Historically, the diagnostic confirmation of placenta previa had been through
retrospective findings at cesarean delivery54 or on palpation during double set-up examination. However, because of
difficulties in defining the exact location of the placental bed during
an emergency operation and the obvious implications of the double set-up
exam, sonography has become the most accurate and commonly used method
for diagnosing placenta previa.54,55 In fact, most cases are diagnosed incidentally at mid-trimester ultrasound. Before
Gottesfeld and co-workers56 first reported on the usefulness of transabdominal sonography for localization
of the placenta, angiography, radiography, radioisotope scanning, and
digital examination of the placenta were used for diagnosis of
the placenta previa.57,58,59 Although far from perfect, with false-positive rates of 3% to 7% and false-negative
rates up to 2%, transabdominal sonography is currently the
standard in the diagnosis of placenta previa.56,60,61,62 Explanations proposed to account for the false-positive error rate, summarized
by Langlois and colleagues,53 include placental conversion, overdistention of the urinary bladder, low-lying
myometrial contraction, or leiomyomas and extraembryonic blood
clots. Placental conversion is the main source of the false-positive results of
the first and second trimester diagnosis of placenta previa. Various
studies have indicated that the incidence of placenta previa in mid-gestation
is more frequent than at term.56,64,65,66 The explanation of placental conversion, supported by most authors, is
based on the theory that the uterus grows at a faster rate than the placenta
as pregnancy progresses. This differential growth rate results
in a decrease in the proportion of the inner uterine surface that is
covered by placenta. Thus, with time, an initially diagnosed low-lying
placenta appears to be carried away from the os toward the fundus. Overdistention of the maternal urinary bladder is sometimes cited as a
cause of false-positive diagnosis of placenta previa.39,44,45,46,47 Apposition of the anterior and posterior walls of the lower uterine segment
may decrease the length of this segment and falsely suggest a placenta
previa. Although some authors recommend the routine use of post-voiding
scans, other doubt their usefulness, citing the difficulties
of visualizing the placenta and its relationship to the os without a full
bladder.39 Focal low-lying myometrial contractions may also distort the lower uterine
segment and contribute to a previa misdiagnosis. Myometrial contractions
can either simulate placental tissue or shorten the distance between
the placental edge and the internal os. Townsend and co-workers48 documented myometrial contractions in 16% of the false-positive diagnoses
and recommended repeat scanning after 30 minutes if the myometrial
thickness exceeds 1.5 cm.31,48 Morrison49 demonstrated that the lower uterine segment develops continuously throughout
pregnancy. Normal lower uterine segment varies from 0.5 cm from
the internal os at 20 weeks' gestation to 5 cm at 38 weeks. Therefore, the
fixed limitation of the distance between the lower uterine segment
and the internal os to within 5 cm for diagnosis of placenta previa
will cause several false-positive results early in the third trimester. Low-lying leiomyomas and extraembryonic blood clots can be easily confused
with low-lying placenta and cause false-positive results. Moreover, accurate
localization of placenta via the transabdominal route can be
difficult in the presence of obesity and posterior or lateral placentation. The
acoustic shadow of the fetal head in a vertex presentation
may prevent an accurate localization of a low placenta.72 Transabdominal sonography becomes increasingly difficult as the third trimester
progresses, predominantly because of attenuation of sound by
the presenting part of the fetus.68,73 Several techniques, including external fetal manipulation, overdistension
of the maternal urinary bladder,and Trendelenburg positioning, have
been used to elevate the presenting part of the fetus from the pelvis
and facilitate visualization of the cervix.68,73,74,75 However, such maneuvers can be uncomfortable for the patient, distort
the appearance of the cervix, and are frequently unsuccessful late in
the third trimester.73,74,75 Because of these limitations, alternative techniques are needed to complement
transabdominal sonography for the diagnosis of placenta previa.73 Transvaginal and transperineal sonography are frequently used with transabdominal
studies. There is little doubt that transabdominal sonography will remain as the
first-line diagnostic means for the localization of placenta previa.55 However, an emerging body of evidence suggests the superiority of transvaginal
sonography in this respect and supports its use as a second-line
investigation to avoid the complications of misdiagnosis of placenta
previa due to false-positive or false-negative results. Brown and colleagues first described the use of transvaginal sonography
to evaluate the lower uterine segment and cervix during pregnancy in 1986.76 Transvaginal ultrasound not only circumvents many problems faced by transabdominal
sonography, but also possesses certain inherent characteristics
that improve diagnostic accuracy.55 The sound waves travel a shorter distance from the tip of the vaginal
probe to the pelvic organs than they do from the tip of the abdominal
probe.55,72 This enables the use of a higher frequency ultrasound wave generator, which
in turn increases picture resolution.55,72 Transvaginal ultrasonography avoids the disturbances caused by body habitus, bladder
overdistention, and acoustic shadowing of fetal parts.55 Tan and co-workers55 demonstrated that transvaginal sonography ruled out placenta previa in 12 cases
out of 70 (17%) thought to be placenta previa by transabdominal
ultrasound. Leerentveld and colleagues77 reported a positive predictive value of 93.3% and a negative predictive
value of 97.6% of transvaginal sonography. Farine and co-workers78 reported a positive predictive value of 71%, but a negative predictive
value of 100%. Despite the higher accuracy of transvaginal sonography, it remains underutilized
in the diagnosis of placenta previa.55 This is mainly because of safety concerns. Vaginal manipulation in cases
of suspected placenta previa runs against the grain of classic obstetric
teaching.55 However, recent data in many studies suggest that sonography is a safe
technique. None of the authors encountered any evidence of vaginal bleeding, preterm
labor, premature rupture of membranes, or vaginitis.55,62,72,77 Tan and co-workers55 attributed the safety of transvaginal sonography to the fact that the
vaginal probe is inserted under direct ultrasonic visualization, and hence
direct contact with the cervix is avoided. There is still a fair
distance between the tip of the vaginal probe and the cervix when the
lower uterine segment comes into focus because the focal range of the
vaginal probe is 2 to 7 cm. Therefore, if the transducer is closer to
the target than its focal length, the image may be blurred and out of
focus.62 Timor-Tritsch and Yunis62 evaluated the safety of transvaginal ultrasonography in the diagnosis
of placenta previa by determining whether the angle between the cervix
and the vaginal probe is sufficient for alignment of the probe with the
cervix. They concluded that the anatomic relationship between the vagina
and cervix, as reflected in the measured angle between the two (greater
than 44°), makes inadvertent insertion of the probe into the
internal cervical os virtually impossible. Transvaginal sonographic placental localization appears to be a simple, reliable, and
safe technique,77 and it is recommended as a second-line diagnosis in patients who are diagnosed
to have minor placenta previa by transabdominal sonography.55 Transperineal sonography is another technique for imaging the cervix during
the third trimester of pregnancy, allowing cervical visualization
in most patients in whom transabdominal sonography of this area is unsuccessful.73 Although transvaginal ultrasound is more commonly used to complement transabdominal
studies, a transperineal approach provides a more convenient
means of imaging the cervix and lower uterus without requiring specialized
equipment, vaginal penetration, or external fetal manipulation.79 Hertzberg and colleagues79 demonstrated that the greatest value of transperineal sonography was in
helping to exclude placenta previa in patients in whom the cervix was
not seen on transabdominal sonography. In such cases, transperineal
sonography will usually show the internal surface of the cervix without
overlying placental tissue, allowing confident exclusion of placenta
previa. In a significant minority of patients with placenta previa, however, transperineal
sonography will show a placenta previa that was
not seen with transabdominal sonography. The cervix is almost always seen on transperineal sonograms, but the lower
edge of the placenta may be beyond the field of view.79 Therefore, transperineal sonography is a valuable procedure to complement
transabdominal studies, but not to replace them. Accurate interpretation of transperineal sonograms requires the same precautions
as in the evaluation of transabdominal sonograms,79 and inherent in the procedure are the same sources of false-positive results. The
potential value of magnetic resonance imaging (MRI) for placental
localization has been investigated in several centers.80,81 MRI offers two major advantages over ultrasound that may make it particularly
suitable for evaluating third trimester bleeding and diagnosis
of placenta previa. These advantages are potentially better tissue differentiation
and an ability to highlight blood.82 Excellent maternal soft tissue definition can be obtained; both placenta
and cervix have a characteristic appearance. Therefore, the relationship
of the lower edge of the placenta to the internal cervical os can
be accurately determined. Despite the encouraging research results, MRI diagnosis of placenta previa
is still an experimental technique and is not widely used in a clinical
setting. Disadvantages associated with MRI for diagnosis of placenta
previa include: (1) safety concerns regarding moving a patient from
labor and delivery to a radiology suite; (2) the relatively lengthy
examination (typically 30 to 60 minutes); (3) long-term safety in pregnancy
has yet to be established; and (4) MRI scans are more costly than
ultrasound examination.82 Although there is some evidence for using MRI as a complementary technique
to ultrasound, these barriers effectively preclude its use in most
patients. The diagnosis of placenta accreta usually is made at delivery, when it
becomes apparent the placenta is abnormally adherent. The diagnosis can
be confirmed after surgery with histopathologic examination of the uterus
or by biopsy of the placental bed. The characteristic histopathologic
feature of this condition is absence or poor development of decidua
basalis.83 Diagnosis before delivery would allow adequate surgical preparation to
decrease maternal morbidity and mortality. Although it is possible to
do so, placenta accreta is rarely diagnosed antenatally.84 The sonographic characteristics of a placenta accreta are the absence of
the normal retroplacental clear space, placental tissue contiguous with
myometrium, and prominent placental venous lakes and uterine vascularity.83,84 Absence of the hypoechoic zone is thought to represent a defect in decidua
basalis and adjacent myometrium, whereas the vascular changes may
be a result of alternative vascular patterns associated with an abnormal
basal plate.83,85 Rosemond and Kepple84 described a case in which abnormal sonographic findings were appreciated
only with transvaginal color Doppler sonography. When Doppler flow
studies of the normal retroplacental clear space are performed, multiple
venous flow signals are seen in this area.84,86 Absence of this space represents abnormal placentation. In a case of placenta
percreta diagnosed at our institution (Fig. 2), the interface between the placenta and the maternal urinary bladder
is essentially absent. Transabdominal color Doppler studies were diagnostic
in this patient, and placenta percreta was subsequently confirmed
at cesarean delivery. 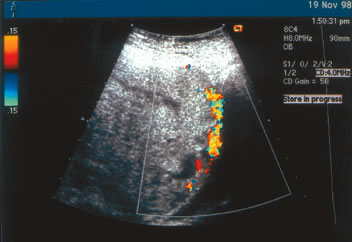 Fig. 2. Placenta percreta diagnosed antenatally by transabdominal color Doppler
ultrasound. Note the loss of interface between the placenta and the maternal
urinary bladder.(J.S. Sholl, MD; ultrasonographer) Fig. 2. Placenta percreta diagnosed antenatally by transabdominal color Doppler
ultrasound. Note the loss of interface between the placenta and the maternal
urinary bladder.(J.S. Sholl, MD; ultrasonographer)
|
Color flow Doppler sonography is also particularly useful in identifying
hypervascularity beneath the placental attachment site. This technique
highlights the areas of increased vascularity and reveals a continuum
of lacunar flow from the placenta through the myometrial layer without
an intervening clear space. However, sonographic findings are not
pathognomonic for placenta accreta.84 Callen and Filly33 documented absence of a retroplacental clear space in 27 of 100 cases
examined prospectively; but none of these patients had placenta accreta. Antepartum diagnosis of these invasive placental conditions may allow for
the placement of arterial catheters preoperatively in case it is necessary
to perform uterine artery embolization by interventional radiology, which
is designed to lessen the risk of catastrophic hemorrhage. Foreknowledge
of placental invasion can also provide the opportunity
for additional patient counseling, blood product availability, and an
appropriate surgical team to be assembled for the delivery. |