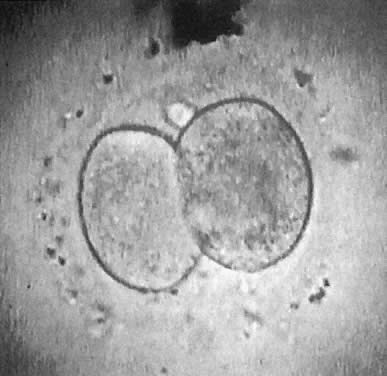
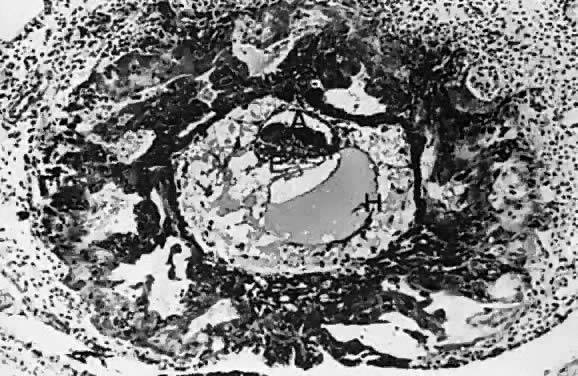
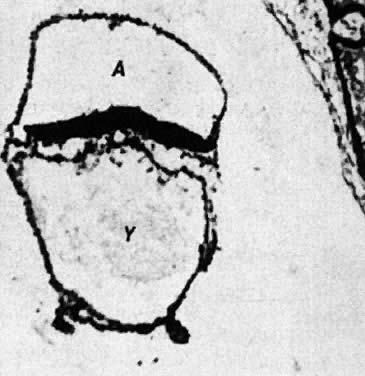
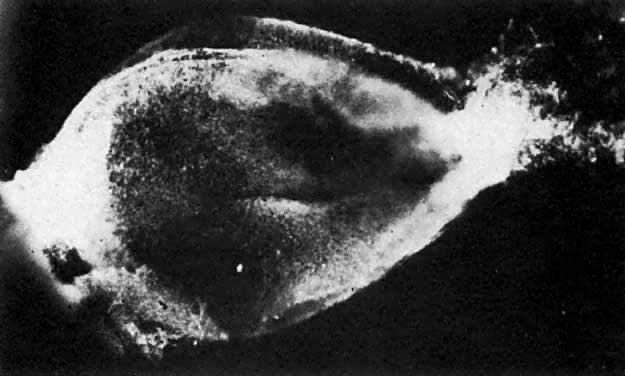
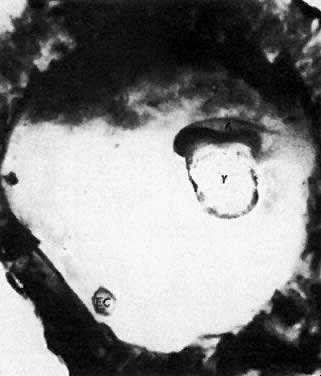
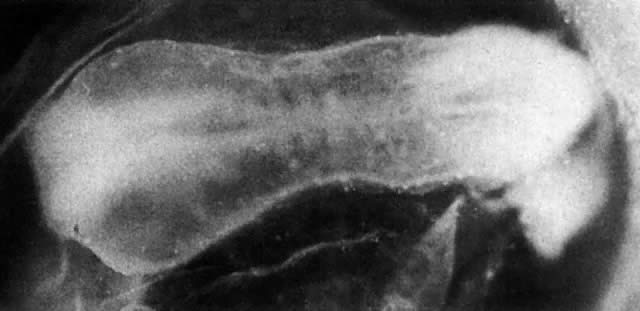
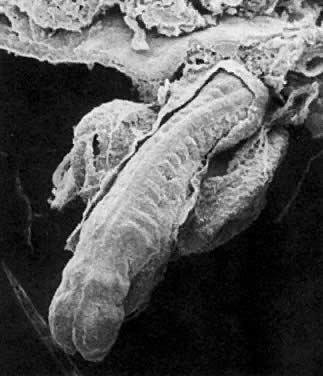
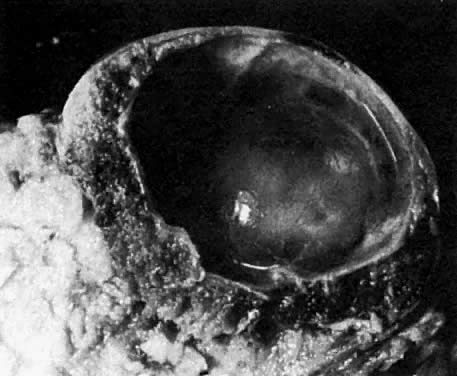
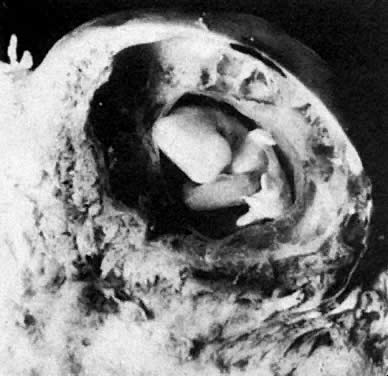
At fertilization, the oocyte is round, approximately 150 μm in diameter, and enclosed by zona pellucida. Zona pellucida is approximately 10 μm thick, composed of glycoproteins contributed by the oocyte during its growth, which occurs at the beginning of follicular growth.11,12 The cleavage of the oocyte and the formation of the early blastocyst take place in the space limited by the zona pellucida. During the pre-embryonal stage (days 1 to 4) the conceptus is transported by the oviduct into the uterine cavity. During days 5 to 6, the blastocyst increases in size, and the zona pellucida ruptures and is rejected (hatching of oocyte). The diameter of the “free blastocyst” is approximately 200 μm. On day 7, the blastocyst implants into the edematous, compact (superficial) layer of the undecidualized functional layer of endometrium.13 The bilaminar embryonal disc, aged 7 to 14 days, is round and 0.2 to 0.7 mm in diameter. During blastogenesis, the term ectoblast, endoblast, and mesoblast are used. Beginning with organogenesis (somite formation), these terms are replaced by ectoderm, endoderm, and mesoderm.
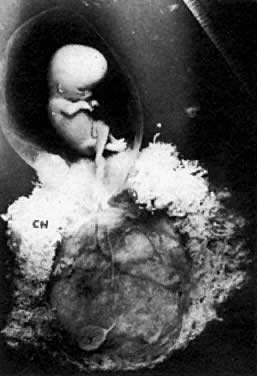
The trilaminar embryonal disc, aged 14 to 20 days, is pear-shaped and 0.5 to 2.5 mm long. Organogenesis begins with the formation of somites.
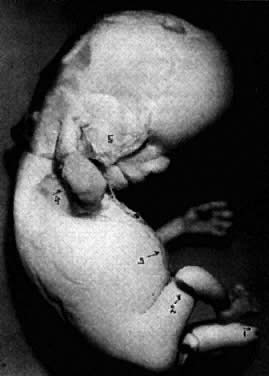
During stage 6, characterized by formation of somites and closure of the neural tube, the embryo becomes delineated by anterior, lateral, and posterior amnionic folds. The embryo becomes cylindric and 2 to 3 mm long. After closure of neural tube, as the primordium of central nervous system grows faster than the other portions of the embryo, the embryo bends ventrally and is C-shaped. At stage 7, the head, neck, trunk, and limbs become evident. The primary lip closes (substage 7–6), and human features are conclusive.
At the end of the embryonal period, the eyelids grow and (days 56 to 60 postconception) their rims finally fuse, closing the eye fissure.
During the fetal period (gestational weeks 10 to 26, stage 9), the eyes of the fetus are closed. At the early human fetal period (gestational weeks 11 to 12), male and female differentiation of external genitalia occurs. The fetal weight increases from approximately 5 to 50 g. During the human midfetal period, the TBW increases from 51 g to approximately 250 to 300 g. During the late fetal period, the fetus weighs more than 300 g and less than 500 g (if live born), or less than 1000 g if aborted dead. Fetal increase in the weight of brain, lung, kidney, adrenal, thyroid, and placenta14 is presented in Table 2. Clinically, embryonal and fetal growth can be followed with great accuracy using ultrasound anthropometry.15,16,17
| Organ Weight (g) | ||||||
Gestational | TBW (g) | CRL (mm) | CHL (mm) | ||||
Weeks | (average) | (average) | (average) | Adrenals | Brain | Heart | Kidneys |
16 | 117 | ||||||
| 120 | 175 | 0.86±0.08 | 30.7±5.6 | 1.38±0.57 | 1.72±0.57 | |
| 200* | ||||||
20 | 325 | 175 | 240 | 2.5 | 45–50 | 4.0 | |
24 | 500* | 1.96±1.2 | 71.0±15 | 3.60±1.5 | 4.42±1.65 | ||
| 640 | 215 | 295 | 3.2±3.8 | 112±36 | 9.0±4.0 | |
| 800* | 2.8±2.0 | 112.2±13.5 | 5.75±2.35 | 7.75±2.67 | ||
28 | 1000* | 3.32±2.5 | 139.2±29.0 | 7.19±3.0 | 10.1±3.4 | ||
| 1150 | 250 | 350 | 3.9–4.1 | 169±50 | 13±5 | |
32 | 1810 | 280 | 400 | 4.8–4.9 | 283±50 | 17±5 | |
36 | 2650 | 320 | 450 | 6.2–6.9 | 354±70 | 22±5 | |
40 | 3200 | 360 | 500 | 8.0–8.5 | 400±70 | 30±9 | |
* According to Shepard et al14
TBW, total body weight; CRL, crown-rump length; CHL, crown-heel length.
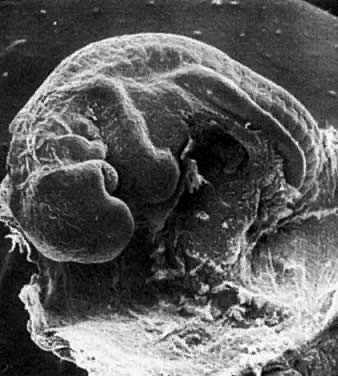
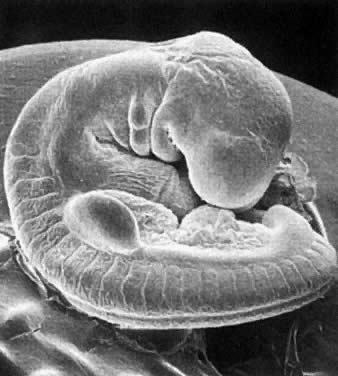
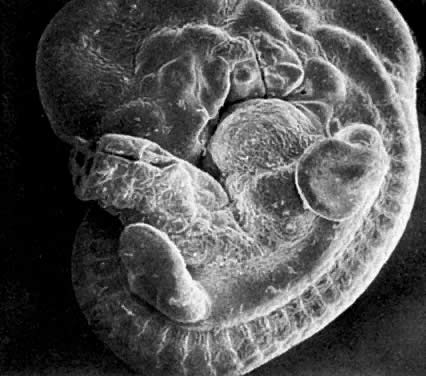
Application of the same criteria for staging prenatal development of human, macaque monkey, rat, and mouse, which are commonly used in teratogenic experiments and drug testing, is given in Table 3. Human embryos at different developmental stages are depicted in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14, Figure 15, and Figure 16.
TABLE 3. Human Prenatal Development: Comparison with Experimental Animals
| Human | Macaques | Rat | ||||
Comparative Stages (Jirásek) | Length | Age | Length | Age | Length | Age | |
Characteristic | Stage | (mm) | (days) | (mm) | (days) | (mm) | (days) |
Unicellular | 1 | 0.2 | 0–2 | 0.15 | 1 | 1 | |
Blastomeric (16–20 | 2 | 0.2 | 2–4 | 0.15 | 2–4 | 2–3 | |
blastomeres) | |||||||
Blastodermic | 3 | 0.4 | 4–6 | 0.2–0.3 | 7–9 | 4–5 | |
Bilaminar embryo stage | |||||||
Bilaminar plate | 4–1 | 0.1 | 6–14 | 0.3 | 10–13 | 6–7 | |
Primary yolk sac | 4–2 | ||||||
Secondary yolk sac | 4–3 | 0.2–0.4 | |||||
Trilaminar embryo stage | |||||||
With primitive streak | 5–1 | 0.4–1.0 | 15–17 | 0.5 | 17–19 | 8–9 | |
With notochordal process | 5–2 | 1.0–2.0 | 17–20 | 1.5 | |||
Early somite stage | |||||||
Completely open neural | 6–1 | 1.5–2.0 | 20–21 | 1.9 | 21–24 | 9½ | |
groove | |||||||
Neural tube closing, both | 6–2 | 1.5–4.0 | 21–26 | 4.0 | 24 | 1.3–3.0 | 10–10¾ |
ends open | |||||||
One or both neurospores | 6–3 | 3–5 | 26–30 | 4.0–6.0 | 24–26 | 3–4.1 | 11 |
closed | |||||||
Stage of limb development | |||||||
Bud of proximal extremity | 7–1 | 4–6 | 28–32 | 6.0 | 26 | 4–4.5 | 11 |
Buds of proximal and distal | 7–2 | 5–8 | 31–35 | 8.0 | 27 | 4–6 | 11.5 |
extremities | |||||||
Proximal extremity two | 7–3 | 7–10 | 35–38 | 5.8–8 | 13 | ||
segments | |||||||
Proximal and distal | 7–4 | 8–12 | 37–42 | 7–9 | 8–9.5 | 13½ | |
extremity two segments | |||||||
Digital rays, foot plates | 7–5 | 10–14 | 42–44 | 10 | 34 | 10 | 14 |
Digital tubercles | 7–6 | 13–21 | 44–51 | 9–11 | 36 | 12.5 | 15½ |
Digits, toe tubercles | 7–7 | 19–24 | 51–53 | 19 | 41 | 16 | 17 |
Late embryonal stage | |||||||
Differentiated extremities | 8–1 | 22–23 | 52–56 | 22 | 44 | 19 | 17½ |
Fusing eyelids | 8–2 | 27–35 | 56–60 | 39–44 | 52 | 22 | 19½ |
Fetal period | 9 | 31–200 | 60–182+ | 40–260 | 53–170 | 25 | 19–21 |
Perinatal period | 10 | 201–450 | 180–266+ | postnatal | postnatal | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
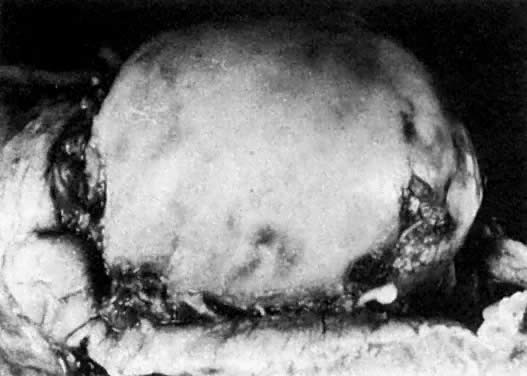
Prenatal development is influenced by various external factors. The prenatal sensitivity of an individual to external influences, including xenobiotics, varies according to the stage of development. Generally, if the mammalian embryo is exposed to damaging agents during blastogenesis (corresponding to human gestational weeks 1 to 5), either the embryo is killed and subsequently aborted, or it survives without any anatomic defects. If the embryo is exposed to nociceptive influences during the organogenic period (corresponding to human gestational weeks 6 to 10), the probability of anatomic malformations is high. Mechanical injuries causing amnionic ruptures and bleeding into the amnionic cavity, especially during gestational weeks 8 to 12, may produce limb amputations and facial disruptions related to blood clotting and, consequently, fibrin cords and deposits on the surface of the embryo or fetus (Fig. 17). The affected embryo usually survives and is not aborted, but it is malformed. During the fetal and perinatal periods, the probability of anatomic malformations decreases; however, damage to the brain and the sense organs (ears, eyes) can never be ruled out. Fetal death followed by abortion can occur at any stage of prenatal development. In humans, serious brain damage during the perinatal period becomes manifested relatively late postnatally as psychomotoric and mental retardation.
The intrauterine survival of the conceptus depends mostly on uteroplacental and fetoplacental circulations. Fetal brain and kidneys are unimportant for intrauterine survival: fetuses with anencephaly or agenesis of both kidneys may develop till term. The early extrauterine survival of the newborn depends on respiratory and circulatory adaptations and on neurovegetative integrations. Obstruction of the intestinal passage and urination are tolerated in newborns for a period of 2 or 3 postnatal days.