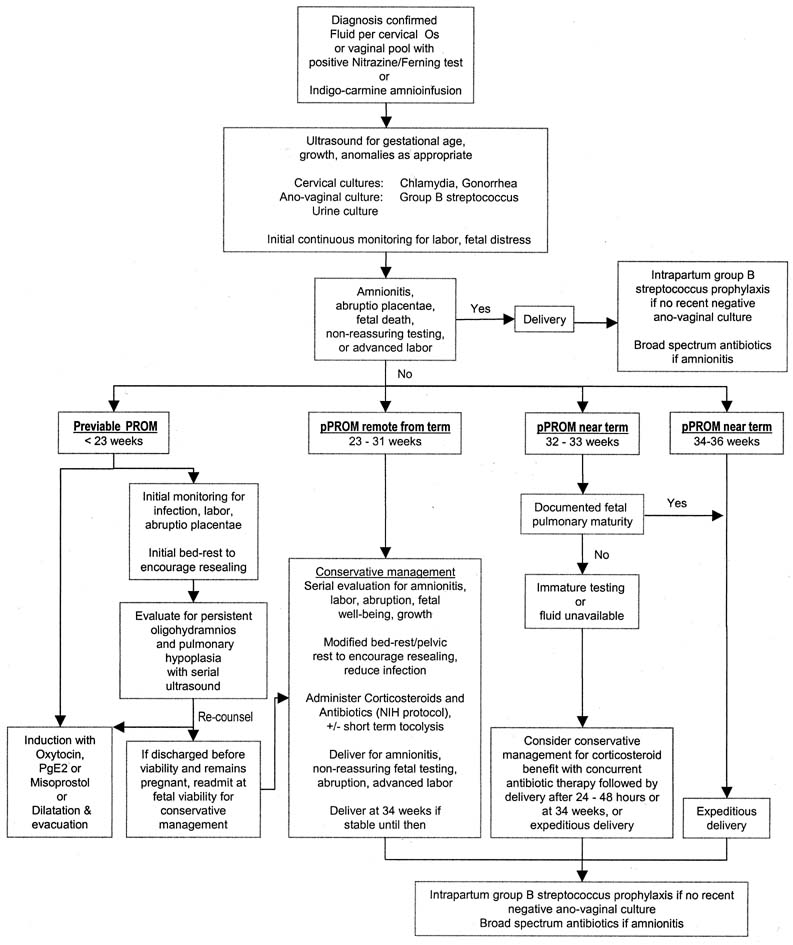
Assessment of Fetal Pulmonary Maturity From Vaginal Pool Specimens Fetal pulmonary maturity testing is helpful when pPROM occurs near term. Clinical
studies suggest a useful role for vaginally collected amniotic
fluid specimens for assessment of fetal pulmonary maturity. Neither
lecithin nor sphingomyelin have been isolated in significant amounts
from vaginal lavage fluid when the membranes are intact.39 This group found the L/S ratio to be unaffected by vaginal cervical
saline washes, and others have found no differences in the L/S ratio
or lecithin between vaginal and amniocentesis specimens.40 Golde found the L/S ratio, PG, and PI levels to be similar when collected
directly from the vagina or collected over a period of hours from
perineal pads.41 Shaver and coworkers found a close correlation between L/S ratios
obtained from vaginal pool and amniocentesis and an 89% concordance
regarding fetal pulmonary maturity.42 The mean L/S ratio from vaginal pool was not significantly higher
than from amniocentesis (2.56 vs. 2.3:1, p = .06). In that study, all patients with a positive amniocentesis
result also had PG present in the vaginal pool. In an evaluation
of 447 women with PROM, PG determinations from vaginal fluid collected
via perineal pads were found to be highly predictive of fetal pulmonary
maturity (.8%) and similarly predictive of pulmonary
immaturity (34%) to specimens collected by amniocentesis.43 No cases of RDS occurred after a mature L/S ratio, PG, or TDx FLM
result greater than 50 mg/g from vaginal fluid collection.44 A study of 153 women with pPROM at 30 to 36 weeks found a mature S/A
ratio (55 mg/g or more) to carry a predictive value of 98%, whereas 24% of infants with an immature result less
than 40 mg/g had RDS.45 Nonpulmonary phospholipids in blood may alter the results of nonspecific
fetal pulmonary maturity tests for pulmonary phospholipids. Because
maternal serum has an intrinsic L/S ratio of 1.3 to 1.5:1, significant
blood contamination could falsely elevate an immature result and
lower a mature result;46 however, a mature L/S ratio result should be reassuring, as blood
contamination would not be expected to increase to an immature result
to a mature level. Blood contamination can falsely lower the TDx FLM assay
result, but a mature result reliably predicts fetal pulmonary maturity.47 Because red blood cell phospholipids may interfere with the TDx FLM result, some
elect not to perform a TDx FLM II assay if there is blood in
the specimen.48 Meconium contamination increases the L/S ratio by .1 to .2:1 in preterm
infants and by as much as .5:1 after 35 weeks.49 The PG test is not affected by blood or meconium contamination. Practically, if
significant blood or meconium is present in a vaginal pool specimen, serious
consideration should be given to expeditious delivery
for fetal indication, rather than conservative management. Amniocentesis Amniocentesis may be particularly helpful in the setting of pPROM, assisting
in the diagnosis of membrane rupture, evaluation of fetal pulmonary
maturity, and assessment of intrauterine infection, when appropriate. Amniocentesis
and amnioinfusion should be performed under ultrasound
guidance. Particular attention should be paid to avoid the umbilical
cord, which may be confused with an amniotic fluid pocket in the case
of severe oligohydramnios. Amnioinfusion of indigo carmine dye will effectively confirm or rule out
the diagnosis of membrane rupture if noninvasive testing is equivocal. One
technique is to infuse 10 mL of sterile saline mixed with .5 to 1 mL
of indigo carmine dye into the amniotic fluid using a 22-gauge
spinal needle under direct ultrasound visualization. The absence
of blue discoloration on a perineal pad worn for several hours effectively
rules out the diagnosis of membrane rupture. Methylene blue should
not be injected for this purpose because it can exacerbate methemoglobinemia
and anemia in individuals with glucose-6-phosphotase
deficiency, among other potential side effects. Amniocentesis may be performed to obtain an adequate fluid volume for fetal
pulmonary maturity testing if there is inadequate vaginal fluid for
collection. There is no reason to believe that amniocentesis fluid
after pPROM will yield less predictive results than that obtained at amniocentesis
with intact membranes. The diagnosis of intrauterine infection is not always clear. The combination
of fever (temperature exceeding 38°C or 100.4°F) with
uterine tenderness and/or maternal or fetal tachycardia are
suggestive of chorioamnionitis in the absence of another source of infection. However, maternal fever is not always present early in the course
of infection. While a rising maternal blood leukocyte count may
be useful in the presence of suspicious clinical findings, the count may
be artificially elevated if antenatal corticosteroids have been administered
within 5 to 7 days. Should maternal symptoms, physical findings, and/or
hematological findings be equivocal, amniocentesis may
be useful in evaluating for intra-amniotic infection. An amniotic
fluid glucose concentration less than 16 to 20 mg/dl, a positive
Gram stain, elevated interleukin levels, and positive amniotic fluid
culture have been associated with increased early delivery and perinatal
infectious morbidity.50,51,52,53 However, positive tests are not 100% predictive and may take time
or be unavailable at certain centers. A positive result should be correlated
to clinical findings before action is pursued, and definitive
intervention should not be delayed for a test result if the clinical
diagnosis of infection is clear. Assessment of Fetal Well-Being MATERNAL PERCEPTION OF FETAL ACTIVITY Fetal testing may be useful in the identification of fetal compromise, umbilical
cord compression, fetal infection, or labor. Fetal activity
does not decrease significantly after membrane rupture.54The simplest form of fetal testing is maternal observation of fetal activity. A
maternal perception of decreasing fetal activity is helpful in
identifying patients who require intensive or prolonged fetal evaluation. FETAL HEART RATE TESTING Fetal heart rate monitoring, including the nonstress test, is particularly
well-suited for the evaluation of pregnancies complicated by
pPROM. Such testing may identify umbilical cord compression by the presence
of variable-type heart rate decelerations, fetal compromise
by the presence of a nonreactive nonstress test, fetal tachycardia
and/or recurrent late-onset decelerations, and infection
by the presence of fetal tachycardia and/or a lack of fetal heart
rate reactivity.50,55,56 Such testing also offers the opportunity for evaluating maternal contractions
by external tocodynamometry. Occasionally, recurrent variable
decelerations will be identified before the identification of symptomatic
uterine contractions.35,56,57 BIOPHYSICAL PROFILE TESTING Fetal biophysical activity not only is affected by fetal compromise but
also is affected by intrauterine infection. A number of studies have
demonstrated decreasing fetal breathing activity, movement, and tone before
maternal and neonatal infection.58,59,60,61 Other studies have found the relationship between fetal biophysical activity
and intrauterine infection to be weak.62,63 Because fetal nonstress testing has a high false-positive rate, biophysical
profile testing is a useful adjunct that may allow continued
expectant management in the face of a nonreactive nonstress test. Such
testing is particularly useful remote from term, when fetal heart
rate accelerations are less likely to be seen. Antibiotic Therapy GROUP B STREPTOCOCCUS PROPHYLAXIS Intrapartum antibiotic prophylaxis against group B streptococcus is indicated
for women with pPROM who are known group B streptococcus carriers
or whose carrier status is unknown.64 Intrapartum group B streptococcus prophylaxis with intravenous penicillin
as a 5-million unit initial bolus followed by 2.5 million units
every 4 hours or ampicillin 2 g followed by 1 gram intravenously every 4 hours (erythromycin, 500 mg
intravenous every 6 hours or clindamycin, 900 mg intravenous every 8 hours, if
penicillin-allergic) should be administered
during labor or before cesarean section after pPROM unless there is
a negative anovaginal culture obtained within 5 weeks of delivery. Because
of increasing resistance of group B streptococcus to erythromycin
and clindamycin, the Centers for Disease Control have recommended intravenous
treatment based on culture sensitivities, cefazolin or vancomycin
therapy for those with documented penicillin allergy. Vancomycin
should be considered for those with a significant likelihood of anaphylaxis
or compromise with penicillin exposure, unless sensitivity to erythromycin
or clindamycin has been demonstrated.65,66,67 Because latency is generally short and delivery may occur rapidly in the
face of nonreassuring fetal testing, it may be prudent to initiate
group B streptococcus therapy pending the receipt of a negative culture. Group
B streptococcus carriers who are treated and have prolonged latency
may have persistent vaginal colonization despite treatment or may
become recolonized from the rectum. Therefore, women with group B streptococcus
colonization on admission should be administered prophylaxis
again in labor. THERAPEUTIC ANTIBIOTICS Antibiotic therapy should be administered during conservative management
of pPROM remote from term to treat or prevent ascending decidual infection. Such
treatment has been shown to prolong pregnancy and offer the
opportunity for reduced neonatal infectious and gestational age-dependent
morbidity. More than two dozen randomized trials in this
regard are addressed in detail elsewhere.68,69,70,71,72 These studies are generally small and use varied approaches to antibiotic, corticosteroid, and
tocolytic administration, making direct comparison
difficult. Two large multicenter clinical trials have adequate power
to evaluate the usefulness of adjunctive antibiotics after pPROM
in reducing infant morbidity and approached this issue differently.73,74 The combination of these studies, and specific details from individual
trials, offer valuable insights. The National Institutes of Child Health and Human Development Maternal
Fetal Medicine Units (NICHD-MFMU) research network studied
women with pPROM from 24 to 32 weeks' and 0 days gestation.73 This study was designed to determine if antibiotic therapy would reduce
the number of infants who were acutely ill after birth. These investigators
gave initial aggressive intravenous therapy (48 hours) with ampicillin (2 g intravenous every 6 hours) and erythromycin (250 mg
intravenous every 6 hours), followed by limited duration oral therapy (5 days) with amoxicillin (250 mg orally every 8 hours) and
enteric-coated erythromycin base (333 mg orally every 8 hours). These agents offered the benefits
of broad-spectrum antimicrobial coverage and demonstrated
safety in pregnancy. The duration of therapy was limited to minimize selection
of resistant bacteria that could be more difficult to treat should
neonatal infection occur. All group B streptococcus carriers were
treated with ampicillin for 1 week and then again in labor, regardless of whether they were assigned
to antibiotic or placebo therapy. Antibiotic treatment improved
neonatal health by reducing the number of babies with one or more major
infant morbidity (composite morbidity: death, RDS, early sepsis, severe
IVH, severe necrotizing enterocolitis) from 53% to 44% (p < .05). Antibiotic therapy also led to significant reductions
in individual infant morbidities including RDS (40.5% vs. 48.7%), stage 3 or 4 necrotizing enterocolitis (2.3% vs. 5.8%), patent ductus arteriosus (11.7% vs. 20.2%), and bronchopulmonary dysplasia (13.0 vs. 20.5%, p = 0.05 or less) for each. Regarding infectious morbidity, the
antibiotic study group had a lower incidence of neonatal group B streptococcus
sepsis (0 vs. 1.5%, p = .03), amnionitis (23% vs. 32.5%, p = .01), neonatal sepsis (8.4% vs. 15.6%, p = .009), and pneumonia (2.9% vs. 7.0%, p = .04) among those who were not group B streptococcus carriers. Similar
to other studies, antibiotic treatment increased the likelihood
that patients would remain undelivered after 7 days of treatment
two-fold. Despite discontinuation of antibiotics at 7 days, treated
women were more likely to remain pregnant for up to 3 weeks after
randomization, suggesting that antibiotic therapy actually successfully
treated subclinical infection rather than just suppressing it. Mantel-Haensel
chi square analysis of randomized trials with initial
broad-spectrum parenteral therapy for women with pPROM at
less than 34 weeks70,71,72,73,75,76,77,78,79,80,81 reveals that antibiotics increase the likelihood of women remaining pregnant
more than 1 week (odds ratio [OR] and 95% confidence
interval [CI]: 2.52 and 1.92 to 3.30), reduce
amnionitis (OR: .60; 95% CI: .47–.78), reduce
neonatal sepsis (OR: .47; 95% CI: .33–.67), reduce
RDS (OR: .76; 95% CI: .60–.96), reduce IVH (OR: .70; 95% CI: .52–.95), without an increasing
the risk of necrotizing enterocolitis (OR: 1.28; 95% CI: .84–1.98). Kenyon and associates performed a large multi-arm, multicenter placebo-controlled
trial of oral antibiotics for pPROM at less than 37 weeks.74 Treatment included oral erythromycin, oral amoxicillin-clavulanic
acid, both, or placebo for up to 10 days. Oral erythromycin led to
brief pregnancy prolongation (not significant at 7 days), reduced
need for supplemental oxygen (31.1% vs. 35.6%, p = .02), and reduced positive blood cultures (5.7 vs. 8.2%, p = .02), but not other morbidities. In singleton gestations, erythromycin
treatment reduced oxygen dependence at 28 days (6.9% vs. 8.9%, p = 0.03), positive blood cultures (5.3% vs. 7.4%, p = .04), abnormal cerebral ultrasonography (3.0% vs. 4.6%, p = .04), and composite morbidity (11.2% vs. 14.4%, p = .02). Oral amoxicillin-clavulanic acid prolonged
pregnancy (43.3% vs. 36.7% undelivered after 7 days, p = .005) and reduced the need for supplemental oxygen (30.1 vs. 35.6%, p = .05), but increased the risk of necrotizing enterocolitis (1.9% vs. .5%, p = .001) without reducing other neonatal morbidities. Similar
findings were seen with the combination of oral amoxicillin-clavulanic
acid and erythromycin. The authors concluded that oral erythromycin
had significant effects on major neonatal disease and might have
a substantial health benefit, but raised concern regarding the increased
incidence of necrotizing enterocolitis with oral amoxicillin-clavulanic
acid. The latter finding is at odds with the NICHD-MFMU
trial finding of reduced stage 2 or 3 necrotizing enterocolitis
with aggressive antibiotic therapy in a higher-risk population. In summary, aggressive antibiotic therapy with erythromycin and amoxicillin/ampicillin
during conservative management of pPROM remote from
term appears to offer benefit in reduction of infant infectious and
gestational age-dependent morbidity. If aggressive therapy is not
administered, oral erythromycin may offer some potential benefit. The
combination of oral erythromycin and extended-spectrum ampicillin-clavulanic
acid in a lower-risk population near term
does not appear to be beneficial and may be harmful. This latter regimen
is not recommended. Because of the potential negative effects raised
in the Kenyon trial, consideration to the benefits of expeditious
delivery rather than conservative management with antibiotic treatment
should again be considered when pPROM occurs near term. Antibiotic therapy of positive amniotic fluid cultures in the asymptomatic
patient remote from term is attractive, but controversial. One case
report has suggested the efficacy of such therapeutic antibiotic intervention
in a patient with a positive amniotic fluid culture for Bacteroides bivius, Veillonella parvula, and Peptococcus at 29 weeks' gestation.82 However, there are no properly conducted trials to address the safety
and efficacy of therapeutic antibiotics in this setting. Induction of Fetal Pulmonary Maturity The current NIH consensus conference recommendations regarding corticosteroid
administration in the setting of pPROM are that a single course
of betamethasone (12 mg intramuscular every 24 hours for 2 doses) or dexamethasone (6 mg intramuscular every 12 hours for 4 doses) be administered
during conservative management of pPROM before 30 to 32 weeks' gestation
because of the potential for reduction of IVH.83 The efficacy of corticosteroids in the setting of pPROM has been questioned. While
it has been suggested that women with pPROM will deliver
too quickly to accrue the potential benefits of antenatal steroids, that
pPROM itself might induce fetal pulmonary maturation, thereby making
corticosteroids unnecessary, and that antenatal corticosteroids might
increase the risk of perinatal infection, data in this regard are not
convincing. Approximately 80% of women with pPROM remote from
term will remain pregnant for at least 24 hours, if antibiotic therapy
is administered. RDS remains the most common morbidity after preterm
birth caused by pPROM remote from (41% in the NICHD-MFMU
trial).73 Studies of antenatal steroid administration performed before the era of
antibiotic treatment during conservative management of pPROM revealed
conflicting results regarding neonatal infection. However, two recent
trials have assessed antenatal corticosteroid administration concurrent
to adjunctive antibiotic administration and found no increase in infectious
morbidity with antenatal corticosteroid administration. Lewis
and associates found antenatal corticosteroids to reduce the incidence
of RDS (18.4% vs. 43.6%, p = .03) without increasing perinatal infection (3% vs. 5%, p = not significant [NS]), when antibiotics were administered
concurrently.84 In a second trial, women with pPROM at 28 to 34 weeks' gestation
received amoxicillin and metronidazole for 5 days and were randomized to antenatal dexamethasone or placebo.85 No increase in maternal or neonatal infectious morbidity was evident with
corticosteroids. Among those remaining pregnant at least 24 hours, the
corticosteroid group had less perinatal death (1.3% vs. 8.3%, p = .05) The most recent meta-analysis in this regard
found corticosteroid administration after pPROM to reduce the risks of
RDS (20% vs. 35.4%), IVH (7.5% vs. 15.9%), and
necrotizing enterocolitis (.8 vs. 4.6%; P < .05 for each), without increasing the risks of maternal (9.2% vs. 5.1%) or neonatal infection (7.0% vs. 6.6%; P > .05 for both).86 Based on current information, a single course of antenatal corticosteroids
with concurrent adjunctive antibiotic therapy is recommended during
conservative management of pPROM before 32 weeks. Similar treatment
could also be considered if conservative management is pursued for suspected
fetal pulmonary immaturity at 32 to 34 weeks' gestation. Absent
adequate data regarding safety and efficacy, repeated administration
of antenatal corticosteroids is not recommended. Tocolysis The use of tocolysis in the face of pPROM remains controversial. Both prophylactic
and therapeutic tocolysis have been used to prolong the latency
interval.87,88,89 Some clinicians consider labor to be a presumptive sign of intrauterine
infection and therefore a contraindication to tocolytic therapy. In 1980, Christensen
administered Ritodrine intravenously to patients with
pPROM in labor and compared this with a placebo for a 24-hour
course.87 None of the patients delivered while administered the intravenous infusion. The
difference between the two groups was statistically significant
at 24 hours. Levy and Warsof randomized 42 women to a prophylactic
oral ritodrine group versus a control group and found a significant pregnancy
prolongation at 48 hours (OR: .19; 95% CI: .05 to .72) but
not at 10 days.90 They did not demonstrate any benefit in terms of respiratory distress, perinatal
mortality, or neonatal infection. Another study by Weiner and
colleagues indicated a significant delay in delivery only among pPROM
patients undergoing tocolysis before 28 weeks' gestation.91 Alternatively, Garite and associates, in a study randomizing women on
admission to conservative management or to an intent to treat with tocolytics
should labor ensue, found no improvement in pregnancy prolongation.88 Similarly, improved neonatal outcome has not been conferred with a practice
of tocolysis and serial assessment of fetal pulmonary maturity followed
by delivery.92 While no study has found tocolytic therapy to alter infant outcomes, such
studies have not evaluated tocolysis when corticosteroids and antibiotics
are administered concurrently. It is plausible that prophylactic
tocolysis could increase the time for corticosteroid action on the
fetus and allow more time for antibiotics to act against subclinical decidual
infection. It is not unreasonable to initiate tocolysis concurrent
to attempts to prevent infection, prolong pregnancy, and induce fetal
pulmonary maturity in pregnancies at high risk for infant morbidity. However, this
approach should not be considered an expected practice
as further research is needed. |