The patient is exposed to short radiofrequency fields to stimulate magnetized protons to yield an NMR signal. A by-product of this process is deposition of radiofrequency energy in the body as heat.16 In addition to transmitted radiofrequency power level, several factors influence the amount of tissue heating; these include radiofrequency coil design, electric properties of tissue, pulse sequence, and size and shape of the body part being scanned. The body's ability to dispose of additional heat is an important consideration in evaluating safety. Tissues having low thermoregulatory blood supply, such as the testes and lens of the eye, may be more susceptible to damage by radiofrequency power deposition. Excessive localized heating may also occur in areas near conductive metals such as metallic implants and in constricted current paths such as the armpits and groin.
When a radiofrequency pulse is applied, the body experiences an oscillating magnetic field. Just as with a pulsed magnetic field gradient, the body responds to the oscillating magnetic field with internal electric currents that generate heat by resistive losses. The measure of power deposition is the specific absorption rate (SAR), defined as the amount of power deposited per kilogram of tissue.16 It can be shown that power deposition in whole-body NMR increases with current path radius (body size), frequency of the radiofrequency pulses, and pulse duty cycle.2 In NMR the frequency of radiofrequency pulses is proportional to the static magnetic field strength. Consequently, tissue heating is a greater concern in high field MRI systems. This is particularly true in body imaging, in which high-frequency radiofrequency penetrates less deeply into the body so high-powered radiofrequency pulses are required.
The total radiofrequency power absorbed by the body can be determined empirically using the following equation:5
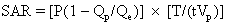
where P is the root mean square power input to the radiofrequency coil, Qp is the coil quality factor with the patient in the coil, Qe is the quality factor with the coil empty, T is the duration of the radio-frequency pulse, and t-1 is the number of radiofrequency pulses per second. For the body average SAR, Vp represents the total body mass, or as a more conservative and accurate measure Vp is only the tissue mass contained within the coil. The quality factor is an electrical quantity indicating the ratio of inductance to resistance. The quantity (1 - Qp/Qe) represents the fraction of total resistive heat losses that is deposited in the patient.
Usually an imaging sequence requires more than one type of radiofrequency pulse. For example, the popular two-echo spin-echo sequence requires two of the more powerful 180° radiofrequency pulses for each 90° radiofrequency pulse. The total SAR is the sum of contributions from each type of pulse. An SAR calculation for a medium-field-strength two-echo spin-echo sequence is shown below:
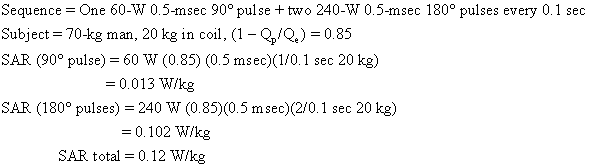
As we shall see, this body average SAR is well below guideline limits.
The power requirements for head scanning is one tenth or less that of body scanning; therefore, it is not limited by tissue heating.
Peak SAR refers to the maximum power deposited per kilogram averaged over any 1 g of tissue. Estimating peak SAR involves models of tissue geometry and electric characteristics.2 For cylindrical and spherical models peak SAR (at the skin surface) is two to three times the average SAR. It is emphasized, however, that actual peak SAR may be significantly higher depending on body geometry and actual electric properties.
The temperature rise of tissue for given SAR and exposure time (texp) is given by the equation
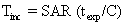
where C is the heat capacity of tissue (0.83 kcal/kg °C).5 Tissue exposed to 2 W/kg for 20 minutes would be expected to rise in temperature by 0.7°C. Temperature increases of this magnitude are not observed clinically owing to the body's thermoregulatory system (acquisition time for MRI sequences varies from subminute to several tens of minutes).
Currently, static magnetic field strength and time-varying magnetic field gradients do not place limits on routine NMR procedures for reasons of patient safety. Field strengths in excess of 2 T are technically difficult to achieve on whole-body NMR systems and may not offer advantages in image quality or diagnostic value. Also, magnetic field gradient pulses currently used are believed to be within safe limits and are adequate for virtually all magnetic resonance procedures. However, as new techniques are developed that require stronger gradient pulses, such as rapid scanning, further careful investigation of safety of time-varying magnetic fields is warranted. The remaining issue of radiofrequency power deposition does restrict the type of pulse sequences that are usable in body scanning at high magnetic field strengths. Considerable effort has gone into radiofrequency coil design and modes of radiofrequency polarization to minimize the percentage of protocols that exceed safety guidelines. In the event the selected protocol exceeds radiofrequency power deposition limits, the examination may be partitioned into two or more acquisition sequences that individually do not exceed power deposition limits. The body's ability to continually transfer heat to the environment allows many pulse sequences to be applied sequentially as long as no one sequence exceeds the guidelines. This is a fundamental distinction between radiofrequency power deposition in MRI and ionizing radiation dosage in x-ray procedures, in which the total examination dose is the primary concern.