Glucose intolerance is the single consistent abnormality in a protean array of syndromes or conditions that have been collectively titled DM. To ensure meaningful provider-consumer and interdisciplinary communication, it is essential that terminology be precise and contemporary.
The American Diabetes Association (ADA) recommended a new classification of diabetes based on its cause.4 This classification is shown on Table 1. New diagnostic criteria for diabetes were also proposed and are shown on Table 2. A group of patients not meeting those criteria, but with blood glucose levels still above normal, were defined as follows:
TABLE 1. Etiologic Classification of Diabetes Mellitus
- Type 1 diabetes* (β-cell destruction, usually leading to absolute
insulin deficiency)
- Immune mediated
- Idiopathic
- Immune mediated
- Type 2 diabetes* (may range from predominately insulin resistance with
relative insulin deficiency with relative insulin deficiency to a predominately
secretary defect with insulin resistance)
- Other specific types
- Genetic defects of β-cell function
- Chromosome 12, HNF-1α (MODY3)
- (Chromosome 7, glucokinase
- Chromosome 20, HNF-4α (MODY1)
- Mitochondrial DNA
- Others
- Chromosome 12, HNF-1α (MODY3)
- Genetic defects in insulin action
- Type A insulin resistance
- Leprechaunism
- Rabson-Mendenhall syndrome
- Lipoatrophic diabetes
- Others
- Type A insulin resistance
- Diseases of the exocrine pancreas
- Pancreatitis
- Trauma/pancreatectomy
- Neoplasia
- Cystic fibrosis
- Hemochromatosis
- Fibrocalculous pancreatopathy
- Others
- Pancreatitis
- Endocrinopathies
- Acromegaly
- Cushing's syndrome
- Glucagonoma
- Pheochromocytoma
- Hyperthyroidism
- Somatostatinoma
- Aldosteronoma
- Others
- Acromegaly
- Drug or chemical induced
- Vacor
- Pentamidine
- Nicotinic acid
- Glucocorticoids
- Thyroid hormone
- Diazoxide
- β-adrenergic agonists
- Thiazides
- Dilantin
- Interferon-α
- Others
- Vacor
- Infections
- Congenital rubella
- Cytomegalovirus
- Others
- Congenital rubella
- Uncommon forms of immune-mediated diabetes
- “Stiff-man” syndrome
- Anti-insulin receptor antibodies
- Others
- “Stiff-man” syndrome
- Other genetic syndromes sometimes associated with diabetes
- Down's syndrome
- Klinefelter's syndrome
- Turner's syndrome
- Wolfram's syndrome
- Friedreich's ataxia
- Huntington's chorea
- Laurence-Moon-Biedl syndrome
- Myotonic dystrophy
- Porphyria
- Prader-Willi syndrome
- Others
- Down's syndrome
- Genetic defects of β-cell function
- Gestational diabetes mellitus (GDM)
*Patients with any form of diabetes may require insulin treatment at some stage of their disease. Such use of insulin does not classify the patient.
Clark C Jr: Report of the Expert Committee on the diagnosis and classification of Diabetes Mellitus. Diabetes Care 22:57, 1999.
TABLE 2. Criteria for the Diagnosis of Diabetes Mellitus*
- Symptoms of diabetes plus casual blood glucose concentration
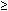 200 mg/dl (11.1 mmol/L). Casual is defined as any time of day without regard
to time since last meal. The classic symptoms of diabetes include
polydipsia, and unexplained weight loss.
200 mg/dl (11.1 mmol/L). Casual is defined as any time of day without regard
to time since last meal. The classic symptoms of diabetes include
polydipsia, and unexplained weight loss. - FPG
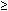 126 mg/dl (7.0 mmol/L). Fasting is defined as no caloric intake for at
least 8 hours.
126 mg/dl (7.0 mmol/L). Fasting is defined as no caloric intake for at
least 8 hours. - Two-hour PG
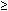 200 mg/dl (11.1 mmol/L) during an OGTT. The test should be performed as
described by the World Health Organization,2 using a glucose load containing the equivalent of 75-g anhydrous glucose
dissolved in water.
200 mg/dl (11.1 mmol/L) during an OGTT. The test should be performed as
described by the World Health Organization,2 using a glucose load containing the equivalent of 75-g anhydrous glucose
dissolved in water.
*In the absence of unequivocal hyperglycemia with acute metabolic decompensation, these criteria should be confirmed by repeat testing on a different day. The third measure (OGTT) is not recommended for routine clinical use. FPG, fasting plasma glucose; OGTT, oral glucose and tolerance test; PG, postload glucose.
Clark C Jr: Report of the Expert Committee on the diagnosis and classification of Diabetes Mellitus. Diabetes Care 22:57, 1999.
- Impaired fasting glucose (IFG): fasting glucose of more than 110 but less
than 126 mg/dl.
- Impaired glucose tolerance (IGT): 2-hour value of the glucose tolerance
test (GTT) of more than 140 but less than 200 mg/dl.
The perinatal implications of IFG and IGT are discussed later. As far as pregnancy is concerned, we only need to address three types of diabetes: gestational diabetes mellitus (GDM), type 1 diabetes, and type 2 diabetes.
Screening and Diagnosis of Gestational Diabetes Mellitus
Classically, screening has consisted of a blood glucose determination 1 hour after the ingestion of a 50-g glucola load. If the blood glucose concentration exceeds 140 mg/dl, a 3-hour oral GTT has been recommended. This two-tiered approach, once recognized as the gold standard, has come under considerable scrutiny and criticism.5–7
The cutoff values used in the GTT were derived by the National Diabetes Data Group by converting the original blood glucose concentrations obtained by O'sullivan and Mahan8 to plasma glucose. Unfortunately, the factor used for the conversion overestimated the plasma values, resulting in cutoff values that were too high. Carpenter and Coustan9 and Sacks and colleagues10 recalculated the conversion factor and arrived at cutoff plasma values that are considerably lower, with a resultant increase in the number of patients identified as having glucose intolerance.
At the Fourth International Conference on Gestational Diabetes, held in 1997, the values reported by Carpenter and Coustan were adopted as cutoff values of blood glucose. These values are given in Table 3. The ADA now recommends that the screening 1-hour challenge test be administered between 24 and 26 weeks of gestation only to pregnant women meeting one or more of the following criteria:
Time | Blood Glucose (mg/dl) | Blood Glucose (mmol/I) |
Fasting | 95 | 5.3 |
1 hr | 180 | 10.0 |
2 hr | 155 | 8.6 |
3 hr | 140 | 7.8 |
ADA: Summary and recommendations of the 4th International Workshop--conference on GDM. Diabetes Care 21(Suppl 21):B163, 1998
- Twenty-five years of age or older
- Less than 25 years of age and obese
- Family history of diabetes
- Belonging to an ethnic group with a high prevalence of diabetes (e.g. Hispanic, Native American, African American, Asian American)
Data reported by Franz and coworkers11 suggest that in the absence of those criteria universal screening is not cost effective. Controversy also exists as a result of the World Health Organization (WHO) criteria for diagnosing GDM. In that system, a 75-g load is used, and a 2-hour value of more than 162 mg/dl is considered diagnostic. Most countries use this approach on pregnant women without a preceding screening 50-g test. The WHO's approach is simpler and more attractive than the National Diabetes Data Group's 3-hour GTT and is more acceptable to patients.
Management of Gestational Diabetes Mellitus
The management of GDM is based on identification of perinatal outcomes associated with the abnormal glucose tolerance. In the absence of a need for insulin therapy, the only clinically significant adverse perinatal outcome is excessive fetal growth leading to increased risk for trauma during delivery and an increased incidence of cesarean section.4,12 The recommended initial therapeutic approach should be dietary manipulation and prescribed exercise in an effort to maintain euglycemia and to prevent excessive fetal growth. The goals are to maintain the fasting blood glucose (FBG) at less than 106 mg/dl and the 2-hour postprandial level at less than 120 mg/dl. Some investigators recommend that the FBG cutoff value for insulin therapy be 95 mg/dl.13,14 Other investigators have recommended that prophylactic insulin therapy be initiated even if those values are not exceeded.15,16 Their data indicate a significant reduction in the incidence of macrosomia and cesarean section. Our group also has found, in a prospective, randomized, double-blind study a reduction in birth weight among gestational diabetic patients treated with insulin prophyllactically (Table 4).
TABLE 4. Prophylactic Insulin Administration and Birth Weight Among Patients
with Gestational Diabetes Mellitus
| Placebo | Insulin |
Birth weight (mean grams ± SD)* | 3480 ± 671 | 3254 ± 963 |
Insulin (mean μIU/ml ± SD) | 22.4 ± 11.9 | 38.0 ± 21.9 |
Glucose (mean mg/dl ± SD) | 99.5 ± 17.5 | 87.9 ± 10.5 |
*Post hoc power analysis indicated that 210 patients are needed in each group to achieve statistical significance for the difference in birth weight.
Buchanan and associates17,18 suggested the use of the sonographically measured fetal abdominal circumference as criterion to determine if insulin therapy is indicated. Based on their observations, they recommend that the abdominal circumference be estimated at 28 weeks' gestation and that insulin be started if it exceeds the 75th percentile. Their findings of a decreased incidence of macrosomia and cesarean section give support to this approach.
Langer and colleagues19 proposed an intermediate step before starting insulin therapy. In a randomized study of gestational diabetics, one group received insulin, and the other group was treated with glyburide. There were no differences observed in maternal blood glucose levels and perinatal outcomes between the two groups. The investigators conclude that, because the primary goal in managing GDM is to achieve normoglycemia, glyburide provides a cost-effective alternative to insulin therapy. If these findings are confirmed by other studies, the use of glyburide may become a desirable substitute for insulin in the management of GDM.
Glucose Tolerance Test During Pregnancy
Many physicians appear to have discarded the oral GTT in favor of simpler techniques. Davidson and coworkers,20 McCance and associates,2 and Stolk and colleagues22 have challenged the continued use of the GTT as the basis for diagnosis and have instead suggested the use of FBG and glycated hemoglobin as the standard for the diagnosis of DM. In their recommendations, the ADA suggests the use of the 2-hour value of the GTT to diagnose abnormal glucose tolerance.4
During pregnancy, concerns have been raised over the practicality of the two-tier system currently advocated as the standard for diagnosing GDM. In our own program, my colleagues and I have found that many patients fail to complete the screening or diagnostic tests, and in essence, they go unscreened throughout pregnancy. Most physicians use glucola for the testing load, but it has been our experience that many patients find this product unpalatable and have difficulty ingesting it, and as a result, they frequently fail to have the test done. We have found that a 100-g carbohydrate breakfast is better tolerated and patients are more likely to complete the test. Other investigators have used similar approaches with test meals and glucose polymers with equally satisfying results.23,24
Several investigators have found a relationship between adverse perinatal outcomes and one abnormal value for the GTT.25,26 It appears that treatment of such patients results in better perinatal outcomes than no treatment.27 Our own data showed that the 2-hour value of the GTT is as reliable as the complete GTT in identifying risk for excessive fetal growth.28 The use of a single blood glucose measurement is more cost effective than a complete GTT. It seems reasonable to use a single blood glucose determination 2 hours after a 100-g carbohydrate load to identify risk for excessive fetal growth. Patients with an abnormal 2-hour value can receive dietary treatment and an exercise prescription, with the need for insulin therapy to be determined by the FBG and 2-hour postprandial blood glucose concentration obtained with that regimen, the sonographically measured fetal abdominal circumference, or both measurements.
Impaired Fasting and Impaired Glucose Tolerance
The perinatal implications of impaired fasting and impaired glucose tolerance are significant because both are associated with increase risk for excessive fetal growth. These patients are identified by the blood glucose concentration defined earlier and should undergo dietary and exercise treatment during pregnancy. From a practical point of view, most of these patients should be identified by a single abnormal value in the GTT or by an abnormal value 2 hours after a 100-g carbohydrate load.
Insulin-Requiring Diabetics
With the emphasis on tight control of blood glucose levels, congenital anomalies have become the leading cause of perinatal mortality among infants of diabetic mothers. Because organogenesis is completed by the seventh to eighth week of gestation, the embryo is most vulnerable to the teratogenic effects of hyperglycemia early in gestation, frequently before the patient is first seen prenatally. Our experience has been that most diabetic women present to prenatal care in poor glycemic control, and therefore the only way to minimize the teratogenic effects of hyperglycemia is to achieve optimal blood glucose concentrations before the patient becomes pregnant. Preconceptional control has become an important and highly desirable aspect of diabetic care in pregnancy. Unfortunately, it is not easy to achieve.
Several investigators29,30 have implemented programs of diabetic education aimed at identifying diabetic women before they become pregnant and guide them toward a metabolic status characterized by euglycemia and normal glycated hemoglobin levels. There are considerable data demonstrating that among diabetic women entering pregnancy with glycated hemoglobin values below 8%, the incidence of congenital malformations is no different from that of nondiabetics.31–33 In our program, my colleagues and I follow diabetic women contemplating pregnancy with the same intensive management plan as we do during pregnancy.
The goal of most investigators is to achieve mean blood glucose levels of less than 120 mg/dl and a HbA1C level of less than 6% before conception. We use a mean blood glucose concentration of less than 150 mg/dl in an effort to minimize hypoglycemic episodes. Other issues that must be addressed in the management of insulin-requiring diabetics are discussed in the following sections.
EDUCATION.
Most insulin-requiring diabetic patients have had the condition for several years and are knowledgeable about the disease. However, most of them are unaware of the profound hormonal and metabolic changes that occur during pregnancy and significantly affect carbohydrate metabolism. These patients need extensive education on dietary requirements, insulin needs, frequency and quality of meals, exercise, prenatal care, and fetal surveillance.
The educational process has to be carried out in such a way that the patient does not resent it, because many of them feel that they know all that is needed. The development of a team approach, as discussed later, facilitates this process. The major objective of the educational process should be the empowerment of the patient to take control of the management process. The women need to be comfortable with making decisions about dietary and insulin changes, exercise frequency and intensity, and other aspects of the care plan.
The main educational goal for the provider is understanding and accepting the concept of patient empowerment. Our experience has been that many of our patients depend entirely on their provider to make changes in their management plan. We spend a significant amount of time and effort preparing the patients so they can become able to manage their diabetes. This approach increases our rapport with patients, and we find that compliance is significantly enhanced. Patient empowerment can promote efforts at long-term diabetic control with lower risks of microvascular complications.
DIET
Desirable perinatal outcomes are affected to an important degree by nutrient intakes sufficient to meet pregnancy requirements. It is well established that energy is the most important nutrient determinant of weight gain during pregnancy. Extra energy is required during pregnancy, and it has been recommended that between 200 to 300 kcal per day be added to nonpregnant requirements.34 The actual caloric intake needed during pregnancy can be modified by the rate of weight gain.
Because the body mass index (BMI) is the best indicator of maternal nutritional status, adequate dietary intake and weight gain during pregnancy can be calculated based on the BMI. We use a modification of the recommendations of the Institute of Medicine34 (Table 5).
TABLE 5. Recommended Total Weight Gain for Pregnant Women
|
| Second and Third Trimester |
| Body Mass Index | (lb/wk) Total (lb) |
Underweight (<19.8) | 2 | 28–40 |
Normal (19.8–26) | 1 | 25–35 |
Overweight (>26) | ½ | 15–25 |
Institute of medicine, National Academy of Sciences: Nutrition During Pregnancy.
Washington, DC: National Academy Press, 1990.
The diet should be a well-balanced one, with healthy meals for pregnancy having a nutrient distribution of 40% to 50% carbohydrate, 15% to 20% protein, and 30% fat. It should include four servings of dairy products, three servings of protein, and five servings of fruit and vegetables daily. We teach our patients carbohydrate counting to monitor their intake and to help adjust the insulin dose.
The diet is distributed in three meals and three snacks to minimize the accelerated starvation of pregnancy. More frequent feedings lower the need for insulin. A typical daily diet for our patients consists of the following servings of carbohydrate (1 serving = 15 g of carbohydrate):
Breakfast: 2 to 3 servings (30 to 45 g of carbohydrate)
Lunch: 4 servings (60 g of carbohydrate)
Dinner: 4 servings (60 g of carbohydrate)
Three snacks: 1 to 2 servings (15 to 30 of g carbohydrate per snack)
INSULIN.
Together with dietary intake, insulin forms the mainstay of treatment. There is no universal formula, and treatment must be individualized. The desired dosage schedule should be one that resembles insulin production in the normal patient as closely as is technically possible. We prefer a regimen of multiple injections consisting of rapid-acting insulin before each meal and at bedtime, with the latter mixed with an intermediate-acting insulin.
We have not found any significant advantages with the use of insulin pumps, and we use them only in special cases in which the patient is already using one or for the occasional patient who has difficulties injecting herself four times each day.
We calculate the insulin dose to be between 0.5 and 1.0 units/kg depending on the trimester of pregnancy and the patient's weight. This total daily dose (TDD) is given as rapid-acting insulin as follows: 40% before breakfast, 30% before lunch, 20% before dinner, and 10% at bedtime. In addition, 5–10 units of NPH or Lente is given at bedtime. The bedtime doses may need to be lowered or discontinued if the patient has hypoglycemia during the night.
We discontinued the use of regular insulin in favor of a rapid-acting human analog (Lispro). We find the latter to be associated with better 2-hour postprandial blood glucose levels and a lower incidence of hypoglycemia, making good control easier to achieve with better patient compliance (Table 6). We also use the rule of 1500 and the carbohydrate-insulin ratio35,36 to make further adjustments in their insulin dose, depending on the preprandial blood glucose and the amount of extra carbohydrates anticipated to be eaten during a meal. For instance, if the patient weighs 100 kg and is in the third trimester, TDD = 1 U/kg × 100 = 100 units 75 U (75%) to be given by boluses of insulin Lispro as follows:
TABLE 6. Effect of Regular and Lispro Insulin on Blood Glucose Concentration
| Mean Blood Glucose | |
| Concentration | |
| (mgm/dl ± SD) | |
| Regular | Lispro |
FBG | 136 ± 68 | 117 ± 15 |
2 hr after breakfast | 132 ± 31 | 116 ± 13 |
2 hr after lunch | 126 ± 42 | 114 ± 18 |
2 hr after dinner | 149 ± 57 | 128 ± 24 |
Total average | 135 ± 36* | 119 ± 16* |
Glycated hemoglobin (%) | 7.9 ± 1.0 | 8.3 ± 2.0 |
Maternal hypoglycemia | 4 | 0 |
*p < 5.05. FBG, fasting plasma glucose.
Zuspan F, Quilligan EJ: Use of an ultrafast insulin analog (Lispro) in pregnancy. Am J Obstet Gynecol 180:1:S39, 1999.
Before breakfast: 40% of 100 U; 40 U
Before lunch: 30% of 100 U; 30 U
Before dinner: 20% of 100 U; 20 U
Bedtime: 10% of 100 U; 10 U + 5–10 U NPH
By the rule of 1500: 1500 ÷ 100 (TDD) = 15 mg/dl. This is the amount 1 U of insulin can decrease blood glucose concentration. The preprandial blood glucose would determine how much additional insulin is required. By the carbohydrate-insulin ratio: 5 to 15 g of carbohydrate are covered by 1 U of insulin. The number of extra servings of carbohydrate determine how much additional insulin is to be injected.
EXERCISE.
Regular exercise is an essential component of the management of diabetes. Blood glucose concentration is lowered by exercise resulting in lowering the need for insulin. We recommend to our patients that they exercise at least 3 days each week, but preferably every day for 0.5 to 1 hour. The exercise should be anaerobic and its intensity can be calculated at 60% of the maximal heart rate as determined by the patients age. The maximum heart rate = 222 – patient's age. We prefer that the type of exercise be swimming, walking, or jazzercise. These types of exercise are well tolerated by pregnant women and most of our patients enjoy them.
PSYCHOLOGIC ISSUES.
The medical management necessary to safeguard pregnancy complicated by diabetes seriously affects a patients lifestyle and creates significant stresses. It is important to recognize and address these stresses to help the patient and her family deal with the demands of the pregnancy without jeopardizing her mental health. We have found that having a social worker or counselor and a case manager as members of the care team is extremely helpful in addressing these issues properly.
FETAL SURVEILLANCE.
Because large and small for gestational age infants are a concern, serial monitoring of fetal growth by ultrasound is mandatory. We recommend that the fetal weight be monitored carefully, along with the head and abdominal circumferences. These latter two measurements can help determine whether the abnormal fetal size is symmetric or asymmetric. Together with the weight estimate, the ratio of the head circumference to the abdominal circumference may help in predicting possible shoulder dystocia. We also recommend an early ultrasound around 8 to 10 weeks for dating purposes and to promote bonding between the patient and the fetus. In our experience, this early bonding is helpful in promoting compliance. A targeted ultrasound between 18 to 20 weeks is recommended to exclude fetal anomalies.
Daily kick counts should be started around 28 weeks. We recommend to our patients that if eight kicks have not been felt over a period of 2 hours, they must come to our fetal testing unit for further evaluation. We routinely begin biweekly non-stress testing (NST) at 30 to 32 weeks. Biophysical profiles (BPP) and amniotic fluid index (AFI) are done as clinically indicated.
GLYCATED HEMOGLOBINS.
Most investigators have not found a significant correlation between glycated hemoglobin (GHb) concentrations and perinatal outcome. As a marker for adequate preconceptional control, GHb is extremely useful and is clearly related to the incidence of congenital malformations. It can also be of use in confirming good control as indicated by the blood glucose concentrations reported by the patient.
TEAM APPROACH.
Management of the pregnant diabetic is a complex issue, and a single provider cannot take care of all aspects of the care. It is in the best interest of the patient to have a team of providers working together with her to facilitate accomplishing all the goals of treatment. Our team includes a perinatologist, a nurse and a nutritionist (both of whom are certified diabetic educators [CDE]), a social worker or counselor, and a case manager. The most important member of the team is the patient, and all other members must work with her to facilitate her achieving the goals outlined. Failure to recognize the central role of the patient threatens the success of the management plan.
GOALS.
The most important goal is to achieve a successful perinatal outcome. To maximize the chances for success, most investigators follow identified targets to guide the daily management of the patient. From a metabolic point of view, the key indicator of good control is the blood glucose concentration. Freinkel and Metzger37 championed the concept of multiple fuel derangements in diabetes, but from a practical point of view, blood glucose is the most easily available indicator.
The advent of reflectance meters has made the use of blood glucose concentration on a daily basis a reality. We provide all our patients with a meter and instruct them to measure their blood glucose before and 2 hours after each meal. The ADA recommends that insulin and diet be manipulated to keep the FBG less than 96 mg/dl and the 2-hour postprandial level less than 120 mg/dl.38 Our experience with these targets, especially with type 1 diabetics, is a high frequency of hypoglycemic episodes, which tend to discourage patients and make them less compliant. To avoid hypoglycemia, we use as targets a FBG of less than 120 mg/dl and a 2-hour postprandial less than 150 mg/dl. As seen in Table 7, the perinatal outcomes we have achieved with this approach are comparable to those reported by other investigators using the lower targets.39,40 Leveno and coworkers41 also reported good perinatal outcomes in women with mean preprandial blood glucose values as high as 143 mg/dl. In the Diabetes Control and Complications Trial (DCCT), the mean blood glucose concentration among intensively controlled type 1 diabetics was 155 mg/dl and was associated with delayed onset of microvascular complications.42 It appears that diabetic women can expect good perinatal outcomes even while maintaining blood glucose concentrations higher than what is currently recommended, thereby avoiding the problem of hypoglycemia. It is rare among our patients to have significant episodes of hypoglycemia. Nevertheless, they and their significant others are instructed in the use of glucagon for possible emergencies.
TABLE 7. Perinatal Outcomes Among 125 Insulin-Dependent Diabetic Patients
Average blood glucose concentration | 133 mg/dl |
Average glycated hemoglobin | 8.4% |
Average birth weight | 3.2 Kg |
Macrosomia (>4 kg) | 11% |
Large for gestational age | 17% |
Cesarean section | 27% |
Neonatal hypoglycemia | 14% |
Respiratory distress syndrome | 4.9% |
Fetal deaths | 3 |
Class B-incompetent cervix → PROM | Delivered at 27 wk |
Class F-chronic hypertension w/severe preeclampsia | Delivered at 28 wk |
Class F-severe hypertension w/delivery at 26 wk |
|
Neonatal deaths | 4 |
Congenital anomalies (2 class C, 1 class B) |
|
Class B-PROM at 26 wk → severe respiratory distress syndrome |
|
PROM, premature rupture of membranes.
We encourage our patients to test their urine for ketones on a daily basis with instructions to check their blood glucose if the urine is positive for ketones. Depending on the blood glucose concentration, dietary or insulin manipulation would be encouraged.
ADDITIONAL EVALUATIONS.
We routinely obtain cardiac, ophthalmologic, and renal evaluations on all our pregnant type 1 and 2 patients at their first prenatal visit. Further evaluation depends on the results of the initial evaluation and the presence or absence of vascular complications. In patients with renal involvement, the renal function tests may need to be repeated as often as every month.
The study by Klein and associates43 suggests that proliferative retinopathy may be aggravated by pregnancy. Its presence, however, is not a contraindication to pregnancy but requires intensive and frequent evaluation of the retina with photocoagulation therapy as clinically necessary. Prompt laser photocoagulation treatment of retinopathy during pregnancy is associated with similar results as in the nonpregnant patient.44,45
NEEDS OF TYPE 1 AND 2 DIABETICS.
The U.K. Prospective Diabetes Study (UKPDS) demonstrated that type 2 diabetics are prone to the same microvascular complications as type 1 diabetics.45 We use the same aggressive management approach for types 1 and 2 diabetics. We have found no significant differences in outcome between the two types when adequate control is achieved. Langer and colleagues46 have reported similar outcomes. Type 1 patients need more intensive monitoring and vigilance, but as long as the metabolic control is adequate, a good outcome can be expected in both groups.
It appears that more important than the type of insulin-requiring diabetes is the presence of vascular complications. The latter increases the risk for preeclampsia, fetal malnutrition, and fetal distress. Nevertheless, our experience has been that, if the patient is compliant and good metabolic control is achieved, perinatal outcome is good.
DELIVERY.
Maturity of the fetus must be ensured before delivery, except when temporization is life threatening to the mother or fetus. In diabetic pregnancies dated by an ultrasound before 20 weeks, we have not had any infants born after 37 weeks of gestation develop respiratory distress syndrome.47 If ultrasound is available to date the pregnancy, we do not believe amniocentesis is necessary to assess fetal lung maturity. We have also found that the prognostic significance of a mature lecithin sphingomyelin (L/S) ratio and the presence of phosphatidyl glycerol (PG) in a well-controlled diabetic is no different from that of nondiabetic patients. Reliable estimates of fetal lung maturity can be derived from measurements of those phospholipids in the amniotic fluid when the need arises. As a general rule, in the presence of good metabolic control and fetal surveillance, we deliver our insulin-requiring diabetics between 39 and 40 weeks.
Intrapartum Management
The main objective of the intrapartum control of maternal blood glucose is the prevention of neonatal hypoglycemia. We have recommended an infusion of 10% Invert sugar (5% glucose and 5% fructose) and a separate intravenous infusion of regular insulin by pump.48 The Invert sugar is infused at a rate of 125 ml/hour, and the insulin is started at 1 to 2 units/hour. The rate is changed as necessary to maintain a blood glucose concentration of 70 to 100 mg/dl for at least 8 hours before delivery.
Our studies on the relative importance of the antenatal and intrapartum blood glucose concentration in the prevention of neonatal hypoglycemia have documented the crucial role of the intrapartum control.48 These relationships are shown are shown in Table 8.
| Mean Prenatal 2-Hour | |
| Postprandial Concentration | |
Mean Intrapartum Concentration | <150 mg/dl | >50 mg/dl |
<100 mg/dl | 10%* | 16%* |
| 43% | 71% |
*p < 0.05 when compared with groups
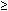 100 mg/dl.
100 mg/dl.Curet LB, Izquierdo LA, Gilson GJ et al: Relative effects of antepartum and intrapartum maternal blood glucose levels on incidence of neonatal hypoglycemia. J Perinatol 17:113, 1997.
We compared the use of D5W to 10% Invert sugar and found no significant difference in the incidence of neonatal hypoglycemia (Table 9). Suggesting that the nature of the substrate administered is not as important as the maintenance of the blood glucose concentration of less than 100 mg/dl.
TABLE 9. Effect of 10% Invert Sugar and D5/LR on Neonatal Blood Glucose
Concentration
| Invert Sugarn = 13 | D5/LR n = 13 | P |
Average intrapartum maternal blood glucose mg/dl* | 90.7 ± 11.1 | 82.6 ± 12.2 | NS |
Initial neonatal blood glucose | 45.3 ± 19.3 | 43.5 ± 9.9 | NS |
½ Hour neonatal blood glucose | 44.5 ± 9.4 | 45.0 ± 10.9 | NS |
1 Hour Neonatal blood glucose | 48.2 ± 9.9 | 51.8 ± 9.0 | NS |
Average neonatal blood glucose | 48.3 ± 9.1 | 48.2 ± 6.8 | NS |
Incidence of neonatal hypoglycemia | 15.4% | 23.1% | NS |
*Average blood glucose in mother for the 8 hours before delivery.
Postpartum Management
After delivery has been accomplished, specific issues need to be addressed for each type of diabetes.
GESTATION DIABETES MELLITUS.
If the patient did not require insulin during pregnancy, it is not likely that she will require any further treatment. We only recommend that these patients be followed by a primary care provider with monitoring of their blood glucose. Studies are underway to place these patients on metformin in an effort to delay the development of overt diabetes.
If the patient required insulin, we routinely discontinue it and monitor the blood glucose concentration. If the need arises, we recommend the use of oral hypoglycemic agents that in most of these patients can normalize the blood glucose concentration. Medical follow-up of these patients must be more intense as they are more likely to remain or progress to overt diabetes.
TYPE 1 AND 2 DIABETICS.
Data from the UKPDS45 demonstrate that long-term microvascular complications occur in type 2 diabetics. The trial also showed that intensive management of diabetes can prevent those long-term complications. These findings are similar to the findings of the DCCT on type 1 diabetics.42 Because of the increase in insulin sensitivity that occurs after delivery, we decrease the insulin dose by one half and instruct the patient to continue to decrease or increase the dose as during pregnancy, and we instruct our patients to continue the intensive approach used during pregnancy.
To ensure adequate follow-up, these patients need to be referred to an endocrinology program with extensive experience in intensive management of diabetic patients. This approach also can enhance the probability of the patient being in optimal control when she becomes pregnant again.