Airway mismanagement accounts for one third of cases of fatal aspiration
and 50% of anesthetic-related maternal mortality. The most recent studies
in England, the United States, and other parts of the globe show
that the incidence of failed intubation has remained at one in 250.16–18 Editorials, articles, and reports refer to the problem as intractable, unsolvable, or
irreducible.19–21 There are several reasons for this: - There is no accurate method to predict difficult intubation.
- The current technology or techniques are not suited for handling the situation
of a failed rigid laryngoscopy.
- Failed intubation occurs rarely, so few practitioners can gain experience.
- General anesthesia is the method of last resort when regional anesthesia
fails.
- Anesthesiologists ignore the data and belie their experiences.
- Maternal physiology is not conducive to surviving prolonged periods of
apnea.
For the past 30 years, rapid sequence induction reigns as the unchallenged
standard of care to secure the airway because of the urgency of obstetric
emergencies. There is a tendency to underestimate that which is
quantifiably small, leaving awake intubation as an underutilized technique. Surveys
show a wholesale undermining of safety. Even in the presence
of a known difficult intubation, 60% of anesthesiologists prefer
to secure the airway with the patient asleep.22 Prediction The current state of predicting difficult intubation is similar to the
situation in the 1970s when x-ray pelvimetry was considered to be the
gold standard for predicting successful vaginal delivery.23 Every few years, articles are published stating that if one uses a specific
set of airway measurements derived from physical examination, x-rays, computed
tomography, or magnetic resonance imaging, one can predict
difficult intubation within a reasonable degree of certainty.24,25 Later, these studies are refuted and replaced by a different technique
or method that purports greater accuracy and reproducibility.26,27 None of these tests have been validated by large prospective studies, and
thus the reports are the scatologic equivalent of worthless. Patients
intubated fiberoptically because of abnormal external anatomy prove
to be easy when intubated at a later date. Patients who appear normal
often are found to be difficult. Only 6.6% of patients with Mallampati
IV classification often were found to be difficult.28 In the Confidential Enquiry into Maternal Mortality (CEMD) series from 1967 to 1984, 83% (44/53) of the deaths due to failed intubation occurred
in patients with normal-appearing airways. Technology and Technique The current practice of general anesthesia requires intubation for management
of the obstetric airway. This can be accomplished with a rigid
laryngoscope in 99.6% (249/250) of the cases. Although no single company
or person has a monopoly on the correct technology or equipment to
solve the problem of failed intubation, a panoply of apparatus is promoted. Thus, the
anesthesiologist is inundated by a host of pulse oximeters, end-tidal
carbon dioxide detectors, Combitubes, cuffed oropharyngeal
airways, laryngeal mask airways, light-wands, jet ventilators, and
retrograde wires. These devices are popular because they require minimal
training and skill. Conversely, the reviews by Pierce and Cheney29,30 of the American Society of Anesthesiologists (ASA) Closed-Claim Database
conclude that increased monitoring will not prevent all cases of airway
disasters. Whereas cases of death due to unrecognized esophageal
intubation have virtually disappeared, deaths due to airway mismanagement
still occur. Despite the myriad of proposed solutions, the best epistemologists
of our specialty are stymied by the problem of preventing
maternal death after failed intubation. With the invention of the LMA, they
recommend that our efforts should focus on ventilation instead
of intubation.31 It is difficult using only positive pressure and a mask to ventilate the
parturient with an abdomen distended by a 9-month gravid uterus. Whether
the patient weighed 140 to 300 lb (65 or 140 kg) when the LMA was
successful, spontaneous ventilation returned for the duration of the
cesarean delivery.32,33 When positive pressure ventilation is planned, the LMA is best suited
as a helpful guide for sedated endotracheal intubation before the administration
of muscle relaxants.34 There is little time to react to the problem of failed intubation because
of the speed by which anesthesia induces apnea, with subsequent loss
of the airway, and oxygen desaturation. To be of any value, it is imperative
that the LMA and other airway aids are physically present in
the operating room before induction. It has long ago been recognized that more important than the technology
are the approach and technique.35 Rather than hire a tailor with a different style to make new clothes from
old cloth for the emperor, we are tempted to buy new fabric to effect
a sartorial result.36 With minimal sedation, liberal topicalization, and maintenance of spontaneous
ventilation, the airway can be secured before rendering the patient
unconscious. This was first suggested by Walts in 196537 and is now a recommended option in the ASA Task Force for management of
the difficult airway.38 Older solutions to airway management were forgotten and abandoned because
the technology was not appropriate for the task. Species are most
successful when their genetics promote a very active atavism, enabling
certain traits to disappear when the environment becomes hostile and
allowing their re-emergence in later generations when conditions become
more favorable. The same rules apply to engineering, when newer technology
ensures the success of older solutions. The fiberoptic laryngoscope is ideally suited for the task of securing
the airway with the patient awake, although a recent editorial did not
even mention its existence.19. Fiberoptic skills are not connatal; they must be developed. Similar to
following ASA pathways, guidelines, algorithms, or drills, training
and practice are the keys to successful outcome. Keeping the fiberoptic
laryngoscope locked in a cabinet for use only in emergency situations
leads to failure and frustration. Nor should its use be limited to the
sacerdotal services of attending physicians training novices in academic
centers. Other than isolated case reports, there is no large series
of using fiberoptics before induction of anesthesia to reduce maternal
mortality.39–42. Rare Occurrences Failed intubation accounts for 20% of the obstetric anesthesia malpractice
cases in which severity of outcome determines both physician reviews
and jury awards.43,44 There are few articles from major institutions on this subject because
errors of judgment concerning the airway are clothed in secrecy for fear
of being misconstrued as cases of medical malfeasance.45 In a recently published American study, fiberoptics were used preinduction
of general anesthesia in only 1.3% (7/538) of the parturients and
the incidence of failed intubation remained at one in 269.17 Of the two cases of failed intubation, one resulted in a maternal death. When
compared with the British data from Lyons' study, in which no
fiberoptics were used, the ratio of the two standardized incidence ratios
is 0.97.46 Why not take a passive approach and use the fiberoptic only after failed
rigid laryngoscopy? This question is of eschatologic magnitude because
one in every 87 (40/3333 = 1/87) cases of failed intubation results
in a maternal death.21 Following the old adage of closing the barn door after the horse escapes
leads to maternal death since 1.2% (40/3333) of failed intubations
are in parturients who are impossible to mask ventilate. This is 13 times
the incidence in the nonobstetric patient.21 When prospective studies rather than mortality statistics are reviewed, impossible
mask ventilation occurs in 9% of failed intubations whether
the patient is pregnant (2/23) or not (1/11). Analysis shows that the
combination of failed intubation and impossible mask ventilation occurs
in nonobstetric patients with risk factors that include body-mass
index higher than 26 kg/m2 and presence of sleep apnea.47 Pharyngeal edema frequently occurs during pregnancy with gain in body
weight.48 The linkage of difficult mask ventilation with failed intubation is roughly
the same in the two populations: 36% (4/11) in the nonobstetric
patients compared with 30% (7/23) in the obstetric patients. In contrast to the CEMD series, 26% of the patients presenting for general
anesthesia in an obstetric population from South Africa had difficult-appearing
airways.28 They were described as having a short neck and swollen face or tongue
and as being obese. Unfortunately, the endomorphic parturient who weighs
more than 300 pounds is no longer a rare event in the United States, in
which 17% of the population is overweight and medical complications
from obesity account for more than 300,000 deaths annually.49,50 In the United States, Endler and coworkers51 in Michigan found that 80% of general anesthesia deaths were caused by
obesity. In a different American population of parturients weighing more
than 300 pounds (Hood, North Carolina), difficult intubation was encountered
in 35% (6/17) of the cases.52 Only one patient was intubated awake with rigid laryngoscope. After induction, two
patients required multiple attempts at securing the airway. The relative scale of the problem must be brought into perspective. Greater
concern should be made of managing the maternal airway and less worry
about neonatal respiratory depression. The 300-pound parturient at 28 weeks' gestation
presenting for emergency cesarean delivery due to
a prolapsed cord is better served by a sedated fiberoptic intubation
than a failed rapid sequence induction. It is easier to intubate an 800-g
neonate than to struggle with the airway of the mother. The neonate
will be intubated and ventilated anyway because of fetal lung immaturity. An
order-of-magnitude mentality can easily mislead. Although the
neonatal airway is much smaller than the adult, intubation equipment
is appropriately scaled to the task. Larger laryngoscope blades are used
in adults. However, this does not guarantee success because of the
challenge of aligning less plastic airway anatomy with the usual field
of the intubator. Gaining Experience There is increasing concern that as the number of cesarean deliveries to
patients receiving general anesthesia decreases, there will be insufficient
cases for training.53 Because of the acuteness of obstetric care, there must be a designated
fiberoptic assigned to labor and delivery. Unlike the situation in other
institutions, the fiberoptic cannot be shared with respiratory care
or the general operating room.54 In emergency situations or when the abdomen is already open, it is appropriate
for an attending physician to perform the fiberoptic intubation. When
facing a nonurgent situation, such as a retained placenta or
termination, it is appropriate for a tyro to try his or her skills with
fiberoptics. How can we ensure that attending physicians covering obstetric patients
are qualified in the use of fiberoptics? Experience performing three
awake fiberoptic intubations per month is considered the minimum to maintain
proficiency.55 It is doubtful whether most of the attending staff in this country who
are responsible for covering labor and delivery suites currently possess
these skills. Age of the physician seems less of a handicap in using the fiberoptic laryngoscope
than lack of hand-eye coordination. When using a straight
or curved laryngoscope blade, presbyoptics are at a disadvantage because
it is impossible to focus on an anterior larynx through their lower
set of bifocal lenses. By enhancing the visual acuity of the optically
challenged physician, the fiberoptic soon becomes an indispensable part
of their armamentarium. The video game generation quickly grasps the use of the fiberoptic because
the controls on the handle are similar to the controls of a joystick. Simulators
are being developed to take advantage of the generation's
youthful proclivity in navigating through virtual environments
from Mario World to the Virtual Larynx.56 Promoting Regional Anesthesia The use of general anesthesia for cesarean deliveries has fallen steadily
from 50% to 5% during the past 25 years. However, regional anesthesia
may fail in situations in which it is needed the most, or the opposite
may happen: too high a level is reached in a parturient with a difficult
airway. Denying the Data Some contest the veracity of Medic-Alert periapts emblazoned with “difficult
intubation.” Others hold firm to their inveterate beliefs
and inveigh a litany of complaints against sedated fiberoptic intubation. They
protest that the hypermetric use of the fiberoptic exposes
patients to the very dangers we are trying to avoid: (1) delays in
delivery, (2) lower neonatal Apgar and umbilical cord gases, (3) maternal
aspiration and asphyxiation, and (4) psychological trauma. The data show the following: - Time from transtracheal injection to intubation is less than 2 minutes.
- When stratified by gestational age and indication for cesarean delivery, there
is no difference between rigid and fiberoptic groups.
- No cases of maternal aspiration have occurred in the fiberoptic groups. Parturients
with a full stomach, sedated but breathing spontaneously, who
can cough do not aspirate.57
- Because general anesthesia results in 17 times the mortality of regional
anesthesia, it is no surprise that there is great fear of its use, even
when appropriate.20 During the past quarter of a century (1975 to 2000), more patients have
had to endure cesarean deliveries with poor anesthesia because of an
inadequate regional block than the few patients who reported a brief
choking sensation after fiberoptic intubation.
Surviving Prolonged Periods of Apnea The physiologic changes of pregnancy include increased oxygen consumption
and cardiac output but a decrease in alveolar volume. During apnea, these
factors shift the maternal hemoglobin de saturation curve to the left. The curve is shifted to the right by increased
maternal blood volume. Overall, there is a faster rate of hemoglobin de saturation in the gravid patient compared with the nongravid patient58 (Fig. 5). The nonpareil benefit of preoxygenation only adds 4 to 6 minutes before
hemoglobin desaturation to 65%. Benumof59 showed that return to 50% twitch height after succinylcholine can extend
beyond 8 minutes. A rapid sequence induction without preoxygenation
should be proscribed. The whole scenario of failed intubation and impossible
ventilation can be avoided by securing the airway before induction. Maternal, and
thereby fetal, oxygenation then can be maintained
up to the time of delivery. In preparation for fiberoptic intubation, a
nasal cannula should be placed on the mother before sedation and topicalization. Even
in the presence of high maternal PaO2, the fetal pvO2 cannot exceed maternal mixed venous PO2.60 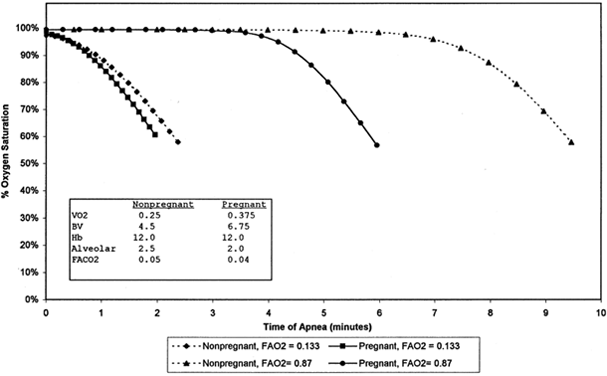 Fig. 5. Hemoglobin desaturation versus time pregnant and nonpregnant breathing
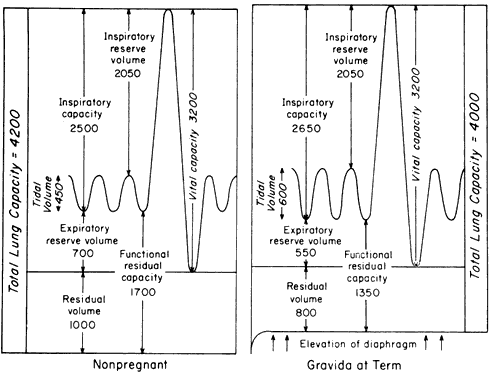
room air or 100% oxygen before apnea. Fig. 5. Hemoglobin desaturation versus time pregnant and nonpregnant breathing
room air or 100% oxygen before apnea.
|
| 
 /
/ ), and small airway closure. Because small airway closure has been suggested
as the major mechanism for hypoxemia during labor, it deserves special
consideration. Small airways in dependent portions of the lung
are known to collapse during forced exhalation, a mechanism intended to
protect the small, dependent alveoli from collapsing. This small airway
closure phenomenon is studied by the closing volume (CV) pulmonary
function test. CV measurements in the supine pregnant patient show that
one third of patients have airway closure develop during normal tidal
ventilation
), and small airway closure. Because small airway closure has been suggested
as the major mechanism for hypoxemia during labor, it deserves special
consideration. Small airways in dependent portions of the lung
are known to collapse during forced exhalation, a mechanism intended to
protect the small, dependent alveoli from collapsing. This small airway
closure phenomenon is studied by the closing volume (CV) pulmonary
function test. CV measurements in the supine pregnant patient show that
one third of patients have airway closure develop during normal tidal
ventilation