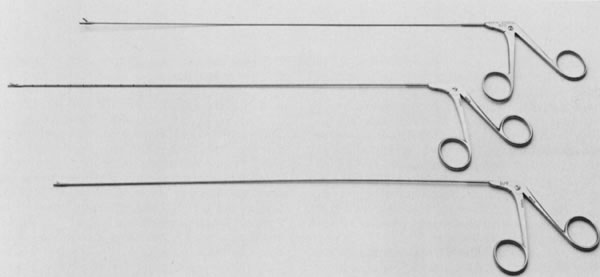
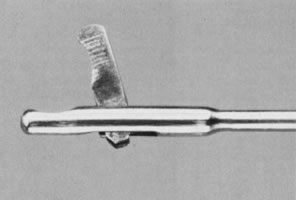
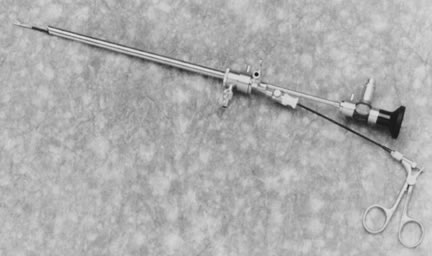
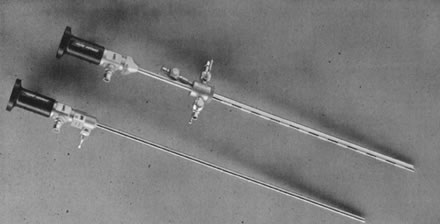
The therapeutic applications of hysteroscopy are rapidly expanding, in
great part because of the improvement in instrumentation, improvement
in energies and delivery systems of the distending media, and availability
of video systems that enhance visualization with added magnification (Table 1). Table 1. Therapeutic Applications of Hysteroscopy
Targeted biopsies
Removal of endometrial polyps
Removal of submucous leiomyomas
Division of uterine septa
Removal of “lost” IUDs and other foreign bodies
Lysis of intrauterine adhesions
Endometrial ablation(laser, electrosurgery)
Tubal cannulation (tubal obstruction)
Chorionic villus sampling
Tubal occlusion (electrocoagulation, cryocoagulation, chemical mechanical)
Hysteroscopic Biopsies Under Visual Control Biopsy of an abnormal endometrial lesion is essential, particularly a lesion
suspected to be malignant or premalignant. Hysteroscopy offers the
possibility of choosing the area in need of biopsy. A biopsy forceps
of sufficient diameter to allow appropriate tissue sample should be
used; when appropriate, several biopsies of the same lesion should be
taken to obtain adequate specimens. Removal of Intrauterine Foreign Bodies Hysteroscopy is the best method of removing partially embedded or fragmented
intrauterine devices (IUDs) misplaced within the uterine cavity. Using
a semirigid or rigid grasping forceps, this can be accomplished
easily by grasping the device or its tail, and withdrawing the hysteroscope
once the device is fixed at the end of the endoscope. Removal of
these devices under visual control prevents unnecessary trauma to the
uterus by blind manipulations and permits visual appraisal of the condition
of the device regarding embedment or fragmentation (Figs. 19 and 20).7  Fig. 19. Hysteroscopic removal of partially embedded Dalkon Shield IUD. Fig. 19. Hysteroscopic removal of partially embedded Dalkon Shield IUD.
|
 Fig. 20. Hysteroscopic removal of partially embedded Lippes-loop IUD. Fig. 20. Hysteroscopic removal of partially embedded Lippes-loop IUD.
|
Hysteroscopic Removal of Endometrial Polyps and Submucous Leiomyomas A polyp can be removed by blind methods, including forceps or curettes, but
the diagnosis can be obtained consistently only by direct visualization. By
visually detecting the polyps, it is easy to detach them from
the endometrial cavity by hysteroscopy, and their removal is simple. By
whatever method they are removed, it is important to check that the
total polyp has been removed, because often only a portion of the polyp
is detached from its anchoring pedicle.
Although large endometrial polyps, particularly when pedunculated, are
easily removed by hysteroscopic guidance, the removal of submucous leiomyomas
requires more experience. Several methods are available. When the leiomyoma
is smaller than 2 to 3 cm and is pedunculated, mechanical transection
of the pedicle expedites its removal. Nonetheless, myomas larger than
3 cm with thick pedicles, or sessile myomas, must be removed by segmental
shavings with a resectoscope and a cutting loop. This is performed systematically
until the uterine wall is reached, when resection should stop. With experience,
the endoscopist learns tactile appraisal provided by the resecting loop
to distinguish fibrous myomatous tissue from myometrium. In addition,
he or she can visually appraise the fibrotic tissue and the fascicularis
aspect of the myometrium. When myomas penetrate deeply into the uterine
wall, laparoscopy should be considered to monitor the hysteroscopic-resectoscopic
operation transabdominally (Color
Plate 1A to F; Figs.
21, 22, 23,
24, 25,
and 26).8–17
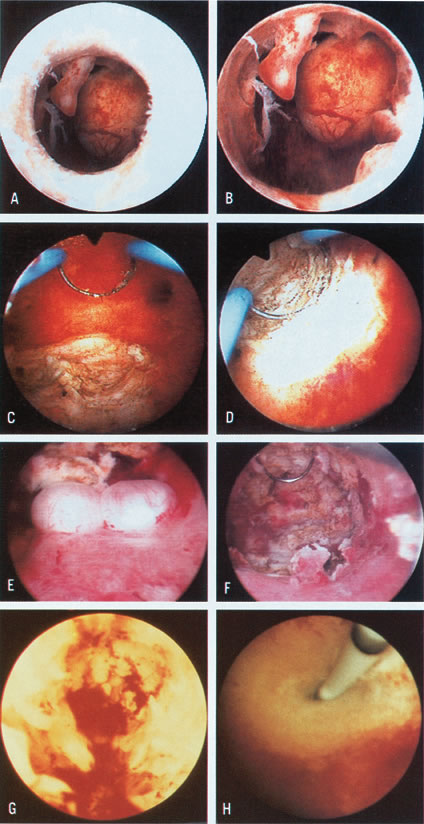 Color Plate 1. A. Endometrial polyp and submucous myoma, as seen just before crossing the
internal os. B. Closer view of endometrial polyps and pedunculated submucous myoma. C. Resection of posterior submucous myoma. D. Selective coagulation of the myoma bed after resection. E. Small submucous myomas in the posterior uterine wall. F. Hysteroscopic view after resection of posterior submucous myomas. G. Hysteroscopic view after initial treatment of severe intrauterine adhesions
completely occluding the uterine cavity. The remaining fibrotic
stumps on lateral walls are visible. H. Hysteroscopic ostial tubal cannulation using a 1-mm OD catheter. Color Plate 1. A. Endometrial polyp and submucous myoma, as seen just before crossing the
internal os. B. Closer view of endometrial polyps and pedunculated submucous myoma. C. Resection of posterior submucous myoma. D. Selective coagulation of the myoma bed after resection. E. Small submucous myomas in the posterior uterine wall. F. Hysteroscopic view after resection of posterior submucous myomas. G. Hysteroscopic view after initial treatment of severe intrauterine adhesions
completely occluding the uterine cavity. The remaining fibrotic
stumps on lateral walls are visible. H. Hysteroscopic ostial tubal cannulation using a 1-mm OD catheter.
|
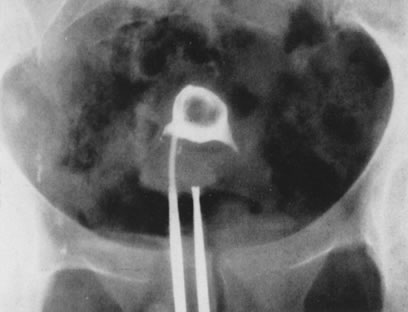 Fig. 21. Hysterosalpingogram shows an occupying lesion distorting the uterine cavity
symmetry. Fig. 21. Hysterosalpingogram shows an occupying lesion distorting the uterine cavity
symmetry.
|
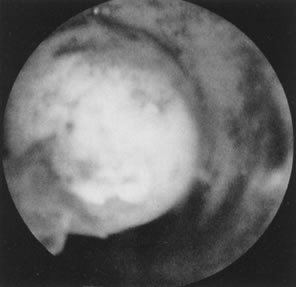 Fig. 22. Hysteroscopic view of pedunculated submucous myoma. Fig. 22. Hysteroscopic view of pedunculated submucous myoma.
|
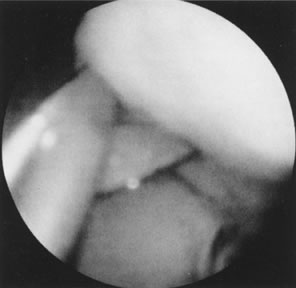 Fig. 23. Hysteroscopic dissection/removal of myoma with scissors. Fig. 23. Hysteroscopic dissection/removal of myoma with scissors.
|
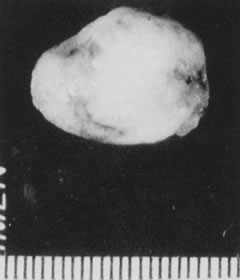 Fig. 24. Myoma specimen after resection. Fig. 24. Myoma specimen after resection.
|
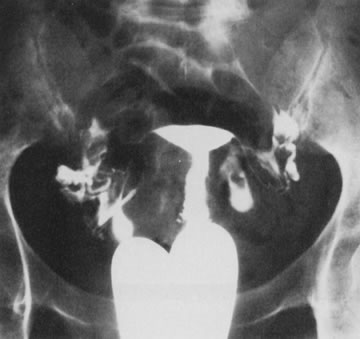 Fig. 25. Follow-up hysterosalpingogram shows normal uterine cavity. Fig. 25. Follow-up hysterosalpingogram shows normal uterine cavity.
|
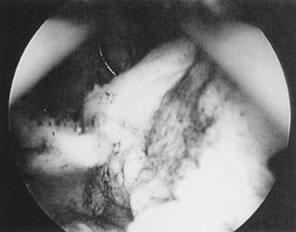 Fig. 26. Large, sessile submucous myoma being resected with wire-loop electrode. Fig. 26. Large, sessile submucous myoma being resected with wire-loop electrode.
|
The Nd:YAG laser or other fiberoptic lasers (e.g., the argon or KTP-532) can
also be used as operating instruments to transect the pedicle of
a pedunculated leiomyoma or to aid in morcellation, particularly for
larger leiomyomas located at the fundal region, which may be difficult
to remove with a resectoscope.18 The advent of gonadotropin-releasing hormone (GnRH) analogues has facilitated
the treatment of larger leiomyomas by reducing their size and vascularity. It
has provided a method of preoperatively controlling the
bleeding in patients with submucous myomas. This allows better preparation
of the uterine cavity for resectoscopy, by causing atrophy of the
endometrium. The patient is able to reestablish preoperatively normal
physiologic iron stores and hemoglobin and hematocrits once bleeding
ceases.19 Another adjunct in the management of patients with submucous myomas has
been vaginal sonography to evaluate the uterine walls for other leiomyomas (size, number, and
location) and to evaluate the submucous leiomyoma
for penetration into the uterine wall, measuring thickness as a guide
for resectoscopic removal to avoid shaving small portions of an intramural
myoma that merely impinge on the uterine cavity (Table 2).20 Table 2. Hysteroscopic Myomectomy for Abnormal Bleeding
|
|
|
Type of Myomas |
|
|
|
|
|
|
|
Author |
No. Patients |
Pedunculated |
Sessile |
Method |
IUD* |
E/P† |
Antibiotics |
Cure (%) |
Recurrent (%) |
|
Haning et al |
1 |
- |
+ |
Resectoscope |
- |
+ |
+ |
1 |
- |
|
(1980) |
|
|
|
|
|
|
|
|
|
|
DeCherney and |
8 |
+ |
+ |
Resectoscope |
Foley |
+ |
+ |
8 |
- |
|
Polan (1983) |
|
|
|
|
|
|
|
|
|
|
Neuwirth (1983) |
28 |
+ |
+ |
Resectoscope |
Foley |
+ |
+ |
17 (60.7) |
8 (28.5) |
|
Lin et al |
13 |
+ |
- |
Resectoscope (9) |
Foley |
+ |
+ |
9 (69.2) |
4 (30.7) |
|
(1986) |
|
|
|
Rigid scissors (4) |
|
|
|
|
|
|
Hallez and |
300 |
+ |
+ |
Resectoscope |
+ |
+ |
+ |
299‡ |
- |
|
Perino (1988) |
|
|
|
|
|
|
|
|
|
|
Baggish (1989) |
23 |
+ |
+ |
Nd:YAG Laser |
Foley (5 patients) |
- |
+ |
NR§ |
NR§ |
|
Valle (1990) |
52 |
+ |
- |
Semi-rigid scissors |
- |
- |
- |
52 (100.0) |
12 (20.0) |
|
Donnez et al |
60 |
48 |
12 |
Nd:YAG laser |
- |
- |
- |
48 (80.0) |
12 (20.0) |
|
(1990) |
|
|
|
|
|
|
|
|
|
|
Loffer (1990) |
53 (10 were |
18 |
25 (2 patients had |
Resectoscope |
NR§ |
- |
- |
40 (93.0) |
3 (6.9) |
|
|
polyps) |
|
2 procedures) |
|
|
|
|
|
|
|
Corson and |
92 |
92 |
- |
Resectoscope |
NR§ |
- |
+ |
65 (81.2)# |
15 (18.7)# |
|
Brooks (1991) |
|
|
|
|
|
|
|
|
|
|
Derman et al |
94 |
94 (2 intraoperative |
- |
Resectoscope |
Rubber balloon |
+ |
+ |
69 (75.0) |
23 (24.5) |
|
(1991) |
|
laparotomies) |
|
|
|
|
|
|
|
|
Wamsteker et al |
51 |
25 |
26 (several patients had |
Resectoscope |
- |
- |
+ |
48 (94.1) |
3 (5.9) |
|
(1993) |
|
|
2–3 procedures) |
|
|
|
|
|
|
|
Emanuel et al |
285 |
73 |
266 |
Resectoscope |
- |
- |
+ |
225 (78.8)¶ |
41 (14.4) |
|
(1999) |
|
|
|
|
|
|
|
|
|
|
Totals |
1040 |
|
|
|
|
|
|
881 (81.2) |
109 (10.5) |
*IUD, Intrauterine device
†E/P, Estrogen/Progesterone
‡1 patient required laparotomy
§NR, Not Reported
#From 80 patients
¶17 lost to follow up
Modified from Siegler AM, Valle RF: Therapeutic hysteroscopic procedures. Fertil
Steril 50:685, 1988.
Division of Symptomatic Uterine Septa Uterine septa may be the cause of reproductive problems, particularly miscarriages, in
about 20% of women affected by these uterine anomalies.21 Hysteroscopy has been used successfully to treat these conditions by simple
transection or by coagulation-resection. Uterine remnants have poor
visualization, seldom bleed upon division, and therefore can be easily
divided under hysteroscopic view. Before hysteroscopy is attempted, however, laparoscopic
or ultrasonographic examination of the uterus
is mandatory to rule out a bicornuate uterus. Furthermore, concomitant
laparoscopy helps the hysteroscopist in the transection of a uterine
septum to avoid possible injury to the fundal area of the uterus, once
the septum has been divided. Division of the uterine septum is relatively
easy when the septum is thin and partial, but broad septa that extend
the total length of the uterine corpus are challenging and require
experience in operative hysteroscopy. The septum is transected systematically
in the midline, avoiding drifting to the posterior or anterior
wall. By advancing the hysteroscopic division of the septum systematically
and visualizing the tubal openings, the hysteroscopist becomes
aware of myometrial thickness. When the junction between the septum
and myometrium begins, small arteries may be seen pulsating. If these
are cut, they bleed upon division, indicating that the septum has been
transected completely. With the symmetric visual view of the uterotubal
junctions and the laparoscopic uniform translucency of the hysteroscopic
light, the hysteroscopist can safely transect the uterine septum
without danger of perforation.
At the completion of the procedure, the intrauterine pressure produced
by the distending fluid may be lowered to less than 50 mmHg, and areas
of bleeding may be observed. Usually, small bleeders stop on their own,
but if the number of active arterial bleeders is significant, these can
be individually coagulated with a pinpoint electrode (Figs.
27, 28, and
29).22–25
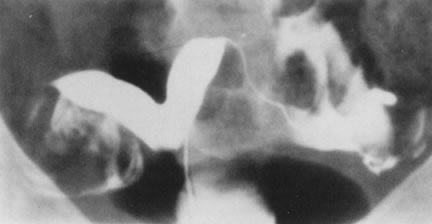 Fig. 27. Hysterosalpingogram shows a divided uterine cavity. Fig. 27. Hysterosalpingogram shows a divided uterine cavity.
|
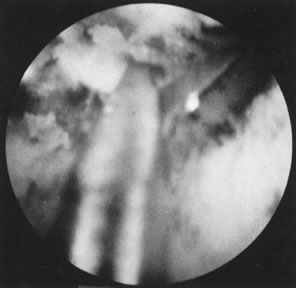 Fig. 28. Hysteroscopic division of septum with semi-rigid scissors. Fig. 28. Hysteroscopic division of septum with semi-rigid scissors.
|
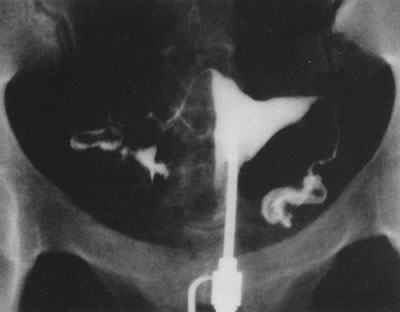 Fig. 29. Postoperative hysterosalpingogram shows normal uterine cavity. Fig. 29. Postoperative hysterosalpingogram shows normal uterine cavity.
|
The resectoscope may also be used to transect the septum, adding the advantage
of coagulation-transection, which is most beneficial when dealing
with very broad septa. A special straight knife or a loop oriented
forward can be used for this purpose, using the blended current for simultaneous
cutting and coagulation. Care should be taken not to overcorrect
the defect, because bleeding may not be a warning sign of invading
myometrium when blended current (cutting/coagulating) is used. Only
fluids without electrolytes should be used, and laparoscopic monitoring
is most helpful (Fig. 30).26 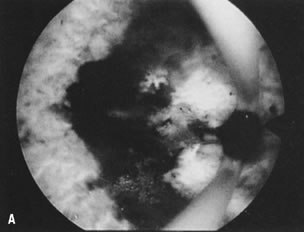 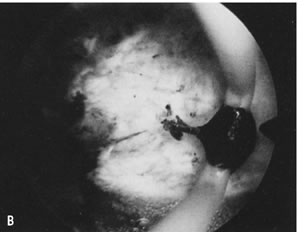 Fig. 30. ( A) and ( B )Resectoscopic division of broad uterine septum with knife electrode. Fig. 30. ( A) and ( B )Resectoscopic division of broad uterine septum with knife electrode.
|
The uterine septum can also be transected using fiber lasers. The Nd:YAG
adapted with a sculptured or extruded tip cuts on contact; the argon
or KTP-532 can also be adapted with a sharp fiber. Precautions similar
to those when using an electrical knife should be undertaken to avoid
uterine perforation.27,28 It is important to evaluate patients with pregnancy wastage who also have
uterine septa, to rule out genetic, endocrine, or metabolic problems. The
best indication for the hysteroscopic treatment of a uterine septum
is pregnancy wastage. This operation is relatively simple, and division
of the uterine septum seems most reasonable in infertile patients
who need to undergo laparoscopy for unexplained infertility, and in
patients who are candidates for insemination and have a uterine septum. Similarly, patients
who require in vitro fertilization or any other
reproductive technology procedure may benefit from hysteroscopic treatment
of the septum, even if they never have achieved a pregnancy. The results of hysteroscopic surgery for the uterine septum in terms of
reproductive outcome have been encouraging, comparing well with and even
surpassing previous results of abdominal metroplasties. The hysteroscopic
treatment of the symptomatic uterine septum is the method of choice
to treat this condition because of the obvious gains in performing
this operation endoscopically, particularly avoiding laparotomy and
hysterotomy and their accompanying morbidity; the decrease in hospitalization
and inconvenience for the patient; the decrease in cost; and
the avoidance of a routine cesarean section (Table 3).29–34 Table 3. Hysteroscopic Metroplasty
|
|
|
|
|
|
|
|
Pregnancy |
|
Author |
No. Patients |
Medium |
Technique |
IUD* |
E/P† |
Antibiotics |
Term |
Premature |
Abortion |
In Progress |
|
Edstrom, (1974) |
2 |
Dextran 70, 32% |
Rigid biopsy forceps |
+ |
+ |
- |
- |
19 weeks |
- |
- |
|
Chervenak and |
2 |
Dextran 70, 32% |
Scissors adjacent to |
+ |
+ |
+ |
1 |
- |
- |
- |
|
Neuwirth (1981) |
|
|
hysteroscope |
|
|
|
|
|
|
|
|
Rosenberg et al |
1 |
Dextran 70, 32% |
Flexible scissors |
N/A‡ |
N/A‡ |
N/A‡ |
N/A‡ |
- |
- |
- |
|
(1981) |
|
|
|
|
|
|
|
|
|
|
|
Daly et al (1983) |
25 |
Dextran 70, 32% |
Flexible scissors |
- |
+ |
- |
7 |
- |
1 |
- |
|
Perino et al |
11 |
CO2 |
Flexible, semi-rigid |
+ |
- |
- |
N/A‡ |
- |
- |
- |
|
(1985) |
|
|
scissors |
|
|
|
|
|
|
|
|
DeChemey et al |
72 |
Dextran 70, 32% |
Resectoscope |
- |
- |
- |
58 |
- |
4 |
4 |
|
(1986) |
|
|
|
|
|
|
|
|
|
|
|
Corson and |
18 |
Dextran 70, 32% |
Resectoscope and |
- |
- |
- |
10 |
1 |
2 |
2 |
|
Batzer (1986) |
|
CO2 |
rigid scissors |
|
|
|
|
|
|
|
|
Fayez(1986) |
19 |
Dextran 70, 32% |
Rigid scissors |
Foley Catheter |
- |
+ |
14 |
- |
- |
- |
|
March and Israel |
91 |
Dextran 70, 32% |
Flexible scissors |
+ |
+ |
- |
44 |
4 |
7 |
7 |
|
(1987) |
|
|
|
|
|
|
|
|
|
|
|
Valle (1987) |
59 |
D5W/Dextran |
Flexible, semi-rigid |
- |
+ |
+ |
44 |
2 |
5 |
- |
|
|
|
70, 32% |
|
|
|
|
|
|
|
|
|
Choe and Baggish |
19 |
Dextran 70, 32% |
Nd:YAG with bare or |
Foley Catheter |
+ |
+ |
10 |
1 |
1 |
3 |
|
(1992) |
|
|
sculptured fibers |
|
|
|
|
|
|
|
|
Fedele et al |
102 |
Dextran 40, 10% |
Semi-rigid scissors(80) |
+(21) |
+(39) |
+ |
45 |
10 |
11 |
NA‡ |
|
(1993) |
|
in normal saline |
Argon laser(10) |
|
|
|
|
|
|
|
|
|
|
|
Resectoscope(12) |
|
|
|
|
|
|
|
|
Valle (1996) |
124 |
D5 in 1/2 saline |
Semi-rigid scissors (98) |
- |
+ |
+ |
84 |
7 |
12 |
- |
|
|
|
Glycine 1.5% |
Resectoscope (20) |
|
|
|
|
|
|
|
|
|
|
|
Nd:YAG Laser (6) |
|
|
|
|
|
|
|
|
Totals |
545 |
|
|
|
|
|
317 (78.3%) |
26 (6.4%) |
43 (10.6%) |
18 (4.4%) |
*IUD, intrauterine device
†E/P, Estrogen/Progesterone
‡NA, Not Applicable
Modified from Siegler AM, Valle RF: Therapeutic hysteroscopic procedures. Fertil
Steril 50:685, 1988.
Treatment of Intrauterine Adhesions Hysteroscopy is the standard method for treatment of intrauterine adhesions; it
allows for selective division of adhesions without damaging the
surrounding healthy endometrium and offers an excellent alternative
to reestablish the symmetry and architecture of the uterine cavity. The
use of semirigid hysteroscopic scissors has improved lysis of adhesions
when the adhesions are extensive and composed of thick connective
tissue and/or are located at the uterotubal cones. In this situation, because
of the thinning of the uterine wall, laparoscopy is mandatory
to monitor the dissections and warn the hysteroscopist of perforation. Tubal
patency is evaluated, and peritoneal and adnexal adhesions are
assessed and frequently treated endoscopically.35–38
Although alternative methods for the surgical transection of adhesions
can be used, such as the resectoscope and/or fiber lasers with a low energy
output, the mechanical approach using hysteroscopic scissors seems more
logical, particularly to avoid peripheral damage to the remaining healthy
endometrium, which will be the reservoir for rapid uterine cavity reepithelialization
after treatment (Figs.
31, 32, 33,
34, and
35; see Color
Plate 1G, ).39,40
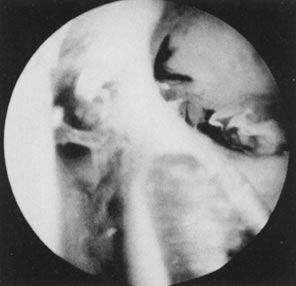 Fig. 31. Lateral intrauterine adhesion at right uterine cornual region. Scissors
approaching adhesion for division. Fig. 31. Lateral intrauterine adhesion at right uterine cornual region. Scissors
approaching adhesion for division.
|
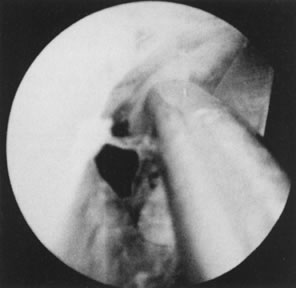 Fig. 32. Hysteroscopic division of adhesion. Fig. 32. Hysteroscopic division of adhesion.
|
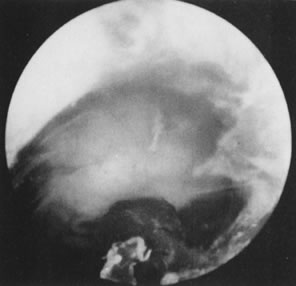 Fig. 33. Uterine cavity symmetry reestablished after hysteroscopic treatment. Fig. 33. Uterine cavity symmetry reestablished after hysteroscopic treatment.
|
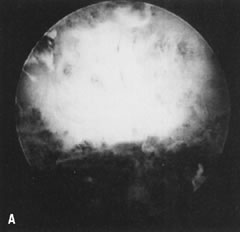 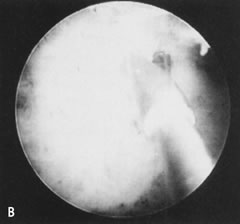 Fig. 34. A. Thick fundal adhesion close to uterotubal cone. B. Hysteroscopic division with scissors. Fig. 34. A. Thick fundal adhesion close to uterotubal cone. B. Hysteroscopic division with scissors.
|
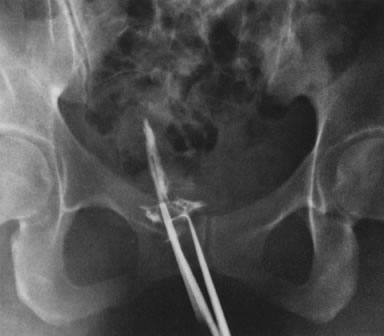 Fig. 35. Hysterosalpingogram shows complete uterine cavity occlusion by adhesions. Fig. 35. Hysterosalpingogram shows complete uterine cavity occlusion by adhesions.
|
When adhesions are extensive and composed of fibrotic connective tissue, a
uterine splint in the form of a no. 8 pediatric indwelling catheter, with 3 or 3.5 mL
of saline, is placed inside the uterine cavity after
the division of adhesions and is left in place for a week. Concomitant
prophylactic antibiotics are used during and after the procedure, particularly
when splints are left in place. Vibramycin is given 100 mg
twice daily orally for a week, or intraoperative cefazolin (Kefzol) 1 g
IV is given, followed by cephalexin (Keflex) 500 mg four times daily
orally for 6 to 7 days. To aid reepithelialization, oral conjugated
estrogens are prescribed (Premarin 2.5 mg twice daily for a 30-day cycle) with
terminal progesterone, medroxyprogesterone acetate (Provera) 10 mg
per day orally for the last 5 days of the artificial cycle to allow
withdrawal bleeding. When total uterine cavity occlusion has been
treated, this cycle may be repeated once or even twice. At the completion of the hormonal treatment, a hysterosalpingogram is performed
to assess the uterine cavity to determine if further surgery
may be necessary or if the patient may be allowed to attempt conception. The cumulative results reported with hysteroscopic treatment of intrauterine
adhesions have shown the reestablishment of normal menstruation
in over 90% of the patients treated. Nonetheless, the reproductive outcome
has paralleled the severity of the disease, with an overall pregnancy
rate of 60% to 70%, demonstrating that the more extensively the uterine
cavity is occluded by adhesions, and the older the adhesions are, the
poorer the prognosis is (Table 4).41–44 Table 4. Hysteroscopic Lysis of Intrauterine Adhesions*
|
|
|
|
|
Reproductive Outcome |
|
|
|
|
|
Menses nl. |
Pregnancy |
Term |
|
Author |
No. Patients |
Medium |
Technique |
No. (%) |
No. (%) |
No. (%) |
|
Levine and Neuwirth |
10 |
Hyskon |
Flexible scissors |
5 (50) |
2 (20) |
- |
|
1973 |
|
|
|
|
|
|
|
Edstrom 1974 |
9 |
Hyskon |
Biopsy forceps |
2 (22) |
1 (11) |
1 (11) |
|
Siegler and |
25 |
CO2 |
Target abrasion/ |
13 (52) |
11 (44) |
12 (44.4) |
|
Kontopoulos 1981 |
|
|
scissors/curettage |
|
|
|
|
March and |
38 |
Hyskon |
Flexible scissors |
38(100) |
38 (100) |
34 (79.1) |
|
Israel 1985 |
|
|
|
|
|
|
|
Neuwirth et al 1982 |
27 |
Hyskon |
Scissors alongside |
20 (74) |
14 (51.8) |
13 (48.1) |
|
Sanfilippo et al 1982 |
26 |
CO2 |
Curettage |
26 (100) |
6 (100) |
3 (50) |
|
Hamou et al 1983 |
69 |
CO2 |
Target abrasion |
59 (85.5) |
20 (51.3) |
15 (38.4) |
|
Sugimoto et al |
258 |
Hyskon/normal |
Target abrasion/ |
180 (69.7) |
143 (76.4) |
114 (79.7) |
|
1984 |
|
saline |
Kelly forceps |
|
|
|
|
Wamsteker 1984 |
36 |
Hyskon |
Scissors/biopsy forceps |
34 (94.4) |
17 (62.9) |
12 (44.4) |
|
Friedman et al 1986 |
30 |
Hyskon |
Resectoscope/scissors |
27 (90) |
24 (80) |
23 (76.6) |
|
Zuanchong and |
70 |
Normal saline |
Biopsy forceps/ |
64 (84.3) |
30 (85.7) |
17 (48.5) |
|
Yulian 1986 |
|
|
flexible scissors |
|
|
|
|
Valle and Sciarra |
187 |
D5 W/Hyskon |
Flexible/semirigid/ |
167 (89.3) |
143 (76.4) |
113 (79.7) |
|
1988 |
|
|
rigid scissors |
|
|
|
|
Lancet and Kessler |
98 |
Hyskon |
Felxible scissors/ |
98 (100) |
86 (87.8) |
77 (89.5) |
|
1988 |
|
|
electosurgery |
|
|
|
|
Pabuccu et al 1999 |
40 |
Glycine |
Murphy probe scissors |
33 (82.5) |
27 (67.5) |
23 (57.5) |
|
Feng et al 1999 |
365 |
Dextrose 5% |
Biopsy forceps/scissors |
294 (83.7) |
156 (83.8)* |
145 (92.9) |
|
Totals |
1298 |
|
|
1060 (87.5) |
718 (72.3) |
603 (87.2) |
*Of 186 desiring pregnancy
Modified from Siegler AM, Valle RF, Lindemann HJ. et al. Therapeutic Hysteroscopy. Indications
and Techniques, p. 103. St Louis, CV Mosby, 1990.
nl, Normal
Hysteroscopic Tubal Cannulation Fallopian tube obstruction is a significant cause of infertility in about 30% of
infertile women. Proximal obstruction of the fallopian tubes
occurs in about 10% to 20% of women who undergo hysterosalpingography
as part of their infertility evaluation.45,46 Laparoscopy is used to rule out physiologic spasms and to evaluate other
fallopian tube or pelvic pathology. When proximal fallopian tube obstruction
is confirmed by laparoscopy, surgical reconstruction can be
used to treat this condition. Because patients operated on for this condition
do not consistently show fibrosis of the occluded area, but often
show simple occlusion or obstruction by debris or proteinaceous material
plugging the tubal lumen, as demonstrated by Sulak and colleagues,47 tubal cannulation has been used as the initial method to treat these patients. New soft, small-caliber catheters have significantly facilitated this procedure, adapting
angiographic techniques with coaxial catheters to cannulate
the fallopian tubes (see Color Plate 1H). The hysteroscopic approach offers the advantage of ruling out tubal spasms
under laparoscopy and guiding catheters directly into the fallopian
tubes under direct vision. The concomitant use of laparoscopy allows
assessment of tubal patency and provides an opportunity to evaluate and/or
treat other pelvic conditions, such as pelvic adhesions and endometriosis. Tubal cannulation has variations, particularly the use of straight coaxial
catheters versus catheters with distal balloons to distend the cornual
tubal regions; from the published data, however, the outcome does
not vary with these techniques, and the simplicity of simple coaxial
catheters to cannulate the fallopian tubes makes them attractive to the
practitioner. Although dilatation of a vessel to flatten an atheroma
is useful, in the intramural portion of the fallopian tubes in which
the obstruction must be released, dilatation is not necessary because
the fallopian tube quickly recovers its normal anatomy after distention.48–52 The results of tubal cannulation are promising. The successful visualization
of the fallopian tubes at cannulation by hysteroscopy has shown
a 70% to 92% patency. The intrauterine pregnancy rate was about 47%, and
the ectopic pregnancy rate about 8%, in 50 patients after a follow-up
period of 12 months.53–58 Only those patients who fail tubal cannulation and who demonstrate true
fibrotic occlusion require microsurgical tubal reconstruction (Table 5).59 Table 5. Results of Hysteroscopic Cannulation of Proximal Tubal Obstruction
|
|
No. Of |
|
Complications |
|
|
Author |
Cases/Failed |
Catheter |
(Perforation) |
Pregnancies |
|
Confino et al50 (1986) |
1/0 |
Balloon |
1 |
— |
|
Daniell and Miller51 (1987) |
1/0 |
Urologic |
0 |
1 |
|
Sulak et al52 (1987) |
2/0 |
Epidural |
0 |
1 |
|
Confino et al53 (1988) |
12/5 |
Balloon |
3 |
2 |
|
Novy et al54 (1988) |
10/1 |
Cornual set |
1 |
2 |
|
Deaton et al55 (1990) |
11/4 |
Urologic |
2 |
6 (3 ectopic) |
|
Lin et al56 (1990) |
10/0 |
Urologic |
— |
5 (1 ectopic) |
|
Flood and Grow57 (1993) |
27/3 |
Cornual set |
4 |
15 |
|
Total |
74/13 (17.5%) |
|
11 (14.8%) |
32 (43.2%) | Although most tubal cannulations can be performed with a rigid operative
hysteroscope, the introduction of flexible operative hysteroscopes with
a 4.9-mm OD offers a useful alternative for tubal cannulation, particularly
when the tubal openings are angulated and difficult to localize
with rigid endoscopes, and anatomic variations in the uterine configuration
make localization difficult. The steerability of these endoscopes
greatly facilitates tubal cannulation by aligning the endoscope
in direct opposition to the proximal tubal ostia, simplifying the procedure
and reducing failures. Endometrial Ablation Significant numbers of patients undergo hysterectomy for treatment of abnormal
uterine bleeding of a nonorganic origin that fails to respond
to hormonal treatment. Many attempts to use conservative methods to treat
bleeding without a hysterectomy have failed. Recently, endometrial
destruction by laser energy or electrosurgery has been used to accomplish
this objective in an ambulatory setting. Because the number of women
requiting this treatment is substantial (it has been calculated that
more than 700,000 hysterectomies are performed annually in the United
States, and of those, 20% may be due to dysfunctional uterine bleeding), conservative
alternatives for treatment have a major role.60,61 There are two methods of accomplishing endometrial ablation: laser endometrial
ablation and electrosurgical endometrial ablation. LASER ENDOMETRIAL ABLATION. Laser endometrial ablation is performed with the Nd:YAG laser, with a wavelength
of 1064 nm, in the near infrared (invisible) portion of the
light spectrum. This laser is useful for endometrial ablation both because
of its penetration of 4 to 5 mm and because it can be transferred
through fluids without losing its energy. Because of its special property
of frontal, lateral, and back scattering, which creates deep craters
in the tissue when applied in contact with the tissue, it helps in
the destruction of the endometrium and of the superficial portion of
the myometrium. The technique of laser endometrial ablation requires an operative hysteroscope
and fluids with electrolytes, specifically sodium, because of
the time required for the procedure and the large amount of fluids necessary
during this procedure. The “bare” quartz fiber, 0.6 mm
in diameter, is introduced, after it has been tested for spot size
and functioning, and the endometrial ablation begins. Two techniques
are available to complete this task. One is the “dragging” or
touch technique, accomplished by direct contact between the bare quartz
fiber and the tissue: first the uterotubal cornual junctions and
then the anterior and posterior uterine walls. Care should be taken not
to destroy the endocervical tissue to avoid cervical stenosis (Fig. 36).62 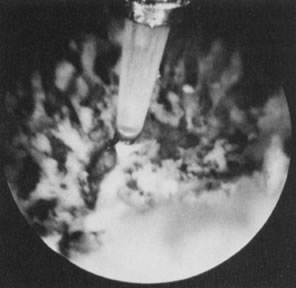 Fig. 36. Endometrial ablation with Nd:YAG laser by the contact technique. Fig. 36. Endometrial ablation with Nd:YAG laser by the contact technique.
|
The second technique, the nontouch or “blanching” technique, can
be used by firing the laser with a quartz fiber 1 or 2 mm away from
the tissue. This is particularly useful at the uterotubal cones, where
the thinnest portion of the myometrium may reach only 3.5 to 4 mm. With
blanching, it is often difficult to differentiate between tissue
that has been coagulated and tissue that has not been coagulated; therefore, it
is important to divide the uterine cavity in segments to permit
more systematic endometrial destruction. Many physicians prefer
a combination of these two techniques, using the nontouch technique at
the cornual regions and accomplishing the rest by the dragging or touch
technique (Fig. 37).63–65 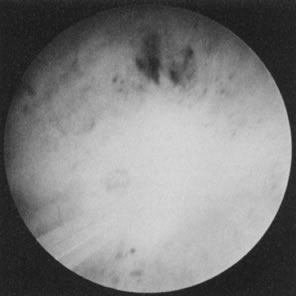 Fig. 37. Endometrial ablation with Nd: YAG laser with the noncontact technique. Fig. 37. Endometrial ablation with Nd: YAG laser with the noncontact technique.
|
The results of endometrial ablation with the Nd:YAG laser show a 95% resolution
of the abnormal bleeding and about a 5% failure rate. The aim
of this procedure is not to create amenorrhea but to resolve the abnormal, excessive
uterine bleeding.66 ENDOMETRIAL ABLATION BY ELECTROSURGERY. Using the resectoscope and a special electrode, the endometrium may be
resected or coagulated. The initial technique to accomplish endometrial
destruction was resection with use of the loop electrode. The difficulty
in maintaining a uniform depth of resection makes this technique
cumbersome, however. Furthermore, because resection could predispose to
more fluid intravasation and immediate postoperative bleeding, physicians
were prompted to use the alternative method of endometrial coagulation
with thicker roller-bar or roller-ball electrodes, which can accomplish
endometrial destruction just by coagulation. This technique is
simpler, faster, and as effective as resection, and the instrumentation
and electrical power units required are less expensive than those
required for laser ablation. In general, pure coagulating current of 40 to 50 W
or, alternatively, pure cutting current of 100 to 120 W, can
be used to accomplish this procedure. Because the electrical current
used is unipolar, grounding of the patient is necessary to complete the
electrical circuit, and no electrolytes should be used with the distending
media. The possibility of fluid overload and water intoxication
must be carefully monitored, measuring precisely the inflow and outflow
of fluids used and deducting the deficit of fluid not recovered.67–74 The results obtained with endometrial ablation by electrosurgery are similar
to those achieved with lasers. Nonetheless, it seems that the failure
rate is slightly higher (5% to 10%), as one would expect, because
electrosurgery may not be as predictable as the laser energy, particularly
when visual appraisal of the thermal damage cannot predict uterine
wall penetration (Fig. 38).66,74,75 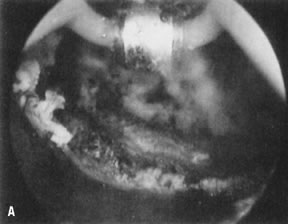 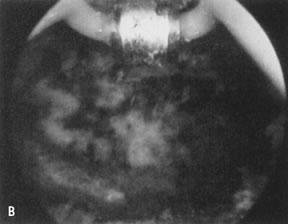 Fig. 38. Endometrial ablation with a roller-bar electrode. A. Line of demarcation can be seen between treated and nontreated area. B. Completed procedure. Fig. 38. Endometrial ablation with a roller-bar electrode. A. Line of demarcation can be seen between treated and nontreated area. B. Completed procedure.
|
All patients undergoing endometrial ablation benefit from hormonal preparation
to stop bleeding and produce atrophy of the endometrium, making
the actual technique simpler and more efficacious. The thinner the endometrium, the
better the chance of penetrating the superficial portion
of the myometrium and destroying those deeper glands that may be the
source of reepithelialization. Although progesterones occasionally have
been used to atrophy the endometrium, the pseudodecidual reaction
they produce impairs visualization, and the response of the endometrium
is somewhat erratic. Danazol 800 mg a day for 4 to 6 weeks has also
been used, although patient compliance is not as uniform because of its
side effects. Leuprolide acetate (Lupron) is an excellent alternative
and can be given in its depot form 3.75 mg IM with two doses 1 month
apart; the operation is performed 2 to 3 weeks after the second injection. Alternatively, a 7.5-mg IM depot injection can be used as a single
dose, although some patients may not achieve enough endometrial atrophy
despite the shortening of the flare-up phase of these agonists.66 Complications of Operative Hysteroscopy Operative hysteroscopy involves the possibility of serious complications, as
compared with diagnostic hysteroscopy. Uterine perforation, fluid
overload from distending media, infection, and associated injuries related
to laser or electrosurgical energies are the most common complications
related to operative hysteroscopy. Operative hysteroscopy requires experience in diagnostic hysteroscopy and
should not be undertaken as the first step in any hysteroscopic therapeutic
procedure. Necessary instrumentation should be available and
precautions taken, such as concomitant laparoscopy for extensive operative
procedures that risk uterine perforation. Operative hysteroscopy
is best performed in the operating room with the patient under general
anesthesia, except when minor interventions are performed, such as the
removal of IUDs, biopsy of some endometrial lesions, and division of
mild filmy adhesions that only partially connect the uterine walls. The manipulation of instruments within the uterine cavity, therefore, should
be performed with caution and delicacy, and when difficult manipulations
are necessary, concomitant laparoscopy should be liberally used. The distending media, particularly low-viscosity fluids, should be carefully
monitored as to the amount infused and the amount recovered, to
measure serially the deficit of these fluids, particularly when fluids
without electrolytes are used. When the operations are prolonged or require
significant dissections, urine output should be monitored, as well
as vital signs and pulse oximetry, in conjunction with the anesthesiologist. Prudent
use of diuretics when necessary may be helpful, and
the operation should be stopped should any of these variables be abnormal.76–79 Bleeding may occur during an operative procedure, particularly during the
division of a uterine septum or the removal of a leiomyoma, and immediate
hemostasis should be accomplished with either electrosurgery or
mechanical tamponade. Although infection has not been a major problem
for operative hysteroscopy, caution should be exercised in patients who
undergo extensive operations, and prophylactic antibiotics should be
used if deemed necessary, particularly in infertility patients and patients
who may be at risk for infection.80,81 Laser energy should not be used without knowledge of the physics and properties
of these lasers as well as the interaction of these lasers with
tissues. Safety measures should be taken to protect the operator from
back-scattering of the fiberoptic lasers, using appropriate filters, safety
goggles, or, alternatively, video systems as protection from
retinal injuries. In addition, precautions should be taken to protect
patients from deeper laser penetration to avoid uterine perforation. Similarly, electrosurgical
energies should be used with caution and understanding
of the biophysical properties of these energies. Specific precautions
should be taken regarding the use of the different electrodes
available and their properties, the effects on tissue, and the drawbacks. When a patient has a background of specific indications and there are no
contraindications, and when meticulous attention is paid to details
in the different aspects of operative hysteroscopy, an operator can perform
these techniques safely and with minimal or no complications. |