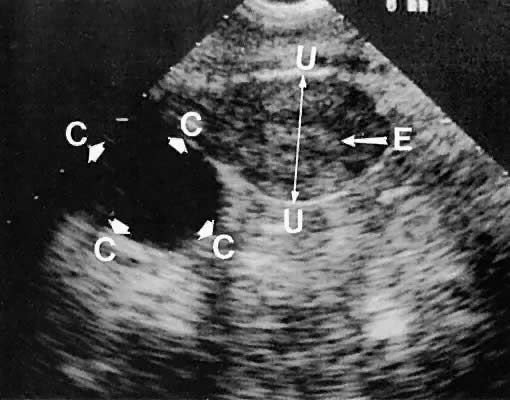 Fig. 35. Unilateral serous ovarian cyst (right ovary). The transvaginal scan depicts
a unilocular, anechoic cyst (C) delineated by the arrowheads; uterine
corpus (U); and endometrium (E). Left ovary is not in this scan plane. Fig. 35. Unilateral serous ovarian cyst (right ovary). The transvaginal scan depicts
a unilocular, anechoic cyst (C) delineated by the arrowheads; uterine
corpus (U); and endometrium (E). Left ovary is not in this scan plane.
|
A more conservative management approach is feasible in patients with reassuring
intracystic morphology (e.g., anechoic, smooth wall, no papillary excrescences). The decreased need
for surgery in children was demonstrated retrospectively in 1989 by Thind
and colleagues,86 who reviewed the cases of 64 children with ovarian cysts. In addition, the
potential also exists for therapeutic cyst aspiration using ultrasound
guidance of selected cysts in young women.87,88 In patients for whom expectant management is elected, comparative studies
after subsequent cycles or hormonal therapy is feasible, especially
with the transvaginal technique. The differentiation between endometriomas and nonendometriotic ovarian
cysts was investigated by Guerriero and associates,89 who evaluated 251 premenopausal, nonpregnant patients using transvaginal
ultrasound. Of the 31 diagnosed as ovarian endometriomas, 24 were confirmed
as endometriomas by histologic assessment. The sensitivity of
transvaginal ultrasound in differentiating endometriotic cysts from cysts
of other etiologies was 83%, and the specificity, 89%. The authors
observe that this was compatible with the diagnostic accuracy of magnetic
resonance imaging. Controversy exists regarding the significance of cystic masses in the postmenopausal
age group. Fleischer and associates90 published an excellent correlation of ultrasonographic findings with subsequent
histopathologic features of 67 ovaries in 37 postmenopausal
patients. A positive predictive value of 92% and a negative predictive
value of 92% were noted in this highly selected population. Luxman and
colleagues91 noted that 2 of 29 ovarian cancers were not detected by transabdominal
ultrasonography. Goldstein and coworkers92 and Andolf and Jorgensen93 noted no malignancies in hypoechoic cysts smaller than 5 cm in more than 190 patients. Therefore, small, nonsuspicious cystic areas in postmenopausal patients
may be followed conservatively with surgical evaluation for persistent
cysts or those that progressively increase in size. The absolute maximum
cystic diameter that may be followed safely is debatable but appears
to be in the 3.5- to 5-cm range. If a noninterventional posture is
selected, the ultrasonographer must realize that a small likelihood of
an unapparent malignancy exists and that close follow-up is mandatory. Ovarian Neoplasms. Germ Cell Tumors. The most common neoplasms of reproductive-age women are germ cell tumors. Cystic
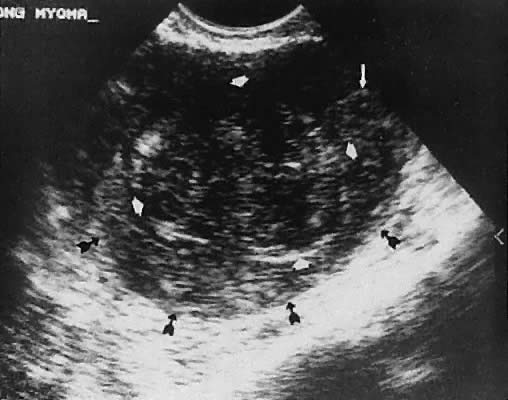
teratomas are wellcircumscribed, complex, cystic masses that
usually are unilateral and exhibit a variably complex, intracystic echo
pattern94–96 (Figs. 36 and 37). This type of tissue characterization is important because these complex
cystic masses require surgical evaluation for definitive therapy more
often than the smaller, unilocular, serous-type cysts, which may be
followed expectantly. Rupture of cystic teratomas during pregnancy is
a major complication that should be avoided.97 Although the specific pathologic diagnosis of an ovarian mass cannot be
made ultrasonographically, ultrasound can be used to categorize and
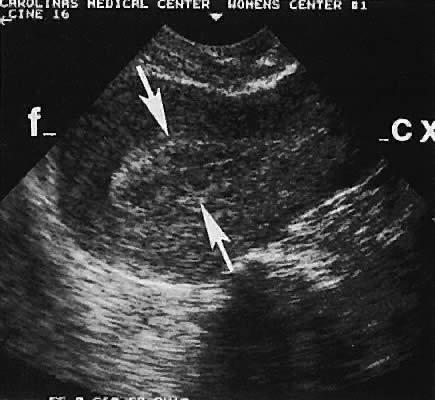
describe these masses to establish a more precise differential diagnosis.98 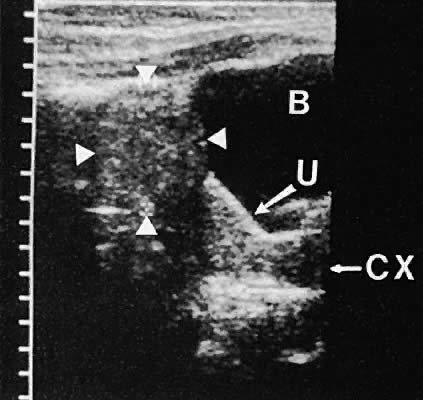 Fig. 36. Cystic teratoma located superior to the uterine fundus. Transabdominal
scan shows cyst demarcated by the four white arrowheads. B, bladder; CX, cervix; U, uterus. Fig. 36. Cystic teratoma located superior to the uterine fundus. Transabdominal
scan shows cyst demarcated by the four white arrowheads. B, bladder; CX, cervix; U, uterus.
|
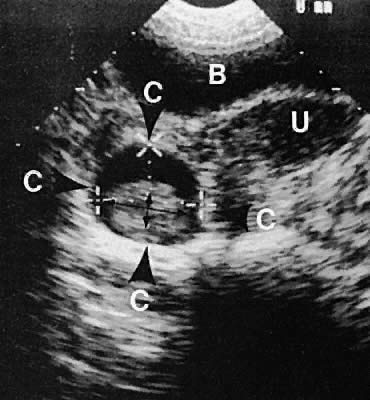 Fig. 37. Cystic teratoma, right adnexa, posterior to the broad ligament. Transabdominal
scan depicts uterus (U), cystic teratoma (C), and bladder (B). Small
thin arrows denote an intracystic hairball. Fig. 37. Cystic teratoma, right adnexa, posterior to the broad ligament. Transabdominal
scan depicts uterus (U), cystic teratoma (C), and bladder (B). Small
thin arrows denote an intracystic hairball.
|
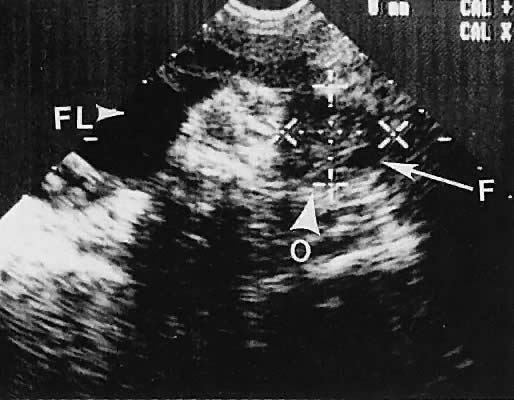
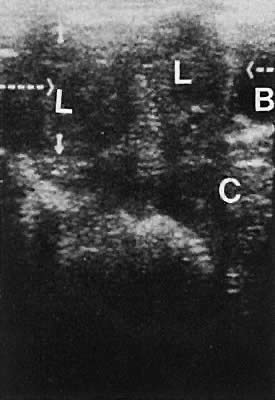
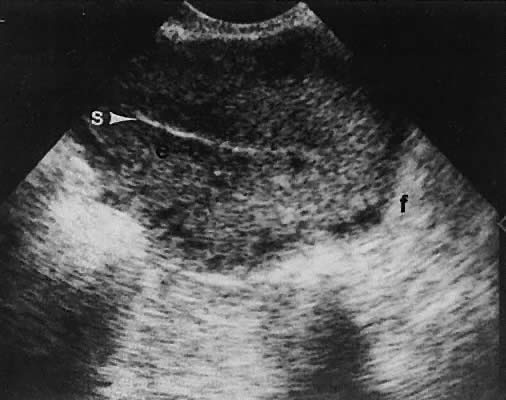
Stromal Neoplasms. Solid ovarian neoplasms include those arising from ovarian stromal cells (fibroma
and thecoma)99 (Fig. 38) and the Brenner cell tumor.100 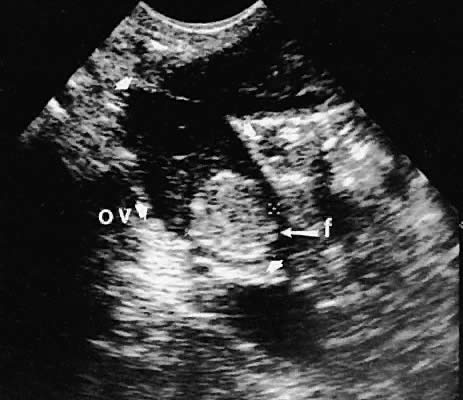 Fig. 38. A transvaginal image in the coronal plane through the right ovary (ov, arrowheads ). The white arrow depicts a small, clinically unapparent, solid-type
stromal tumor (fibroma, f). Fig. 38. A transvaginal image in the coronal plane through the right ovary (ov, arrowheads ). The white arrow depicts a small, clinically unapparent, solid-type
stromal tumor (fibroma, f).
|
Ovarian Carcinoma (Epithelial Ovarian Tumors). Ovarian malignant neoplasms produce a variety of ultrasonographic patterns
that typically produce an image of mixed solid and cystic components, irregular
septations, and coexistent ascites, and they are frequently
bilateral101,102 (Figs. 39 and 40). 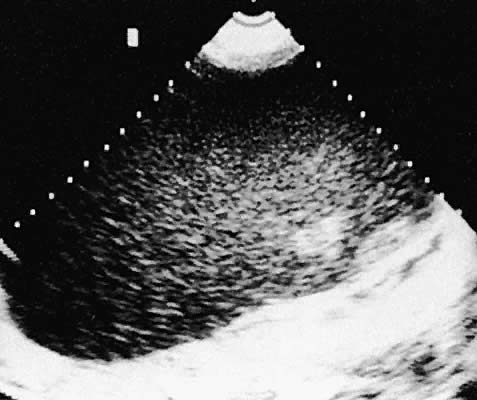 Fig. 39. Ovarian carcinoma, borderline malignancy, seen in transverse, transabdominal
scan. A granular complex echo pattern extends throughout the mass. Fig. 39. Ovarian carcinoma, borderline malignancy, seen in transverse, transabdominal
scan. A granular complex echo pattern extends throughout the mass.
|
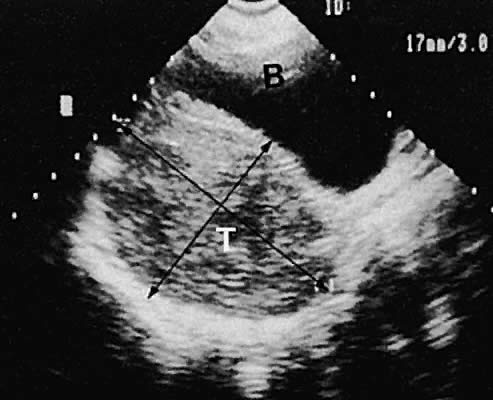 Fig. 40. Longitudinal transabdominal scan of a Sertoli-Leydig cell tumor (T) of
the right ovary. Scan also shows bladder (B). Note the complex intracystic
echoes with bright posterior echoes depicting good sound penetration ( i.e., enhancement). Fig. 40. Longitudinal transabdominal scan of a Sertoli-Leydig cell tumor (T) of
the right ovary. Scan also shows bladder (B). Note the complex intracystic
echoes with bright posterior echoes depicting good sound penetration ( i.e., enhancement).
|
The role of ultrasonography in screening for ovarian cancer is controversial. An
ever-increasing body of literature continues to question the
cost-effectiveness of ultrasound as a screening modality. A thorough
review of this controversy is beyond the scope of this chapter. Ultrasound
screening for ovarian cancer is not widely accepted. The combined
use of tumor-associated antigens (e.g., CA-125) with ovarian imaging by 2D technique and Doppler may offer an
enhanced potential for diagnosis of ovarian malignancies in high-risk
groups.14,103,104 The salient question that must be answered before recommending universal
ovarian cancer screening with this modality encompasses the reliability
and cost-effectiveness of the screening protocol to detect early (stage
I or II) disease. The realization that CA-125 levels are not elevated
in many cases of early stage disease raises a serious question regarding
the efficacy of this serum test as a screening component. The
question of routine ovarian cancer screening remains unanswered. The
role of ultrasonography in staging and follow-up of ovarian cancer continues
to expand. Conte and coworkers105 describe the potential value of ultrasound study in the preoperative staging
of ovarian carcinoma. Although ultrasonography can detect macroscopic
residual disease with acceptable accuracy, its limitation in detecting
small residual disease in lieu of second-look laparotomy has been demonstrated by Murolo and associates.106 Breast cancer and gastrointestinal tract cancer may metastasize to the
ovary. Other ovarian neoplasms have been described ultrasonographically, including
Brenner and Krukenberg tumors.107,108 The Krukenberg tumor has been characterized ultrasonographically as a
complex solid mass and a cystic mass but is unlike the ultrasound picture
of primary ovarian carcinoma.109 Ovarian neoplasms may be imaged more precisely by the vaginal approach. Figure 41 shows the image quality of an ovarian thecoma by transvaginal ultrasonography. 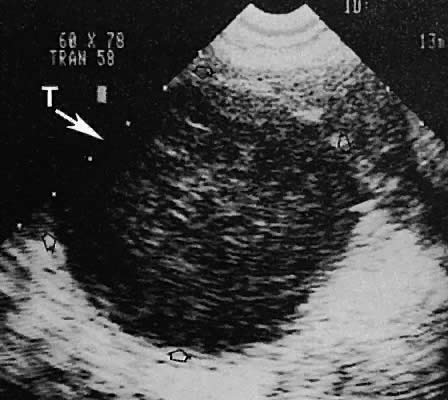 Fig. 41. Transvaginal scan of a right ovarian thecoma (T) with borders shown by
the open arrows. Fig. 41. Transvaginal scan of a right ovarian thecoma (T) with borders shown by
the open arrows.
|
Many articles have been published further describing the ultrasonographic 2D
image characteristics of ovarian cancer. In addition, Doppler interrogation
of small vessels detected by color Doppler imaging reveals
increased diastolic flow velocities and evidence of neovascularization
secondary to tumor angiogenesis. The resultant calculations of waveform
flow velocity ratios are therefore decreased. Multiple scoring systems for 2D findings and 2D and Doppler findings also
have been created. The details of these systems are beyond the scope
of this chapter. The various scoring systems offer the potential of
assigning numeric values to the well-known findings of ovarian malignancy, such
as thickened cyst walls, septations, papillary excrescences, complex
intratumoral echoes, and disorganized echo texture. The addition
of Doppler waveform analysis frequently confirms the concern of nonreassuring 2D
findings but rarely increases concern in patients with
benign-appearing cysts on 2D findings. Brown and coworkers110 evaluated 44 masses with 2D and color Doppler imaging. They noted significant
overlap in the resistance index and pulsatility index between
benign and malignant masses. This finding contrasts with that of other
investigators.111 FALLOPIAN TUBE CARCINOMA. Although less common than ovarian carcinoma, cancer of the fallopian tube
exhibits a similar clinical course. The complex echo pattern of tubal
carcinoma diagnosed by transvaginal ultrasonography has been described.112–114 Pelvic Inflammatory Disease and Endometriosis Other adnexal processes exclusive of neoplastic changes include pelvic
inflammatory disease and endometriosis. Pelvic inflammatory disease most
often results from a primary salpingo-oophoritis that has progressed
to some degree of hydrosalpinx formation with adhesive pelvic disease. A
variety of ultrasonographic findings with differing types of complex
adnexal masses result with frequent visualization of tubal dilation
and intrafallopian tube fluid (hydrosalpinx formation).115 The ability to diagnose and guide aspiration of tubo-ovarian abscesses
with transvaginal ultrasonography has been documented.116–120 Frequently, it is difficult to establish the precise etiology of complex
adnexal masses on an endometriotic or inflammatory basis.121–123 Endometriosis also produces complex adnexal masses with distortion of
the pelvic anatomy typical of adhesive disease (Figs. 42 to 45). In some instances, endometriotic involvement of a nongynecologic structure, such
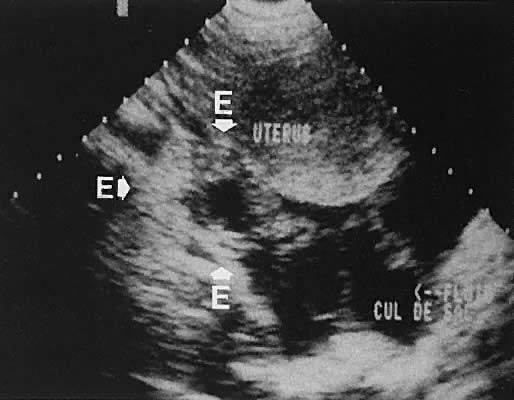
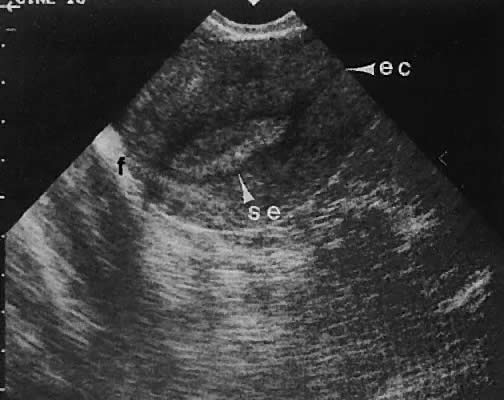
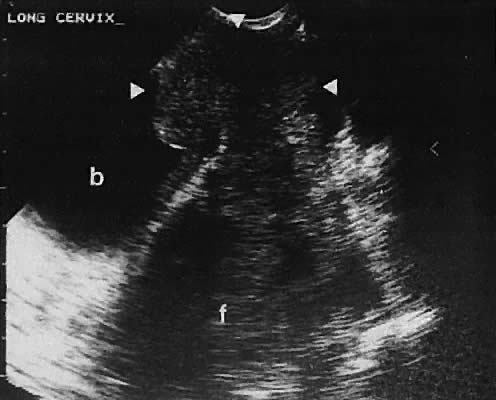
as the urinary bladder or liver, has been noted.124–126 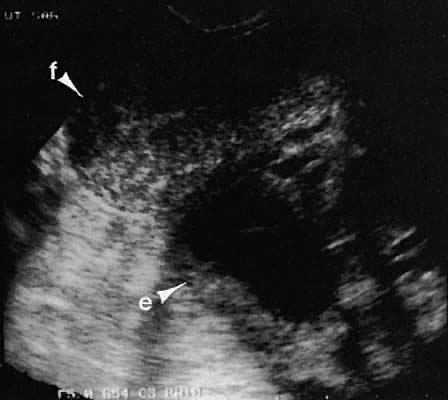 Fig. 42. A sagittal view of the uterus and cul-de-sac endometrioma (e) by transvaginal
ultrasonography. The low-level echoes are characteristic of hemorrhagic-type
cysts within the endometrioma. The uterine fundus (f) is
anteverted. Fig. 42. A sagittal view of the uterus and cul-de-sac endometrioma (e) by transvaginal
ultrasonography. The low-level echoes are characteristic of hemorrhagic-type
cysts within the endometrioma. The uterine fundus (f) is
anteverted.
|
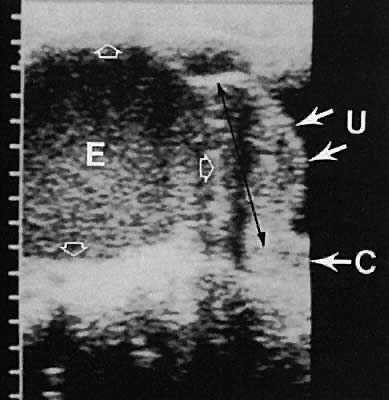 Fig. 43. Endometrioma of the ovary contiguous with the posterior uterine fundus. Longitudinal
transabdominal scan depicts endometrioma (E), uterus (U), and
cervix (C). Note the complex intracystic echoes with bright posterior
echoes ( open arrows ). Fig. 43. Endometrioma of the ovary contiguous with the posterior uterine fundus. Longitudinal
transabdominal scan depicts endometrioma (E), uterus (U), and
cervix (C). Note the complex intracystic echoes with bright posterior
echoes ( open arrows ).
|
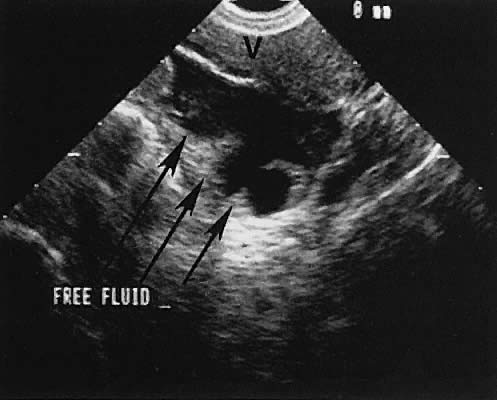
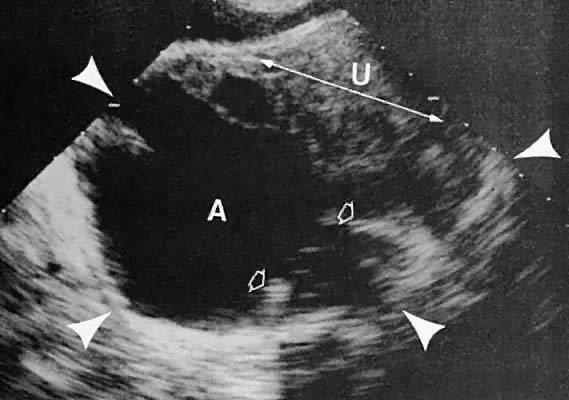 Fig. 44. Transverse transabdominal scan of right tubo-ovarian abscess. Scan depicts
uterus (U) and abscess (A). Small open arrowheads show irregularity
of the abscess wall. Large white arrowheads show the borders of the
abscess. Fig. 44. Transverse transabdominal scan of right tubo-ovarian abscess. Scan depicts
uterus (U) and abscess (A). Small open arrowheads show irregularity
of the abscess wall. Large white arrowheads show the borders of the
abscess.
|
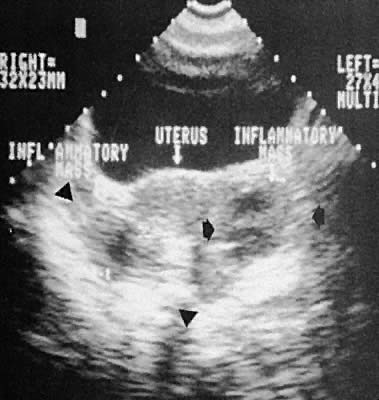 Fig. 45. Bilateral tubo-ovarian complex formation, pelvic inflammatory disease (transabdominal
scan, transverse plane). Black arrowheads delineate borders
of the tubo-ovarian complexes. Fig. 45. Bilateral tubo-ovarian complex formation, pelvic inflammatory disease (transabdominal
scan, transverse plane). Black arrowheads delineate borders
of the tubo-ovarian complexes.
|
Intrauterine Devices Ultrasonographic localization of intrauterine devices has been possible
for years (Fig. 46). The advent of transvaginal scanning increases the accuracy of intrauterine
device localization.127 Removing intrauterine devices under transabdominal ultrasound guidance
offers a more precise and potentially less traumatic removal in difficult
clinical settings.128,129 The ability to extract an intrauterine device that is juxtaposed, but
inferior to, an early intrauterine pregnancy also is occasionally enhanced
by transabdominal ultrasound guidance. 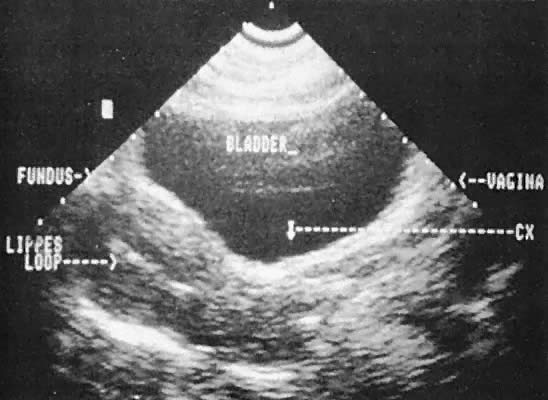 Fig. 46. Transabdominal scan depicts Lippes loop in situ, cervix (CX), fundus, bladder, and vagina. Fig. 46. Transabdominal scan depicts Lippes loop in situ, cervix (CX), fundus, bladder, and vagina.
|
Early Pregnancy INTRAUTERINE PREGNANCY. The most common cause of uterine enlargement is early pregnancy. The advent
of transvaginal ultrasonography offers the capability of extremely
early diagnosis of pregnancy. The prominent echogenicity of decidualized
endometrium is seen clearly on transabdominal or transvaginal scanning
before the visualization of a gestational sac.130,131 With currently available transvaginal imaging techniques, a gestational
sac that is located within the uterine cavity and is developing normally
should be clearly visible, concomitant with a serum human chorionic
gonadotropin (hCG) concentration of 1000 mIU/mL (Second International
Standard [2nd IS]).132 Using the transabdominal technique, the same authors report the discriminatory
zone to be 1800 mIU/mL (2nd IS).133 Notice that the First International Preparation (1st IRP) standard results
in hCG values that are approximately twice the 2nd IS values. Other
publishedanalyses of transvaginal ultrasound show lower discriminatory
zones of 300 mIU/mL (2nd IS),134 450 to 750 mIU/mL (presumed 2nd IS),135 and 1100 mIU/mL (1st IRP).136 From a clinical standpoint, if a quantitative hCG exceeds 1000 mIU/mL
and an intrauterine gestational sac cannot be seen with transvaginal scanning, the
suspicion of an ectopic pregnancy must increase, and serial
follow-up or further evaluation is indicated. Studies of serial scanning
of patients conceiving during in vitro fertilization cycles reveal the presence of a gestational sac at even
lower hCG concentrations.137 The correlation of gestational sac visualization and serum hCG concentration
offers the practicing gynecologist a powerful tool for early diagnosis
of extrauterine pregnancy. The principles of management, evaluation, and
diagnosis presuppose knowledge of the rate of increase of hCG
concentration in normal pregnancy and correlation with ultrasonographic
findings. As a general rule, hCG should double every 2 to 3 days during
the early first trimester.138–141 Many ectopic pregnancies exhibit a subnormal rise in hCG. Early intrauterine pregnancy scanning displays the developing amniotic
membrane, chorion, and yolk sac.130,142 Before visualization of the fetal pole, the assessment of pregnancy status
depends on the rate of growth of the sac and correlation with hCG
levels. Early in gestation, the mean sac growth is 0.9 to 1.13 mm/day.136 A yolk sac should be seen when the mean sac diameter exceeds 2 cm, and
a fetal pole should be visible after the sac exceeds 2.5 cm143 (Fig. 47) using the transabdominal approach. Transvaginal scanning allows visualization
of the embryo with a mean sac diameter of 1.2 cm.144 As the first trimester progresses, the fetus can be outlined easily, and
the cephalic pole, caudal pole, limb buds, and umbilical cord can be
delineated.135 The presence of fetal cardiac activity in early pregnancy scanning indicates
an excellent prognosis for the pregnancy. Fetal cardiac flicker
can be seen in embryos of at least 5 mm with transvaginal scanning and
in embryos of at least 9 mm with the transabdominal approach.144 In published series, the likelihood of spontaneous abortion of ultrasonographically
normal pregnancies in the 8- to 12-week range approximates 2% to 4%.145 Before cardiac flicker becomes apparent, correlation of the gestational
sac size with quantitative hCG assessment provides important predictive
information. If the hCG concentration is clearly abnormal for the
stated gestational sac size, the likelihood of pregnancy progression is
unlikely. The presence of a normal hCG concentration for a specified
gestational sac measurement does not necessarily predict a successful
outcome.146 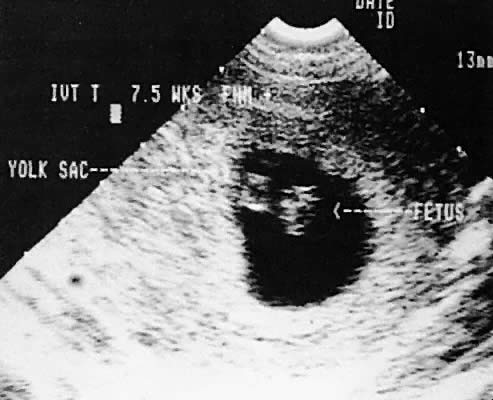 Fig. 47. Transvaginal scan of an early intrauterine pregnancy demonstrating fetal
pole (fetus) and yolk sac. Fig. 47. Transvaginal scan of an early intrauterine pregnancy demonstrating fetal
pole (fetus) and yolk sac.
|
The accuracy of diagnosing pregnancy failure has been enhanced by ultrasonography. Incomplete spontaneous abortion is characterized by disorganized intrauterine echoes, irregularity of
the gestational sac, frequent eccentric sac location, and the evidence
of intradecidual hemorrhage.147 The anembryonic pregnancy (blighted ovum) is characterized by a visible gestational sac (chorion) but
no apparent fetal pole. Frequently, the sac is smaller than expected.143,148,149 When the mean sac diameter minus the crown-rump length is less than 5 mm, 94% of
pregnancies were lost.150 Retention of a discernible embryo that has undergone an early death has
been termed a missed abortion. First-trimester hydatidiform mole may produce bizarre echoes in early pregnancy or may appear initially
as an unremarkable gestational sac.149. Robinson HP: The diagnosis of early pregnancy failure by sonar. Br J Obstet
Gynaecol 82:849, 1975 149 The ultrasound characterization of hydatidiform mole has been well described. The
early diagnosis of molar pregnancy enhances appropriate management
and hopefully minimizes the likelihood of malignant sequelae. Although
variable, the echo pattern suggestive of a molar pregnancy consists
of irregular solid and cystic interfaces within the endometrial
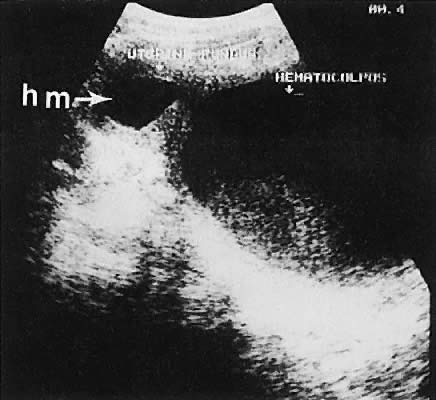
cavity in a patient with signs and symptoms of early pregnancy (Fig. 48). 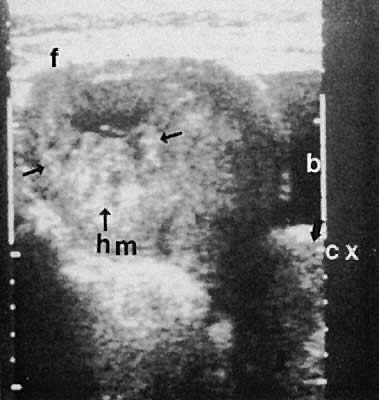 Fig. 48. A transabdominal longitudinal (sagittal) image of a uterine fundus (f) containing
echoes of mixed intensity characteristic of early hydatidiform
mole (hm, small arrows ). A small portion of the urinary bladder (b) is noted to the right of
the image. The uterine cervix (cx) is delineated ( large arrows ). Fig. 48. A transabdominal longitudinal (sagittal) image of a uterine fundus (f) containing
echoes of mixed intensity characteristic of early hydatidiform
mole (hm, small arrows ). A small portion of the urinary bladder (b) is noted to the right of
the image. The uterine cervix (cx) is delineated ( large arrows ).
|
EXTRAUTERINE PREGNANCY. Pregnancies located outside of the intrauterine cavity are referred to
as extrauterine pregnancies. Although these pregnancies include ovarian
pregnancies, abdominal pregnancies, and cervical pregnancies, the most
common location is in the fallopian tube. This section addresses the
preclinical diagnosis of unruptured tubal pregnancies using a combined
approach of ultrasonographic findings correlated with serum hCG concentration. As noted earlier, a discriminatory zone of approximately 1000 mIU/mL is
being used through transvaginal ultrasonography as the level at which
a normal gestational sac should be seen.132 The lack of visualization of the gestational sac with hCG concentrations
greater than 1000 mIU/mL or an inadequate rate of increase of hCG is
suggestive of the diagnosis of extrauterine pregnancy and warrants further
evaluation.132,151 Using transvaginal ultrasound and an hCG discriminatory zone of 1500 mIU/mL (1st
IRP), Barnhart and colleagues152 report that all viable intrauterine pregnancies that were visualized by
ultrasound demonstrated hCG values in excess of 1500 mIU/mL. In a study
of 1253 patients, there were 205 ectopic pregnancies diagnosed by
the combined use of serial hCG measurements and transvaginal ultrasound. Fifty-nine
percent of ectopic pregnancies never produced hCG concentrations
greater than 1500 mIU/mL. In their experience, the sensitivity
of this combined protocol was 100%, and the specificity, 99%. A word of caution: multifetal pregnancies typically demonstrate a higher
discriminatory zone of hCG. Kadar and coworkers153 report that a discriminatory zone of 3000 mIU/mL is required to avoid
misdiagnosis of early, potentially viable multifetal pregnancy. Because the hCG doubling time is relatively short (2 to 3 days), this difference
in discriminatory hCG levels does not translate into a long
time, but as noted in the study mentioned earlier,152 most ectopic pregnancies do not achieve an hCG level of 1500 mIU/mL, much
less 3000 mIU/mL. To minimize the risk of intervening in a potentially
normal intrauterine pregnancy, a discriminatory zone of 3000 mIU/mL
and expected doubling time of 72 hours is preferable. If this protocol is adopted, the physician must be assured of the patient's
reliability and potential for follow-up, having the ability
to intervene rapidly if concerning symptoms develop. This diagnostic protocol
should be applied to patients who are asymptomatic or minimally
symptomatic. Patients with an acute abdomen, vasomotor instability, or
evidence of hemoperitoneum on transvaginal scanning should be treated
surgically. Using the transvaginal approach, an extrauterine conceptus frequently is
delineated with ultrasonography, at which time definitive surgical therapy
may be instituted.154,155 As noted earlier, this technique allows assessment of the cul-de-sac for
fluid accumulation. If evidence of a possible hemoperitoneum is seen, transvaginally
directed culdocentesis can be readily accomplished. Rarely, a combined intrauterine and extrauterine pregnancy (heterotopic pregnancy) is seen.156–159 The incidence of heterotopic pregnancy has been noted to be more frequent
in patients undergoing in vitro fertilization and warrants heightened awareness.160 The role of Doppler assessment to increase diagnostic accuracy is being
evaluated. A sensitivity of 73% for early evaluation was noted in a study
of 96 ectopic pregnancies by Taylor and coworkers161 using Doppler technology. Early diagnosis results in less morbidity, potential
tubal salvage, and the possibility of medical therapy.162 The assessment of eccentric intrauterine implantations is more difficult. The
diagnosis of a cornual or isthmic implantation site frequently
is possible, and in these cases, the early diagnosis alleviates the potential
for catastrophic uterine rupture.163 Chronic and interstitial ectopic pregnancies producing complex adnexal
echogenic masses have been described.164–167 |