Asymptomatic Bacteriuria
A combination of host defense inefficiency, anatomy, behavior, and microbial
virulence factors identifies a cohort of women who have episodes of bacteriuria
throughout their lifetimes.31,32,33,34,35,36
Cross-sectional prevalence studies identify 1% to 8% of
women with asymptomatic bacteriuria.21,22
In longitudinal studies, 30% to 50% of nonpregnant women
with bacteriuria have symptomatic lower urinary tract infections during
3 to 5 years of follow-up.32,33,34 Most episodes cluster over a 3- to 4-month
period, followed by an asymptomatic interval of variable length. A series
of 9- to 19-year follow-up studies37 on
60 asymptomatic bacteriuric school girls (6 to 10 years old) were compared
with studies on 38 nonbacteriuric control school girls matched for age,
race, and school. Episodes of bacteriuria in the 5-year study period for
infected girls and controls were 22% and 3%; episodes during
pregnancy were 64% and 27%. The children of bacteriuric
women were more likely to have urinary tract infections than were the
children of controls.
Of 30% of women who are bacteriuric during pregnancy, 20%
are bacteriuric on long-term follow-up cultures when they are not pregnant.35,36 Radiologic examination at follow-up of women
who were bacteriuric during pregnancy revealed abnormalities in 316 (41%)
of 777 women (range, 5% to 75%). Chronic pyelonephritis
was the most common radiologic diagnosis (47% of abnormalities).
The incidence of bacteriuria during first pregnancies was significantly
greater in women with (47%) than without (27%) renal scarring
from childhood urinary infections. Similar controls who had not had childhood
urinary infections had an incidence of 2%.
The cohort of women with chronic, episodic bacteriuria is identified
by routine screening of urine cultures at the first prenatal visit. The
prevalence of asymptomatic bacteriuria (≥2 cultures having≥105↑
colony-forming units per milliliter) is higher among women with prior
renal/urinary tract disease, diabetes, sickle cell trait/disease, poor
hygiene, high parity, increased age, and lower socioeconomic status.33,39 The overall prevalence varies between 1.9%
and 11.8%, with the lowest prevalence in primiparous patients of
upper socioeconomic class and the highest among indigent multiparas. Although
most women with asymptomatic bacteriuria are identified shortly after
entering prenatal care, approximately 1% to 2% acquire bacteriuria
later in pregnancy.
Uncomplicated, asymptomatic bacteriuria is a significant health risk for
pregnant women but not for nonpregnant women. Asymptomatic bacteriuria
has been associated with pyelonephritis, preterm birth, growth retardation, hypertension, and
fetal neuropathology. The most consistent association
is a greater likelihood of pyelonephritis. Sweet40 reviewed the relationship between asymptomatic bacteriuria and acute pyelonephritis. In 1699 patients with untreated asymptomatic bacteriuria (18 studies), pyelonephritis developed in 471 (27.8%; range, 16% to 65%). Antibiotics in placebo-controlled trials reduced
by 80% the frequency of pyelonephritis in women with asymptomatic
bacteriuria. The incidence of pyelonephritis in the treated groups
ranged from 0% to 5.3%. On the basis of these observations, treatment
of asymptomatic bacteriuria in pregnancy is warranted
to reduce the incidence of pyelonephritis. The association between preterm birth and asymptomatic bacteriuria first
was identified by Kass41 at Boston City Hospital between 1955 and 1960. As is true of many early
studies, prematurity was defined as birth weight less than or equal
to 2500 g, a definition that would include 30% to 50% of
growth-retarded term infants. Kass’ initial study reported that 32 (17.8%) of 179 bacteriuric patients delivered low-birth-weight (LBW) infants, whereas 88 (8.8%) of 1000 nonbacteriuric patients
delivered LBW infants. Since that report, many studies of small
numbers and heterogeneous populations have supported and rejected this
observation. In 1990, Sweet and Gibbs39 reviewed 19 studies that related bacteriuria to LBW infants. In these
studies, 3619 bacteriuric pregnant women delivered 400 (11%; range, 4.4% to 23%) LBW infants. In these same studies, 31,277 nonbacteriuric
women delivered 2725 (8.7%; range, 3% to 13.5%) LBW
infants. Some cohort studies designed to adjust
for socioeconomic demographic variables failed to show a difference in
LBW between women with and without asymptomatic bacteriuria. Perhaps
asymptomatic bacteriuria is not associated with LBW per se, but it is
a marker for low socioeconomic status, which in turn predicts LBW.
When confounding variables are controlled, a strong relationship between
asymptomatic bacteriuria and LBW remains. In 1989, Romero and associates42
reported on the relationship between asymptomatic bacteriuria and LBW.
A meta-analysis was performed to increase the statistical power for primary
and secondary outcome variables and to improve estimations of the effect
of sample size on treatment trials. Previous cohort, case-controlled,
and randomized antibiotic trials, many of which also were reviewed by
Sweet,39,40 were analyzed
for comparable and appropriate study design. Seventeen cohort studies
met their criteria for “good” studies. The typical relative
risk for a nonbacteriuric woman to deliver a LBW infant compared with
a bacteriuric woman was 0.65 (95% confidence interval [CI] =
0.52 to 0.72). One case-controlled study compared the prevalence of bacteriuria
in women delivering at less than 36 weeks (33 of 404 [8.1%]) with
the prevalence of bacteriuria in women delivering at or beyond 37 weeks
(15 of 404 [3.7%]) (p = .0036) after matching for
maternal race, age, parity, smoking habits, physical dimensions, and sex
of the newborn. Eight randomized clinical trials of antibiotic therapy
showed a significant reduction in the frequency of LBW after antibiotic
therapy (typical relative risk = 0.56; 95% CI = 0.43
to 0.73). These analyses support the hypothesis that untreated asymptomatic
bacteriuria is associated directly with a higher incidence of LBW. It
is unclear whether the benefit from antibiotics results from a reduction
in asymptomatic or symptomatic pyelonephritis or from beneficial changes
in abnormal genital tract flora, which are associated with LBW.
A variety of antimicrobial agents and treatment regimens have been used
to treat asymptomatic bacteriuria during pregnancy.43 Most community-acquired pathogens associated with asymptomatic bacteriuria
during pregnancy are sensitive to sulfa drugs (sulfisoxazole, 1 g
four times daily for 7 days), nitrofurantoin (100 mg four times daily
for 7 days), or cephalosporins (cephalexin, 500 mg four times daily for 7 days). Ampicillin (500 mg four times daily for 7 days) is a time-honored, safe, effective, and inexpensive therapy; however, there are
a growing number of resistant E. coli strains. Patient education should accompany any prescription for antibiotics to
treat urinary tract infection. The essentials of behavior intervention
include (1) avoiding the female superior position during sexual activity; (2) avoiding
anal intercourse before vaginal intercourse; (3) voiding
within 15 minutes after sexual activity; (4) avoiding bubble baths
and oils; (5) avoiding vaginal douching or deodorant sprays; and (6) always
wiping the urethra, perineum, and anus from front to back. These
interventions reduce the frequency of recurrent urinary tract infections
in high-risk women.44 Fihn and Stamm45 reviewed 62 treatment trials for uncomplicated urinary tract infections
to assess whether methodologic problems compromised the validity of
the study. These trials fulfilled an average of 56% of 12 standards
necessary for accurate interpretation and comparability. The standards
least often met were sufficient power to detect a meaningful difference (21%), double-blind assignment of treatment regimens (37%), and
clear definitions of cure and failure (35%). Those
deficiencies were especially true when comparing single-dose versus
multidose therapy. None of 14 randomized controlled trials had sufficient
power to prevent a type II error. When roughly comparable studies
were pooled, single-dose amoxicillin (3 g) was significantly less effective
than conventional multidose therapy (69% versus 84%). Until
a larger study is performed, single-dose therapy should not
be used in the treatment of urinary tract infections in pregnancy. Antibiotics sterilize the urine in asymptomatic bacteriuria in 80% to 90% of
women. The cure rate depends on compliance, length
of regimen, preexisting risk factors, asymptomatic renal infection, and
sensitivity of the organism. A test of cure by culture within 2 weeks
after the end of the antibiotic regimen discriminates between relapse
and reinfection. Relapse (a positive test-of-cure culture) has been
associated with complicated asymptomatic bacteriuria. These women may
have urinary tract abnormalities, asymptomatic renal infections, or silent
urolithiasis. Unusual organisms or antibiotic sensitivity patterns
alert the clinician to a reservoir of partially protected bacteria (e.g., renal
abnormality, urolithiasis, or noncompliance). A urine pH
greater than 6.0 (Proteus) and persistent hematuria are clues indicating a stone-related infection. During
pregnancy, a renal ultrasound helps identify a renal stone
as a cause of relapse. A postpartum intravenous pyelogram is warranted
in any case of relapse. Relapse should be treated with another 10-day
course of antibiotics chosen by the sensitivity pattern from the test-of-cure
culture. The therapeutic regimen should be followed by suppressive
therapy. Suppressive antibiotic therapy is effective in reducing recurrent cystitis
in nonpregnant women and recurrent pyelonephritis in pregnant women. The
prophylactic efficacy depends on nightly bactericidal activity
against sensitive reinfecting bacteria entering the bladder urine. Vaginal
colonization with uropathogenic Enterobacteriaceae continues unabated, depending
on the regimen chosen. The rectal reservoir for potential
uropathogen is rarely sterilized by either therapeutic or prolonged
suppressive regimens. One danger of suppressive therapy is the emergence
of antibiotic-resistant strains. High-dose cephalexin (500 mg four
times daily), but not low-dose cephalexin (250 mg four times daily), induces
resistant E. coli strains.36 Nitrofurantoin macrocrystals (100 mg every night) neither reduce the prevalence
of Enterobacteriaceae in rectal or periurethral flora nor induce
antibiotic resistance. Trimethoprim, 40 mg, plus sulfamethoxazole, 200 mg, given
every night reduces the incidence of Enterobacteriaceae
in rectal and periurethral flora, but it generally is not associated
with antibiotic resistance.36 Lincoln and coworkers46 reported resistant urinary infections resulting from sulfonamide suppression
therapy.
In motivated patients, a combination of patient education and urine testing
biweekly for leukocyte esterase and nitrite is just as effective as prophylactic
antibiotic suppression in reducing the frequency of recurrent pyelonephritis
after an initial episode during pregnancy. The frequency of recurrent
pyelonephritis in the antibiotic suppression group was 7% versus
8% in the close surveillance group.47
The latter surveillance regimen may be enhanced further by antibiotic
prophylaxis (nitrofurantoin macrocrystals, 100 mg, or cephalexin monohydrate,
500 mg) after each episode of sexual intercourse or masturbation.36,48
Acute Cystitis Acute cystitis occurs in 0.3% to 2% of pregnancies.39 The reported frequency is only minimally greater than the frequency of
cystitis in sexually active nonpregnant women. The diagnosis is more
difficult to make during pregnancy. Most pregnant women have urgency, frequency, or
suprapubic discomfort. Suprapubic discomfort in pregnancy
often results from pressure from the presenting fetal part or early
labor. Suprapubic discomfort from cystitis is unique, however, and most
women with a history of acute cystitis can discriminate accurately between
cystitis and pregnancy-related discomfort. The most reliable findings
are dysuria and hematuria. Acute dysuria also may result from labial
or perivaginal irritation secondary to vaginitis, vulvitis, herpes
simplex, condylomata acuminata, or genital ulcers. Because of the separate
pregnancy risks associated with these factors, an inspection of
the vulva and vagina is warranted in patients with acute cystitis during
pregnancy. Patients with preterm labor and impending second-trimester loss often present
with signs and symptoms similar to those of acute cystitis. As
the lower uterine segment expands and the presenting fetal part descends, hesitancy, urgency, frequency, and suprapubic discomfort occur. A
bloody vaginal discharge may contaminate and confuse urine testing and
may lead to misdiagnosis of urinary tract infection. Pelvic examination
is warranted in patients presenting with signs and symptoms of lower
urinary tract infection to rule out preterm labor. Treatment of acute cystitis is similar to that of asymptomatic bacteriuria:43 nitrofurantoin, 50 mg four times daily for 7 days; cephalosporin, 500 mg
four times daily for 7 days; or sulfonamide, 1 g four times daily for 7 days. Because
these patients are symptomatic, therapy is initiated
as soon as a midstream, clean-catch urine culture is obtained. Studies
in nonpregnant women suggest that 3 days of antibiotics may be as efficacious
and cost less than a 7-day regimen.49 The comparative efficacy of 3- versus 7-day regimens has not been studied
in pregnancy. A test-of-cure culture is obtained within 2 weeks after
therapy is complete. Of women, 10% to 20% have a positive
test-of-cure culture, representing a relapse. These women should
be retreated with another antibiotic, as determined by bacterial sensitivities. After
retreatment, these patients should be placed on suppressive
antibiotic therapy. Without suppressive therapy, an additional 20% to 30% of women acquire another urinary tract infection (i.e., a
reinfection) during the remainder of pregnancy and the puerperium. Because
of the risk of recurrence, patients with cystitis should
be followed intensively with a urine screen biweekly for nitrite
and leukocyte esterase. The delivery process constitutes a significant risk period for symptomatic
urinary tract infections. Trauma to the urethra, periurethra, and
labia creates swelling and pain that inhibits frequent and complete voiding. Multiple
vaginal examinations and the pumping action of the fetal
head in the second stage inoculate the urine with periurethral flora. Urinary
retention is exacerbated by epidural anesthesia and perineal
trauma. Interventions such as simple in-and-out catheterization to relieve
urinary retention pose a 10% to 15% risk of bacteriuria.50 As a result, 10% to 25% of all pyelonephritis cases associated
with pregnancy occur in the first 14 days postpartum. Acute Pyelonephritis
Acute pyelonephritis is the most common serious medical complication
of pregnancy.39,51 The
incidence of pyelonephritis is 1% to 5%. Often these patients
present for prenatal care in the second half of pregnancy with signs and
symptoms of pyelonephritis. Only 40% to 67% of pyelonephritis
cases occur in patients with a known history of asymptomatic bacteriuria.
Three fourths of women with pyelonephritis present in the antepartum period,
5% to 10% present in labor, and 10% to 25%
present postpartum. Antepartum pyelonephritis occurs mainly after the
first trimester: 10% to 20% during the first trimester,
45% to 70% during the second trimester, and 8% to
45% during the third trimester. The predominance of pyelonephritis
in late pregnancy and the puerperium relates to the partial obstruction
caused by the growing uterus and to trauma or interventions at birth.
The diagnosis of acute pyelonephritis is based on clinical presentation: fever (≥38°C), costovertebral angle tenderness, and either
bacteriuria or pyuria. Among patients meeting these criteria (n = 656),52 12% had fevers greater than 40°C; costovertebral angle tenderness
was on the right side in 54%, on the left side in 16%, and
bilateral in 27%. Chills and back pain were a presenting
complaint in 82% of patients, whereas only 40% had
dysuria, frequency, urgency, or hematuria; 24% had nausea and
vomiting. Overt septic shock or adult respiratory distress syndrome occurs in 1% to 2% of
pregnant women with acute pyelonephritis. Clinical
clues to the development of these life-threatening complications are
leukocytopenia (<6000 cells/mm3), hypothermia (≤35°C), elevated respiratory rate, and widened
pulse pressure. In the late stages, hypothermia, mental confusion, and
symptomatic hyperstimulation of the sympathetic nervous system (cold, clammy
extremities) herald a scenario that often leads to maternal
or fetal death. In all cases, the mother and fetus should be treated
in facilities having the expertise and equipment to handle critically
ill mothers and infants. All pregnant women with pyelonephritis should be hospitalized because of
the additional fetal and maternal risks of acute pyelonephritis in pregnancy. Intravenous
antibiotics (2 g of cefazolin every 6 hours or 2 g
of ampicillin plus 1 g of sulbactam every 6 hours) should be initiated
as soon as possible after urine and blood cultures are obtained. Because
many patients are dehydrated as a result of nausea and vomiting, careful
rehydration is started. The degree of endothelial damage in
the lungs may not be apparent, so careful attention to fluid intake and
output and vital signs, especially respiratory rate, is imperative. Respiratory
symptoms (e.g., an increased respiratory rate), peripheral
cyanosis, and mental confusion prompt an immediate x-ray study and measurement
of arterial blood gases. Colloid oncotic pressure and serum
albumin measurements are important in the fluid management of these critically
ill patients. Endotoxins stimulate cytokine and prostaglandin production by decidual
macrophages and fetal membranes. The ensuing preterm contractions raise
concern for preterm birth. Three major problems confront the physician
at this point. First, although pyelonephritis is often a clear diagnosis, the
presence of lower abdominal pain and contraction raises the
possibility of intra-amniotic infection, a diagnosis that precludes tocolytic
therapy. The presence of white blood cells and bacteria on an
unspun Gram stain of amniotic fluid is sufficiently sensitive in the
diagnosis of intra-amniotic infection to preclude the use of tocolysis. Second, premature
contractions may not indicate labor. Often uterine
irritability ceases after hydration and administration of antibiotics. If
contractions are of sufficient frequency and strength to change the
cervix on serial pelvic examinations (≥2 cm in dilation, ≤1 cm
in length, and ≥50% effacement), the diagnosis
of preterm labor is made. Third, preterm labor must be treated with an
appropriate tocolytic agent if no other contraindication to tocolysis
is present (e.g., intra-amniotic infection, fetal lung maturity, fetal
abnormalities, or rupture of membranes). Ritodrine hydrochloride, the
only FDA-approved tocolytic, exacerbates the cardiovascular effect of
endotoxemia. The risk of pulmonary edema, cardiac toxicity, and adult
respiratory distress syndrome is increased. Magnesium sulfate (4 g intravenous
slow bolus, followed by 2 to 4 g/h) is the tocolytic of choice. Serum
magnesium levels (≤10 mEq/L) and physical signs of toxicity (loss
of deep tendon reflexes) are especially important to follow, however, because
half of patients with acute pyelonephritis have
renal dysfunction.
Maternal hyperthermia (≥38.3°C) should be treated aggressively
with antipyretics, such as acetaminophen. Maternal hyperthermia, hence
fetal hyperthermia (an additional 0.5°C), increases the metabolic
demand of the fetus. Glucocorticoids should not be used to enhance fetal
lung maturity because they may exacerbate maternal infection. Of these
pyelonephritis patients, 80% to 90% become afebrile within
48 hours, and an additional 5% to 15% become afebrile by
72 hours; 5% to 10% are classified as initial treatment
failures. In patients with significant deterioration of their condition
after the first 18 hours of therapy or in patients with temperatures greater
than 38°C at 48 hours of therapy, 1.5 mg/kg of gentamicin every
8 hours should be added. The dosing frequency is extended for serum creatinine
greater than 1 mg/dL (dosing frequency = 8 × the serum creatinine).
Antibiotic therapy should be continued until the patient is afebrile (<37°C)
for more than 24 hours. The patient should finish a 14-day course of antibiotics
with a bactericidal oral medication (500 mg of an oral cephalosporin four
times daily). A test-of-cure urine culture should be performed 2 weeks
after therapy. Without suppressive antibiotic therapy, reinfection is
common in these patients: 20% have asymptomatic bacteriuria, and
23% have recurrent pyelonephritis. Frequent surveillance (nitrate/leukocyte
esterase testing biweekly) or suppressive antibiotic therapy (100 mg of
nitrofurantoin every night) is warranted.47
The risk of recurrent pyelonephritis is less than 10% (with either
regimen).47,51
The differential diagnosis in patients with persistent fever and costovertebral
tenderness at 72 hours of therapy includes a resistant organism, urolithiasis, renal
abscess, complete ureteral obstruction, or another
source of infection (e.g., appendicitis or intra-amniotic infection). A
radiologic evaluation of the urinary tract is warranted after re-examination
of the patient and review of culture and sensitivity reports.53 Many radiologists are unduly concerned regarding the fetal dangers of
intravenous pyelograms during pregnancy and advocate renal ultrasound. A
renal ultrasound is useful for evaluating renal abscess, but not for
evaluating function or ureteral abnormalities, the more common issues
associated with antibiotic failure. A “one-shot” intravenous
pyelogram (no plain film and one 20-minute film) is appropriate. Intra-Amniotic Infection
Intra-amniotic infection is a clinical infection of the amniotic fluid
membranes, placenta, fetus, or uterus that occurs during labor or immediately
after birth (<6 hours). Intra-amniotic infection occurs when an
intrapartum temperature is greater than 37.8°C and two or more
of the following conditions are present: maternal tachycardia (>100
beats/min), fetal tachycardia (>160 beats/min), uterine tenderness,
foul odor of the amniotic fluid, and maternal leukocytosis (>15,000
cells/mm3).13,54
This definition was associated with a higher incidence (81% versus
31%; p < .002) and more virulent organisms (69%
versus 8%; p < .001) cultured from the amniotic fluid
of laboring women.13,54
The incidence of intra-amniotic infection varies between 0.5% and
10% of laboring women and is associated with significant maternal
and neonatal morbidity. Maternal age younger than 20, nulliparas, longer
duration of rupture of membranes, longer duration of fetal scalp sample,
greater number of vaginal examinations, and preterm gestation are risk
factors for intra-amniotic infection.13,55
Potential maternal complications include sepsis, adult respiratory distress
syndrome, prolonged labor, cesarean wound infection, and persistent
fever postpartum.54 As long as intra-amniotic infection is recognized promptly, broad-spectrum
antibiotic therapy reduces additional maternal infectious morbidity
to a minimum. In a randomized trial of intrapartum versus postpartum
antibiotic therapy, intrapartum treatment was associated with a lower
maximum temperature postpartum and fewer postpartum hospitalization
days (4.0 ± 1.0 versus 5.0 ± 1.9; p < .05).56 The duration of labor was not altered by antibiotic therapy; however, the
sample size in this study was small (n = 45). Several cohort
studies showed a deterioration in uterine contractibility with intra-amniotic
infection.54 It is possible that early antibiotic therapy may improve myometrial performance
and reduce the need for cesarean section secondary to dystocia.
In a population of primarily term infants, the neonatal morbidity and
mortality rates of untreated intra-amniotic infection were as follows:
sepsis, 10% to 15%; pneumonia, 1% to 4%; and
perinatal death, 0.5% to 3%.54,57 Intra-amniotic infection in preterm neonates
probably results in higher morbidity rates; however, the observed risk
is reduced by the routine use of intrapartum antibiotics in preterm gestations
complicated by intra-amniotic infection. GBS and E. coli contribute
a disproportionate number of cases of neonatal morbidity, and the goal
of intrapartum therapy is to provide fetal blood levels of antibiotics
to which GBS and E. coli are sensitive.
Reducing fetal neonatal morbidity and mortality is the major focus of
therapy in intra-amniotic infection, and three issues dominate fetal management
in these cases: (1) the need to provide effective antimicrobial therapy
for the fetus; (2) the need to provide direct intravenous antibiotics
to the neonate; and (3) the use of fetal monitoring, antipyretics, and
surgical technique at cesarean section. In the past, obstetric services
delayed antibiotic therapy in intra-amniotic infection until after birth
because the therapy would result in negative newborn cultures and delay
the diagnosis of neonatal sepsis. The results of two retrospective studies58,59 and one prospective, randomized study56
have shown unequivocally a benefit of administering intrapartum antibiotic
therapy as soon as an infection is diagnosed. Overall, intrapartum antibiotics
were associated with 8 (2%) of 389 cases of neonatal sepsis versus
21 (9.3%) of 225 (p < .01) cases among women treated
postpartum. The incidence of neonatal pneumonia (3.2%) reported
in these studies was too small to determine the effectiveness of intrapartum
antibiotics on pneumonia.
The two essential qualities of an antibiotic regimen are the ability to
cover the organisms associated with early neonatal sepsis and intra-amniotic
infection and the ability to cross the placenta in quantities
sufficient to begin fetal/neonatal therapy.60 GBS and Enterobacteriaceae are the most common organisms associated with
early neonatal sepsis and are commonly found in intra-amniotic infection (see Table 2). Ampicillin, 2 g intravenously every 6 hours, plus gentamicin, 1.5 mg/kg
intravenously every 8 hours, or ampicillin, 2 g, plus sulbactam, 1 g
intravenously every 6 hours, provide effective therapy. Ampicillin
is always given first because it crosses the placenta rapidly (<30 minutes) in
high concentrations (ratio of maternal blood to cord blood, 0.71). Gentamicin
is used in higher doses (1.5 mg/kg every 8 hours) in
pregnant women than in nonpregnant women because of the high renal
clearance associated with pregnancy. Anaerobes play a major role in the pathogenesis of preterm birth, the amniotic
fluid flora of intra-amniotic infection (see Table 2), and complications associated with postcesarean endometritis. The addition
of anaerobic coverage has reduced failure rates in postcesarean
endometritis, and because of this finding, we add clindamycin, 900 mg
intravenously every 8 hours, after cord clamping to the primary antibiotics (ampicillin
plus gentamicin or ampicillin plus sulbactam) if the
patient is undergoing a cesarean section. Maberry and Gilstrap60 compared the effect of ampicillin plus gentamicin (n = 69) with
ampicillin, gentamicin, and clindamycin (n = 64) in a randomized, comparative
trial. One infant in each group (1.5%) had positive
blood cultures, and there were no differences in maternal outcomes. The
sample size was insufficient to show a difference in postcesarean
complications (abscess or septic pelvic thrombophlebitis), which may
have been affected by the addition of clindamycin. Modified cephalosporins and penicillins enter the market frequently. Many
have an antimicrobial spectrum including anaerobic coverage, which
would indicate they are effective therapy for intra-amniotic infection. Newer
agents should not be used, however, unless they have limited fetal
or neonatal effects and have been shown to cross the placenta. There
are several alternatives based on safety and the study of their transplacental
pharmacokinetics (Table 15). TABLE 15. Antimicrobial Regimens for Treatment of Intra-amniotic Infection
First Choice - Piperacillin plus tazobactam
- Cefuroxime
- Ampicillin plus gentamicin*
Second Choice- Cefoxitin
- Ampicillin plus sulbactam*
Penicillin Allergy- Cefuroxime
- Cefazolin plus gentamicin*
- Vancomycin plus gentamicin*
*Add clindamycin if cesarean section is performed.
Many clinicians recognize the potential neonatal risks of intra-amniotic
infection and believe that the longer the fetus stays in the infected
environment, the greater the likelihood of neonatal infection or stillbirth. This
urgency may be reflected in a greater risk of cesarean section; however, current
data suggest that this urgency is not warranted. First, intrapartum
antibiotics provide bactericidal concentrations
of antibiotics to the fetus, membranes, and amniotic fluid within 0.5 to 1 hour
after infusion. Second, the average time between diagnosis
of intra-amniotic infection and delivery is 3 to 5 hours.47 It is not likely that 3 to 5 hours will change the neonatal outcome if
the fetus is receiving adequate antibiotics transplacentally. Third, the
duration of infection does not correlate with adverse neonatal outcomes, such
as pneumonia and early neonatal sepsis.54 The use of continuous electronic fetal monito ring is appropriate for observing
the development of fetal compromise in cases of intra-amniotic
infection. The combination of villous edema, hyperthermic stress, and
fetal infection can lead to fetal acidosis. Although no particular pattern
of periodic changes signifies fetal infection, a nonreassuring
tracing (e.g., one with absent variability and late decelerations) predicts
fetal acidosis and poor short-term outcomes. Fetal tachycardia is
a predictor of fetal sepsis or pneumonia but may be due only to fetal
hyperthermia. The use of an antipyretic (e.g., 625 mg of acetaminophen
rectal suppository every 4 hours) is therapeutic and may be diagnostic.61 The lowering of maternal temperature reduces the metabolic stress of fetal
hyperthermia and decreases the fetal heart rate. If the tachycardia
is not due to maternal fever, the acetaminophen will not reduce the
fetal heart rate. In these cases of persistent fetal tachycardia, health
care providers must prepare for a hemodynamically unstable neonate. Preparation
includes the personnel, skill, and drugs required for neonatal
resuscitation in the delivery room. In the past, extraperitoneal cesarean section was recommended in patients
with intra-amniotic infection to reduce the surgical and infectious
complications.62 More recently, the use of extraperitoneal cesarean section was not found
to reduce major complications when compared with traditional transperitoneal
cesarean section.13 Newton63 found no difference in blood loss, duration of surgery, febrile index, or
postpartum hospital stay among patients with antibiotic-treated intra-amniotic
infection who underwent cesarean section compared with patients
without intra-amniotic infection who underwent cesarean section. The
need to continue antibiotic therapy of intra-amniotic infection
after cesarean section has been questioned.64 The sample size was not sufficient to eliminate a beta error based on
persistent endometritis. Antibiotics should be continued until the patient’s
temperature is less than 37.8°C for 48 hours. Prophylactic Antibiotics in Maternal-Fetal Medicine Prophylactic antibiotics generally are used in one of four situations: prevention
of subacute bacterial endocarditis, prevention of GBS sepsis
in the neonate, prevention of endometritis after a cesarean section, and
prevention of recurrent pyelonephritis. Prophylactic antibiotics
for recurrent pyelonephritis was discussed in the section on urinary tract
infection. PREVENTION OF BACTERIAL ENDOCARDITIS. Bacterial endocarditis is a life-threatening complication of pregnancy. Infection
of the heart valves occurs in the presence of bacteremia and
pre-existing injury to the valves (e.g., rheumatic heart disease, congenitally
abnormal valves, artificial valves). Intravenous drug abusers
seem to be at higher risk because of the frequency of bacteremia and, perhaps, their
compromised immune systems. Women with mitral valve
prolapse constitute a large segment (20% to 30%) of the
population of patients with bacterial endocarditis, but the prevalence
of mitral valve prolapse is common (3% to 6% of young women), and
the risk of bacterial endocarditis is small when prophylaxis
is not used. Nevertheless, these women should have bacterial endocarditis
prophylaxis for most obstetric procedures. The incidence of bacteremia during labor and delivery is understudied and
can only be estimated. The major hindrance to study is timing of the
sample in relationship to labor, delivery of the infant, or delivery
of the placenta. Sugrve and coworkers65 found that bacteremia occurred in 3.5% of normal deliveries and
argued that prophylaxis is not routinely indicated. This opinion is in
the minority, however; most authors believe that the seriousness of
bacterial endocarditis outweighs the low incidence of its occurrence and
that the risks of prophylaxis are minimal. In circumstances in which
the risk of bacteremia is higher (e.g., prolonged rupture of membranes, operative
delivery, manual removal of the placenta), antibiotic prophylaxis
is clearly indicated. The recommended regimen for bacterial
endocarditis prophylaxis is ampicillin, 2 g intravenously, plus gentamicin, 1.5 mg/kg
intravenously, 1 hour before any surgical procedure or
every 8 hours after onset of the active phase of labor. One dose is given 8 hours
after the procedure. For patients allergic to penicillin, vancomycin, 1 g
intravenously every 8 hours, replaces the ampicillin. PREVENTION OF EARLY-ONSET GROUP B STREPTOCOCCAL NEONATAL SEPSIS. Early-onset neonatal sepsis is a rare but dramatic complication in the
first 3 to 7 days of life. Early-onset neonatal sepsis occurs in 3.5 per 1000 live
births (GBS, 1.4 per 1000 live births; non-GBS, 0.6 per 1000 live
births).66 The overall case-fatality rate is 16% and varies by gestational
age (<34 weeks, 30%; 34 to 36 weeks, 10%; = 36 weeks, 2%)67 and organism (GBS, 6.7%; non-GBS, 22.3%).66 Intrapartum antibiotics reduce the incidence of early-onset GBS sepsis
by 80% to 90%. Because 90% of births occur at term
and 84% of early-onset GBS sepsis occurs in the term infant,67 most mothers will have had a relatively normal term pregnancy. There is
tremendous opportunity for confusion, anger, and blame on the part of
patients and their family. Consequently, malpractice litigation is common
in these cases. Given these facts, there is tremendous pressure
to identify and treat prophylactically women who carry GBS in their genital
tract.
Of pregnant women, 10% to 25% are carriers of GBS in their
genital tract. The incidence of positive culture is increased with the
use of modified Todd-Hewitt or Lim growth media and simultaneous anorectal
sampling.68 When serially cultured over
a 1-year period, about half of women have persistent colonization, and
about 25% are episodically and transiently colonized. Risk factors
for a higher prevalence of rectovaginal GBS colonization include black
race, age older than 30, not living with family or partner, less than
9 years of school, current tobacco use, and increasing years of sexual
experience.69 The risk factors for invasive
GBS disease in the neonate are gestational age less than 37 weeks, intrapartum
temperature greater than or equal to 38°C, rupture of membranes
18 hours before delivery, GBS bacteriuria during pregnancy, multiple gestation,
and a previous infant with invasive GBS disease.68,70 Of early-onset GBS and non-GBS sepsis, 49%
and 79% are associated with one or more risk factors.66
Since the early 1990s, the American Trial Lawyers Association, the American
Academy of Pediatrics, the American College of Obstetrics and Gynecology
(ACOG), and the Centers of Disease Control and Prevention (CDC) have proposed
different indications for the use of intrapartum antibiotics based on
antepartum culture results, presence of risk factors for early-onset GBS
sepsis, or both.68,70
In mid-2002, a population-based study that was sponsored by the CDC on
629,912 live births in 1998 and 1999 defined much better the advantages
of a screening culture–based approach compared with a risk identification–based
approach.72 The adjusted relative risk of
early-onset GBS neonatal sepsis was 0.48 (95% CI, 0.37 to 0.63)
in favor of the universal screening–based approach. Given the powerful
results of this study, the following management is recommended: A rectovaginal
screening culture is performed on all pregnant women at 34 to 36 weeks’
gestation using appropriate culture media; prophylactic antibiotics are
recommended for any patient with a positive culture or the presence of
a risk factor for early-onset GBS sepsis (see previous paragraph). If
GBS status is unknown, prophylactic antibiotics are recommended regardless
of risk factor status.70
Identification and therapy of GBS carrier status benefit the mother and
the neonate. Many different studies at different institutions have shown
an association between rectovaginal GBS and infectious morbidity in
the mother. In a study involving centers in Houston, Pittsburgh, and
Seattle, 7806 women were cultured at admission to labor and delivery. Of
women, 22% had positive cultures; 5.2% were heavily
colonized. Heavy GBS colonization was associated with intra-amniotic infection (adjusted
odds ratio [OR], 2.0; 95% CI, 1.1 to 3.7) and
postpartum endometritis (adjusted OR, 1.8; 95% CI, 1.3 to 2.6).73 The universal GBS screening and intrapartum antibiotics (CDC-endorsed
method) reduced the incidence of clinical chorioamnionitis when compared
with the risk factor method (ACOG-endorsed)—5.2% of 4453 deliveries(CDC) versus 7.7% of 7917 deliveries (ACOG). Similarly, universal
screening was associated with lower rates of postpartum
endometritis—2.8% (CDC) versus 4.6% (ACOG).74 There are three potential strategies to treat the GBS carrier state: antepartum, intrapartum, or
neonatal therapy. Because GBS is a part of normal
bowel microflora, oral antibiotic therapy has not been successful
in treating antepartum GBS carrier states. The selective pressure may
increase the likelihood of resistant aerobic gram-negative rods. Neonatal
therapy with 50,000 U of aqueous penicillin within 1 hour of birth
has been shown to reduce early-onset GBS sepsis (incidence in treated
patients, 1.1 per 1000 live births).75 The results need to be interpreted with caution, however, because 10% to 20% of
septic neonates present in the first 4 hours of
birth, and this prophylactic therapy may delay definitive therapy in
these neonates. Septic preterm neonates are more difficult to diagnosis; it
is not clear that early neonatal therapy is efficacious. The standard
of therapy is intrapartum therapy occurring more than 4 hours before
delivery.
The recommended prophylactic treatment is penicillin G (an initial dose
of 5 million U followed by 2.5 million U intravenously every 4 hours until
delivery). The treatment reduces neonatal colonization by 80% to
90%, and the efficacy of antibiotics against early-onset GBS sepsis
is 85% (95 CI, 42% to 98%) after adjustment for intrapartum
fever.71 Ampicillin (2 g intravenously every
6 hours) is equally efficacious, but there a risk of sepsis from ampicillin-resistant
gram-negative aerobic rods (E. coli).76,77,78 Penicillin-allergic
mothers should receive clindamycin (900 mg intravenously every 8 hours
until delivery) or erythromycin (500 mg intravenously every 6 hours until
delivery). There is an increasing resistance (15% to 20%)
of GBS to macrolide antibiotics (clindamycin or erythromycin). Consideration
should be given to cefazolin, 2 g intravenously every 6 hours, in cases
in which the reported allergy to penicillin does not include respiratory
distress or immediate urticaria. About 25% of mothers with neonates
who had early-onset GBS sepsis received intrapartum antibiotics (antibiotic
failures).
About 25% of laboring women receive intrapartum antibiotics regardless
of the screening method used. The management of an asymptomatic
term infant whose asymptomatic mother received intrapartum antibiotics
is not clear. Of these neonates, 70% receive a workup or antibiotic
treatment.60 The expense and risk of managing more than 0.5 million neonates per year
in the United States in this fashion is a potential liability. The other hidden risk is that of selection-resistant bacteria caused by
antibiotic usage for GBS prophylaxis. McDuffie and coworkers78 reported a series of four cases of adverse perinatal outcome caused by
resistant Enterobacteriaceae after ampicillin or amoxicillin usage for
preterm premature rupture of membranes or GBS carriage. In very-low-birth-weight
neonates, E. coli is the most common pathogen associated with early-onset sepsis; 85% were
resistant to ampicillin, and it was associated with a higher
death rate (41%).76 These are early observations, and more research is needed, but it is prudent
to use intrapartum penicillin rather than ampicillin for GBS prophylaxis.79 PREVENTION OF POSTCESAREAN ENDOMETRITIS. Multiple studies and reviews have established that prophylactic antibiotics
reduce the incidence of postcesarean endometritis. The reported incidence
of postoperative infection in patients after labor or prolonged
rupture of membranes is 30% to 85%; the incidence in
patients who undergo elective cesarean section without prophylactic antibiotics
is less than 10%.80 Prophylactic antibiotics reduce the incidence of postcesarean endometritis
by 40% to 60%. Table 16 summarizes the results of a review by Swartz and Grolle in 198181 concerning comparative trials of prophylactic antibiotics versus no prophylactic
antibiotics for cesarean section. Prophylactic antibiotics
reduced the risk of endometritis, wound infection, and urinary tract infection. TABLE 16. Effect of Prophylactic Antibiotics on Puerperal Infection After
Cesarean Section
Infection | Control | Prophylactic Antibiotics |
Endometritis | 406/1188 (34%) | 178/1509 (12%)* |
Wound infection | 87/888 (10%) | 32/1115 (3%)* |
Urinary tract infection | 107/709 (14%) | 69/1025 (6%)* |
*p < 0.001 versus controls.
Adapted from Swartz WH, Grolle K: The use of prophylactic antibiotics in
cesarean section. J Reprod Med 26:595, 1981.
Since the 1980s, the focus has been on patient selection, timing of the
first dose, single versus multiple doses, narrow-spectrum versus broad-spectrum
antibiotics, and the development of resistant bacteria after
prophylaxis. Harger and English,82 in a randomized comparison of cefoxitin with placebo prophylaxis in 386 women, identified
maternal age less than 21 years, lower socioeconomic
status, gestational age less than 38 weeks, use of an intrauterine
pressure catheter plus fetal scalp electrode, duration of internal monitoring
greater than 9 hours, and obesity as predictors of postcesarean
endometritis. Cefoxitin reduced the incidence of endometritis in the
presence of one or more risk factors but not in the absence of risk factors (2 of 61 versus 5 of 61; p = not significant). A meta-analysis of the efficacy of prophylactic
antibiotics for nonlaboring patients undergoing cesarean delivery
with intact membranes showed significant reductions in postoperative
fever, endometritis, and wound infection.23 In an animal model83 and in general surgery,84 the use of prophylactic antibiotics concomitantly with skin incision is
associated with a lower rate of endometritis than if the antibiotics
are delayed until after the procedure. Delay in cefazolin prophylaxis
at cesarean section until after the cord clamping was not associated
with a higher rate of endometritis, however.85 The use of antibiotics before cord clamping was associated with more neonatal
intervention and cost.85 As a result of the latter retrospective study,85 it is recommended that prophylactic antibiotics be given after cord clamping. The available literature suggests that a single dose of prophylactic antibiotics
is as effective as multiple doses. At least four studies with
randomized assignment of patients showed equal efficacy between single-dose
and multidose schemes.86 At least three studies with random assignment of subjects to first-generation
agents versus extended-spectrum cephalosporins (cefazolin versus
ampicillin versus cefotaxime, cefazolin versus cefoxitin, cefazolin
versus moxalactam) showed no differences in the incidence of endometritis (2.5% to 7.7%). In 1990, Faro and colleagues87 reported on a large study (N = 1568) with randomized assignment
of subjects to 1 of 10 prophylactic regimens. The rate of endometritis
in each group (n = 142 to n = 217) was as follows: 1 g cefazolin for three doses, 22.5%
1 g cefazolin, 20.3%
2 g cefazolin, 10.6%
1 g cefoxitin, 15.5%
2 g cefoxitin, 16.7%
1 g cefotetan, 6.1%
1 g ceftizoxime, 17.9%
1 g cefonicid, 15.1%
2 g ampicillin, 12.8%
4 g piperacillin, 8.4%
Faro’s study87 showed the following: (1) A single dose of 2 g of ampicillin, 2 g of cefazolin, 1 g
of cefotetan, or 4 g of piperacillin was superior to three
doses of 1 g of cefazolin. (2) Extended-spectrum antibiotics (cefoxitin, cefotetan, ceftizoxime, cefonicid, piperacillin) do not offer a
clear advantage over first-generation cephalosporins (cefazolin) or penicillin (ampicillin). (3) Cephalosporin prophylaxis was associated with
an increase in Enterococcus faecalis colonization of the vagina. Prophylactic antibiotics fail to prevent approximately 20% to 30% of
cases of postcesarean endometritis. In a classic study, Gonik
and associates88 showed what many experts have thought to be true: Postcesarean endometritis
is the postpartum clinical manifestation of subclinical intra-amniotic
infection. Bacteria were shown within the decidua and myometrium
of asymptomatic women at the time of cesarean section and in women in
whom endometritis subsequently developed. These observations supported
the clinical observation that clinical characteristics can predict
the development of postcesarean endometritis or failure of prophylactic
antibiotics. In the large study by Faro and coworkers,87 in which all 1568 women received prophylactic antibiotics, rupture of
membranes lasting more than 4 hours and duration of internal monitoring
predicted failure of prophylactic antibiotics. In a retrospective review
of 766 women who received either ampicillin or cefazolin prophylaxis
for cesarean sections, Chang and Newton89 identified the following predictors of antibiotic prophylactic failure: multiparity (OR, 1.77; 95% CI, 1.26 to 2.48), six or more vaginal
examinations (OR, 3.39; 95% CI, 2.17 to 5.28), gestational
age less than 37 weeks (OR, 1.55; 95% CI, 1.04 to 2.33), and cefazolin
prophylaxis (OR, 1.69; 95% CI, 1.21 to 2.36). The latter
study suggests that in certain clinical situations, prophylactic antibiotics
with a broader coverage and longer duration may reduce postoperative
morbidity. The number of vaginal examinations during labor is
a distinct, objective clinical measure of risk and might be used to select
patients for a more intensive prophylactic antibiotic regimen. A major concern of prophylactic antibiotics has been the changes in genital
tract flora. Four published studies have examined these changes. In 1981, Gibbs
and colleagues90 compared the endometrial flora of 100 patients who were randomly assigned
to receive 2 g of cefamandole intravenously every 4 hours for three
doses or placebo prophylaxis. Cefamandole was associated with significant
increases in aerobic gram-negative rods and isolation of enterococci
from the endometrium postpartum when compared with preantibiotic
amniotic fluid cultures. In 1984, Stiver and associates91 compared the aerobic and anaerobic cervical microflora before and 4 days
after cesarean sections in women randomly assigned to receive one dose
of 1 g of cefazolin, 2 g of cefoxitin, or normal saline for prophylaxis. The
three-dose cephalosporin regimens resulted in statistically
significant increased isolation of Enterococcus from the cervix but no increase in nosocomial infection. In 1990, Faro
and coworkers87 reported changes in vaginal isolates after treatment with prophylactic
antibiotics for cesarean section. A total of 1568 patients were randomized
to one of seven antibiotic regimens. Cephalosporin prophylaxis was
associated with increases in the isolation of vaginal E. faecalis. In 1998, Newton and Wallace9 reported the differences in endometrial microflora in patients with postcesarean
endometritis whether they had received ampicillin, cefazolin, or
no prophylaxis. Patients who had received ampicillin prophylaxis
were more likely to have Klebsiella pneumoniae, E. coli, or any gram-negative rod. Ampicillin prophylaxis was associated with a
decrease in Prevotella bivia or any anaerobe. Women who received cefazolin were more likely to have Enterococcus and less likely to have Proteus. There were no differences between cures; however, cefazolin prophylaxis
followed by treatment with an extended-spectrum cephalosporin was associated
with an increase in wound infection and dehiscence.9 The use of prophylactic antibiotics is recommended for most cesarean sections, especially
for any patient who has a cesarean section with rupture
of membranes. The efficacy of prophylactic antibiotics in women without
rupture of membranes and labor, who are undergoing elective cesarean
section, is not clear. Most authors do not recommend their use. When
prophylactic antibiotics are used, a single dose of 2 g of ampicillin
intravenously (for penicillin-allergic patients, 2 g of cefazolin
or 900 mg of clindamycin intravenously) immediately after cord clamping
is the most cost-effective choice. In high-risk patients having more
than five vaginal examinations in the active phase, an extended-spectrum
prophylactic antibiotic should be given for 24 hours: 2 g of cefotetan
intravenously every 12 hours for two doses or 2 g of ampicillin
plus 1 g of sulbactam intravenously every 6 hours for four doses. Postpartum Endomyometritis
Infection is the most common complication of cesarean section or vaginal
delivery (see Table 1). Some type of infection occurs
in 15% to 20% of postcesarean patients; endometritis is
the most common (15%). Approximately 10% to 20% of
endometritis cases involve severe complications, including septicemia,
pelvic cellulitis, septic pelvic thrombophlebitis, abscess formation,
and pneumonia. The most important single factor in predicting a puerperal
infection is the presence of a uterine wound (OR, 12.84).10
The relative risk of endometritis is increased with internal monitoring,
multiple vaginal examinations, rupture of membranes, and labor. Bacterial
vaginosis organisms in the vagina and amniotic fluid predict a higher
incidence of endometritis.10,11,92
Puerperal infection results from a multiorganism invasion of the endometrium
by vaginal flora (see Table 2). Endometrial cultures show 70% of the isolates to be aerobic. Aerobic
gram-negative rods (E. coli), GBS, and group D enterococci predominate. Of the isolates, 80% have
anaerobic organisms as well (Prevotella, Bacteroides, and anaerobic Streptococcus). Chlamydia and Mycoplasma are present in 5% and 15% of cultures. C. trachomatis is associated with 25% of late-occurring endometritis cases after
a vaginal delivery.93 This observation has not been verified by the examination of post–cesarean
section patients.94 The acute morbidity of cesarean section may mask the low-grade symptoms
of the infection; infertility may result. The diagnosis of a postoperative puerperal infection can be difficult. Normal
postoperative pain and postpartum contractions obscure the discomfort
from mild infection. The clinical markers are fever, uterine tenderness, foul-smelling
lochia, uterine subinvolution, and a persistent
paralytic ileus. Standard febrile morbidity is the occurrence of two
temperature elevations greater than 38°C at least 6 hours apart, taken
by standard technique at least four times daily and occurring
between the 2nd and 10th postpartum days. Despite the definition, fever
in the first 24 hours should not be ignored, and the source should
be sought by complete physical examination and appropriate testing. Possible
causes for early fever include pulmonary atelectasis, mild transient
bacteremia, febrile response to the transfusion of foreign proteins
by placental separation and delivery, and retained products of conception. Pregnancy
and delivery may be coincident with various infectious
diseases. Incentive spirometry and increased activity facilitate pulmonary
recovery. Antipyretics should not be used because they can mask
a more serious infection. A high fever in the first 24 hours (≥38.5°C) is
highly predictive (93%) of subsequent clinical
infection.95 Patients with this sign should have a complete fever workup and appropriate
therapy. Occasionally, early high fever can be associated with extremely
virulent organisms, such as group A streptococci and clostridia. Other clinical signs are useful. A physical examination is important in
localizing an infection, and a thorough pelvic examination is essential. A
baseline pelvic examination records the progress in the management
of the infection. Antibiotic therapy should not be started without
a complete examination. The initial laboratory evaluation is crucial in the diagnosis and management
of postoperative infection. Laboratory data should include a complete
blood count, differential count, serum blood urea nitrogen (BUN), and
serum creatinine determinations. A 25% to 99% increase
in the postoperative white blood cell count from the admission labor
specimen and a greater than 100% increase are associated with
a 70% and a 580% increase in postcesarean endometritis, respectively.96 A high BUN or creatinine changes the dosing frequency of aminoglycosides. A
catheter-obtained urinalysis and urine culture should be obtained
to identify coexisting urinary tract infection. The patient should have
a complete set of cultures, including urine and an endocervical culture. Two
sets of blood cultures from two different sites is recommended
when the fever at diagnosis is greater than 38.8°C; 21.4% have
a positive culture; with a temperature less than 38.8°C, only 0.8% have
a positive culture.92 A Gram stain of the lochia should be obtained in patients with a high
fever (>40°C) within the first 24 hours. Sheets of gram-positive
cocci or gram-positive rods with fragments of muscle are of great
concern and point to streptococcal or clostridial infection. The
delivery record is reviewed for the completeness of the placenta at removal. Retained
products of conception need to be removed if identified. The results of the cultures can alter significantly the cost and management
of postoperative infection. The sensitivities of the organisms within
the particular hospital population help to limit the use of potentially
toxic antibiotics (aminoglycosides). The identification of S. aureus should lead to a prolonged course of antibiotics to prevent metastatic
infection. The isolation of aerobic group A streptococci may indicate
a need for high-dose penicillin therapy and isolation of the patient. The
isolation of enterococci indicates a change in antibiotics, especially
if the primary drug is a cephalosporin. A longer course of therapy
is warranted in enterococcal septicemia. Because puerperal infection is polymicrobial, broad-spectrum empirical
antibiotic therapy with good anaerobic coverage is the cornerstone of
treatment (Table 17). Early studies that lacked adequate anaerobic coverage were associated
with lower cure rates (74%) (Fig. 2).98 The combination of clindamycin, 900 mg, and gentamicin, 1.5 mg/kg intravenously
every 8 hours, has been considered the therapeutic standard
for comparison. Gentamicin, 4 to 5 mg/kg every 24 hours, and clindamycin, 1200 mg
every 12 hours, have been shown to be equally effective as
the three-dose-daily regimens.99 The weighted average cure rate in post–cesarean section endometritis
in 16 studies is 89% (see Fig. 2). This antibiotic combination has some disadvantages, however. First, early
infections (<48 hours) often are associated with gram-positive
organisms (streptococci). In these cases, penicillin, 4 million
U every 4 hours, should be added to the regimen. Second, both drugs have
potentially serious side effects. Diarrhea (6% to 8%) can
occur with clindamycin. Aminoglycoside therapy has the potential
for nephrotoxicity or ototoxicity. Therapeutic aminoglycoside levels may
be difficult to obtain in obstetric patients using a standard dosing
regimen. In patients with poor response, obesity, or renal disease, it
is recommended that aminoglycoside levels be monitored. Third, cost
concerns are increasingly important. Many drugs, frequent doses, and
concurrent laboratory testing make this combination less appealing financially. The
use of the reduced dosing frequency gentamicin/clindamycin
regimen halves the antibiotic costs.99 TABLE 17. Antimicrobial Regimens for Treatment of Postpartum Endometritis
First Choice - Piperacillin plus tazobactam
- Cefotaxime
- Cefotetan
- Cefmetazole
Penicillin Allergy- Cefotetan
- Cefmetazole
- Clindamycin plus gentamicin
Complicated Postpartum Endometritis (10% of cases)- Ampicillin plus gentamicin plus clindamycin
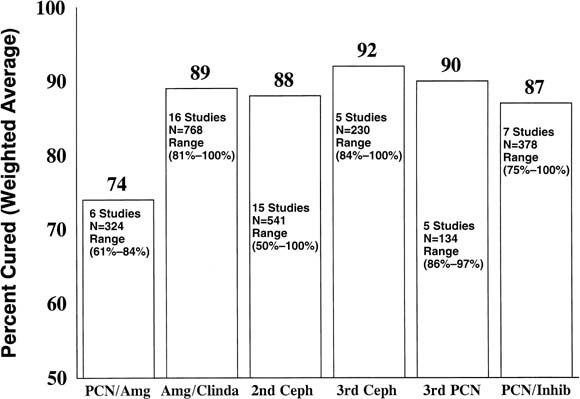 Fig. 2. The weighted average cure rates for various classes of antibiotics used
in the treatment of postpartum endometritis. The classes include penicillin
plus aminoglycoside (PCN/Amg), aminoglycoside plus clindamycin (Amg/Clinda), second-generation cephalosporins (2nd Ceph), third-generation
cephalosporins (3rd Ceph), extended-spectrum penicillins (3rd PCN), and
penicillin plus β-lactamase inhibitor (PCN/Inhib). Fig. 2. The weighted average cure rates for various classes of antibiotics used
in the treatment of postpartum endometritis. The classes include penicillin
plus aminoglycoside (PCN/Amg), aminoglycoside plus clindamycin (Amg/Clinda), second-generation cephalosporins (2nd Ceph), third-generation
cephalosporins (3rd Ceph), extended-spectrum penicillins (3rd PCN), and
penicillin plus β-lactamase inhibitor (PCN/Inhib).
|
Some of the newer synthetic penicillins and cephalosporins have a place
in the treatment of endometritis (see Table 17). Many second-generation and third-generation cephalosporins, extended-spectrum
penicillins, and penicillins plus β-lactamase inhibitors
have favorable activity against genital tract flora. Single-drug therapy, infrequent
dosing (cefotetan), and a wide margin of safety are
attractive features. The weighted average cure rates with these drugs
approach 88% to 92% (see Fig. 2), but the research on them is incomplete. The many studies involve a relatively
few patients, and conclusions may be biased by patient selection (postvaginal
versus postcesarean endometritis), study methodology (definitions
of failure), and dosage definition.
The management of endometritis also includes serial examinations to evaluate
response. The temperature curve is a crucial monitor. The patient should
have a clear response within 48 hours after initiation of therapy and
should be afebrile by the third or fourth day. The breasts, wound, intravenous
sites, and uterus should be examined daily. Uterine tenderness also improves
over a 48- to 72-hour period. A one-time dose of antipyretics should be
used only to treat symptoms. Fever per se has an important antimicrobial
function. Intravenous antibiotics should be continued until the patient
has been afebrile (37.6°C) for 24 to 48 hours. Short-course antibiotic
therapy (3 to 5 days) has been shown to be as efficacious as longer therapy
(7 to 10 days).100,101
Oral antibiotics are not needed if intravenous therapy has been adequate.102
In cases in which bacteremia has occurred, a short course (5 days) of
oral antibiotics may be used, although there is no scientific support
for this management.
Antibiotic failure should be considered after 48 to 72 hours of persistent
high fever (>38.5°C). The causes of antibiotic failure
include (1) retained products of conception; (2) wound infection; (3) pelvic
hematoma, “phlegmon, or abscess; (4) resistant organisms; (5) septic
thrombophlebitis; (6) inadequate drug dose or improper
route of administration; (7) nongenital infection; and (8) a noninfectious
source of fever, such as a drug reaction. The patient should be
examined thoroughly to rule these diagnoses in or out. The workup includes
the following: - Thorough physical examination, including a pelvic examination
- Check of cultures and sensitivity
- Review of dose, route, and levels of antibiotics
- A pelvic ultrasonogram or a computed tomography scan, which can be helpful
in the diagnosis of abscess, hematoma, or retained products of conception
The management of failed antibiotic therapy should consist of triple therapy—an
aminoglycoside, clindamycin, and penicillin. This regimen
may include adding penicillin to the combination of clindamycin and
aminoglycoside or discontinuing a cephalosporin and starting triple
therapy. In patients with a fever, in whom other signs or symptoms are
minimal, a drug fever can be suspected. The presence of eosinophilia
can be helpful in making a diagnosis. In this case, the drugs may be stopped
and the patient observed for 48 hours. If fever recurs, a complete
set of cultures should be obtained and triple therapy initiated. Surgical
intervention is indicated in patients with abscess, myonecrosis, or
septic pelvic thrombophlebitis who are not responding to heparin
and antibiotics. If a triple-therapy regimen has not improved the clinical picture within 24 to 48 hours, septic
thrombophlebitis must be suspected. Intravenous
heparin is started in conjunction with antibiotic therapy. The partial
thromboplastin time should be maintained at 1.5 to 2 times the control. Response
should occur within 48 hours, and treatment should be continued
for 10 to 14 days. Wound infection accounts for 30% to 50% of failed initial
therapy and occurs in 2% to 8% of cesarean sections. Typically, patients
with wound infection present with low-grade fevers (37.8°C
to 38.6°C) and local signs of infection at the wound: erythema, tenderness, induration, or purulent discharge. Uniformly
there is poor healing of the skin and underlying tissue edges; wound
separation and drainage are common. If the infection extends through
the fascia and muscle layer, a more serious deep surgical wound infection
is present. The differential diagnosis is a wound hematoma, seroma, or
infection. A wound hematoma usually occurs in the first 24 to 48 hours, and
although wound pain, tenderness, and erythema are common, pressure
pain and bleeding from the incision allow differentiation from
wound infection. A seroma is most often a wound abscess at a late stage. In
most cases, culture of the fluid is positive, and it should be
treated as a mild wound infection. The treatment of choice is wide opening of the wound and drainage. Subsequent
care includes daily débridement and dressing changes at
least three times daily. Antibiotics should be used only when there are
systemic symptoms (e.g., fever) or cellulitis. Wound cultures reveal S. aureus in 25% to 30% of cases, S. epidermidis in 30% to 40%, Enterococcus in 50%, aerobic gram-negative rods in 25% to 30%, and
anaerobes in 50%. Usually a modified penicillin (e.g., nafcillin) provides
adequate coverage. Occasionally a rapidly advancing cellulitis
requires triple antibiotics and aggressive débridement. Mastitis Mastitis is an infectious process of the breast characterized by high fever (39°C
to 40°C), localized erythema, tenderness, induration, and
heat. Often these signs are associated with nausea, vomiting, malaise, and
other flulike symptoms. Mastitis occurs most frequently
in the first 8 weeks postpartum and at times of marked reduction in
frequency of breastfeeding. Risk factors include maternal fatigue, poor
breastfeeding technique, nipple trauma, and epidemic S. aureus. The most common organisms associated with mastitis are S. aureus, S. epidermidis, streptococci, and occasionally gram-negative rods. The incidence of sporadic
mastitis is 5% to 10% in lactating mothers and less
than 1% in nonlactating mothers. During epidemics involving S. aureus, mastitis may develop in 10% to 20% of lactating mothers.
Until more recently, the management of mastitis was directed by retrospective
clinical reviews of clinical experience. In most cases, management consisted
of bed rest, continued lactation, and antibiotics, with an 80%
to 90% cure rate, a 10% abscess rate, a 10% recurrence
rate, and a 50% rate of breastfeeding cessation. Starting in 1982,
Thomsen and colleagues103,104,105,106
published four important articles concerning pathophysiology, diagnosis,
and treatment of mastitis. They showed that the diagnosis and prognosis
of inflammatory symptoms of the breast are established best by counts
of leukocytes and bacteria in breast milk, which was obtained after careful
washing of the mother’s hands and breasts with a mild soap. The
milk was expressed manually, and the first 3 mL was discarded. When the
leukocyte count was greater than 106/μL and the bacterial
count less than 103/μL, the diagnosis was noninfectious
inflammation of the breast. With no treatment, the inflammatory symptoms
lasted 7 days; mastitis developed in 50%, and of patients with
noninfectious inflammation of the breast, only 21% returned to
normal lactation. In patients with noninfectious inflammation of the breast,
when the breast was emptied frequently by continued lactation, the symptoms
lasted 3 days, and 96% returned to normal lactation. When the leukocyte
count in the breast milk was greater than 106/μL and
bacterial count greater than 103/μL, the diagnosis was
mastitis. Delay in therapy resulted in abscess formation in 11%,
and only 15% returned to normal lactation. Frequent emptying of
the infected breast by continued breastfeeding eliminated abscess formation,
but only 51% returned to normal lactation. Additional antibiotic
therapy increased the return to normal lactation in 97%, with resolution
of symptoms in 2.1 days.
The management of mastitis includes the following: (1) bed rest; (2) breast
support; (3) fluids; (4) assessment of breastfeeding technique; (5) breastfeeding
initiated on the uninfected side first to establish letdown; (6) the
infected side emptied by breastfeeding with each feeding (occasionally
a breast pump helps to ensure complete drainage); and (7) dicloxacillin, 500 mg
every 6 hours for 10 days. Erythromycin may
be used in patients allergic to penicillin. It is important to continue
antibiotics for a full 10 days because abscess formation is more likely
with shorter courses. Hand washing by the mother before each feeding
and by the hospital nurses reduces nosocomial infection rates. Rooming-in
does not reduce the acquisition of hospital strains of S. aureus or infection rates. During epidemics, early discharge may reduce infection
rates. Candidal infection of the nipple is a painful complication
of antibiotic therapy. Therapy consists of rubbing a small amount of
antifungal cream into the nipple after each feed. Breast abscesses are usually the result of lactational failure and delayed
or inadequate therapy.107 The signs include a high fever (39°C to 40°C) and a localized
area of erythema, tenderness, and induration. In the center, a fluctuant
area may be difficult to palpate. The patient feels sick. Abscesses
usually occur in the upper outer quadrants, and S. aureus usually is cultured from the abscess cavity. The management of breast abscess is similar to that for mastitis except
that (1) drainage is indicated, and (2) breastfeeding should be limited
to the uninvolved side during the initial therapy. The infected breast
should be pumped mechanically every 2 hours and with every letdown. Serial
ultrasound-guided aspiration of the abscess is the first choice
for drainage; it is least disruptive to the breastfeeding experience. If
a surgical approach is used, the skin incision should be made over
the fluctuant area in a manner parallel to and as far as possible from
the areolar edge. Although the skin incision follows skin lines, the
deeper extension should be made bluntly in a radial direction. Sharp
dissection perpendicular to the lactational ducts increases blood loss, the
risk of a fistula, and the risk of ductal occlusion. When the
abscess cavity is entered, all loculations are bluntly reduced, and the
cavity is irrigated with saline. American surgeons pack the wound open
for drainage and secondary closure. British surgeons advocate removal
of the abscess wall and primary closure.108 In either case, wide closure sutures should be avoided because they can
compromise the ducts. Patients have a protracted recovery of 18 to 32 days, and
abscess formation recurs in 9% to 15% of cases. Breastfeeding
from the involved side may be resumed if skin erythema
and underlying cellulitis have resolved, which may occur in 4 to 7 days. Adjunctive Antibiotic Therapy to Prevent Preterm Birth
Genital tract infection plays an indirect or direct role in a significant
proportion of preterm births (20% to 50%).109,110 Ample experimental data have shown that
host response through phospholipase A2 or other enzymes or
genital tract bacteria through endotoxins activate prostaglandin synthesis
(preterm labor), lysolecithin (rupture of membranes), and collagenases
(incompetent cervix). A consistent association has been established between
preterm birth and bacterial vaginosis, C. trachomatis, N. gonorrhoeae,
and T. vaginalis. Epidemiologic studies show that women with genital
tract infections in the nonpregnant state are more likely to have preterm
births. Extragenital infections, such as pyelonephritis, bacteriuria,
and appendicitis, can be complicated by preterm labor and birth. Histologic
chorioamnionitis has been associated consistently with preterm birth and
LBW neonates. Histologic chorioamnionitis is identified in 25%
to 75% of placentas from preterm gestations and in 5% to
15% of placentas from term gestations.
Given the strong evidence for infection as a powerful covariable in the
cause of preterm birth, it seems reasonable to use antibiotics to prevent
preterm birth. The trials to date have had three designs: (1) antibiotic
trials conducted in nonlaboring patients at high risk for preterm delivery;
(2) antibiotic trials among women with preterm labor and intact membranes;
and (3) antibiotic trials among women with preterm premature rupture of
membranes but without preterm labor. The antibiotic trials conducted in
nonlaboring patients at high risk for preterm delivery consist of trials
in which patients with a “pathogenic” organism are assigned
randomly to placebo or the appropriate antibiotic. The results have been
mixed.111,112,113,114,115
McGregor and associates115 reported the
results of a prospective, controlled trial of two different management
regimens in two consecutive time periods. The study compared the effect
of antepartum identification and treatment of N. gonorrhoeae, C. trachomatis,
T. vaginalis, or bacterial vaginosis on the incidence of preterm birth.
All patients were screened for organisms at the initial visit, at 22 to
28 weeks, and after 32 weeks. In the control phase, antepartum patients
(n = 559) with N. gonorrhoeae, C. trachomatis, or symptomatic
vaginitis were treated with the appropriate antibiotic. In the test period,
patients who tested positive for a pathogen (n = 579) were treated
whether symptomatic or not. The latter approach resulted in fewer preterm
births (OR, 0.66; 95% CI, 0.04 to 1.0). Two large trials116,117 in high-risk, nonlaboring women with bacterial
vaginosis in one trial116 (metronidazole
versus placebo, n = 1953) and in women with trichomonas in another
study117 (metronidazole versus placebo,
n = 615) showed no reduction in preterm birth or adverse neonatal
outcomes after antibiotic therapy. The issues related to vaginal colonization
and preterm birth are more complex than the simple precepts presumed by
empirical antibiotic therapy. At this time, antibiotic therapy of nonlaboring
women with genital pathogens should be directed at the resolution of symptoms,
public health issues (STD), and prevention of perioperative infection
(i.e., cerclage).
Antibiotic trials that have used empirical antibiotic therapy as adjunctive
therapy in the treatment of preterm labor have not shown benefit.118,119,120,121
A large multicenter European study found a similar lack of benefit with
adjunctive antibiotic therapy.122 A systematic
review of the literature123 supports the
latter findings. Except for GBS prophylaxis, antibiotics are not recommended
in the management of preterm labor with intact membranes.
Rupture of membranes before 37 weeks’ gestation (preterm premature
rupture of membranes) occurs in 1% to 2% of pregnancies
and is associated with 30% to 40% of preterm births. The
central management issues are the increased rate of preterm labor, intra-amniotic
infection, and cord accidents. In untreated populations, 70% at
less than 30 weeks’ gestation and 85% at 30 to 36 weeks’ gestation have delivered by 1 week after premature
rupture of membranes. The rate of intra-amniotic infection appears
to be much higher with earlier gestational age: 40% at or earlier
than 26 weeks, 15% at 26 to 36 weeks, and 4% to 10% at
term.54 A greater proportion of the preterm births at early gestational age are
related to genital tract infections that predated premature rupture
of membranes.
It is reasonable to argue that antibiotic therapy would reduce the incidence
of infection and prolong the time from membrane rupture to delivery. A
variety of antibiotics have been tried, including ampicillin, ceftizoxime,
mezlocillin, erythromycin, and ampicillin-gentamicin-clindamycin. Numerous
reviews124,125,126
and a large trial127 (erythromycin/amoxacillin
versus placebo, n = 4826) show benefit in adjunctive antibiotic
therapy in women with preterm premature rupture of membranes. The large
study127 showed prolonged gestation and
an improvement in neonatal outcomes after erythromycin alone and erythromycin
plus amoxicillin therapies. The use of amoxicillin is recommended with
caution because necrotizing enterocolitis occurred more often in exposed
neonates. Although the incidence of intra-amniotic infection was not reduced,
the incidence of postpartum endometritis was reduced.122,126 Antibiotics are recommended as adjunctive
therapy in preterm premature rupture of membranes. The type, dose, and
duration of the antibiotic therapy are not established, however.
Similar to the adjunctive antibiotic therapy in idiopathic preterm labor, the
routine use of ampicillin prophylaxis for unknown GBS status obscures
the investigation and understanding of the benefits of adjunctive
antibiotic therapy in preterm premature rupture of membranes. Two major
issues that remain unanswered are the development of antibiotic resistance76 and the association between intrauterine infection and cerebral palsy.54 Until the needed research is conducted, a conservative approach is recommended. GBS
prophylaxis with penicillin or erythromycin is recommended
for 72 hours. The results of antibiotic treatment to prevent preterm birth have been
discouraging. Empirical antibiotics should not be given as adjunctive
therapy for preterm labor or preterm premature rupture of membranes beyond
penicillin prophylaxis for unknown GBS status. Prenatal screening
and organism-specific therapy probably are warranted, especially with N. gonorrhoeae, C. trachomatis, and T. vaginalis. When bacterial vaginosis is present in symptomatic patients, patients
at high risk for preterm birth, or patients who are undergoing vaginal
surgery, there is empirical support for antibiotic therapy. Whether
to treat asymptomatic, low-risk pregnant patients with bacterial vaginosis
by either clinical or Gram stain examination remains unresolved. Sexually Transmitted Diseases in Pregnancy The identification and treatment of STDs in pregnancy has important maternal
and fetal implications. The rate of STDs during pregnancy is as
follows: syphilis, 4 to 20 in 1000; gonorrhea, 0.5% to 4%; chlamydia, 3% to 15%; trichomonas, 0.5% to 8%; human
papillomavirus, 10% to 60%; HIV, 0.2 to 40 in 1000 deliveries; and
herpes simplex virus type 2, 10% to 30%. The
public health concerns for venereal transmission should
initiate identification of partners and education of patients and their
partners concerning disease prevention, identification, consequences, and
appropriate treatment. The presence of one STD often (10% to 40%) indicates
the presence of other STDs and other risky behavior (e.g., substance
abuse) that may affect the fetus adversely. Even
after control of other risks, syphilis, gonorrhea, chlamydia, and
trichomonas seem to pose an independent risk for adverse pregnancy outcomes, including
fetal growth restriction, perinatal mortality, and preterm
birth.
The treatment of STDs in pregnant women (Table 18)
is similar to treatment in nonpregnant women with several notable exceptions.128,129 Erythromycin is poorly transferred through
the placenta to the fetus and is inadequate treatment of syphilis in the
penicillin-allergic patient during pregnancy. In these patients, the treatment
of choice is still penicillin, and the patient requires desensitization
to avoid a fatal anaphylactic reaction.130
TABLE 18. Specific Regimens for Treatment of Sexually Transmitted Diseases
in Pregnancy
Syphilis Benzathine penicillin G 2.4 million u IM Primary: 1 dose
Early latent: 3 doses at weekly intervals
Late latent: 3 doses at weekly intervals
Unknown: 3 doses at weekly intervals
HIV-positive, negative CSF: 3 doses at weekly intervals
Penicillin allergy: 3 doses after desensitization
Aqueous crystalline penicillin G 4 million u q 4 h for 14 days Neurosyphilis
Evidence of fetal infection
Secondary syphilis
Gonorrhea Endocervicitis Ceftriaxone 125 mg IM plus azithromycin 1 g PO
Disseminated Ceftriaxone 1 g IV q 8 h until improvement, then cefixime 400 mg PO bid
Chlamydia Azithromycin 1 g PO Erythromycin base 330 mg PO qid × 14 days
Trichomonas First trimester: Metronidazole gel 0.75%, 5 g intravaginally 2 times daily × 5 days
followed by metronidazole 2 g PO 1 dose after 13 weeks
After first trimester: Metronidazole 2 g PO 1 dose
Human papillomavirus Podophyllin and 5-fluorouracil are contraindicated in pregnancy
Trichloroacetic acid 80–90%; apply to warts only, and repeat
weekly
Cryotherapy or electrocautery
Scabies Permethrin cream (5%), applied to body areas (neck down); wash off
in 8 h
Crab louse (Pediculosis pubis) Permethrin cream rinse (1%); wash off in 10 minutes. Re-treat in 7 days. Wash
clothing, bed linens on hot cycle. Treat family members, close
contacts, and sexual partners at same time
Chancroid Ceftriaxone 250 mg IM, 1 dose
Herpes simplex virus Primary Famciclovir 250 mg PO tid × 7–10 days
Valacyclovir 1 gm PO bid × 7–10 days
Acyclovir 400 mg PO 5 times daily × 7–10 days
Recurrent Famciclovir 125 mg PO bid × 5 days
Valacyclovir 500 mg PO bid × 5 days
Acyclovir 200 mg bid PO × 5 days
Frequent recurrent episodes (6/year), third trimester: Famciclovir 250 mg bid
Valacyclovir 500 mg bid
Acyclovir 200 mg PO bid
IM—intramuscularly; PO—by mouth.
SYPHILIS. The second major difference concerns the treatment of syphilis during pregnancy
and the potential for fetal infection. Among patients with symptomatic
or early latent syphilis, the risk of fetal infection is 50% or
greater. Syphilis may result in overt fetal infection, death, or
premature delivery. The fetus is in a relatively “protected” space, and
the infected fetus may be difficult to treat with
standard penicillin doses for nonpregnant patients (CDC, benzathine
penicillin G, 2.4 million units intramuscularly × 1 dose). If the
fetus shows signs of infection or the incidence of spirochetemia is
high (secondary syphilis), intravenous penicillin is the best choice. CHLAMYDIA. C. trachomatis is the most common bacterial STD, and acute infections (IgM positive) have
been associated with preterm delivery and perinatal death. Tetracycline (doxycycline) is
the treatment of choice in nonpregnant women; however, tetracyclines
have been associated with hepatotoxicity in the
mother and bone dysplasia and staining of the decidual teeth in the fetus. Tetracyclines
are relatively contraindicated in pregnancy. Macrolides, such
as erythromycin, azithromycin, and clindamycin, are effective
against Chlamydia and are the therapy of choice in pregnancy. Azithromycin (1 g orally) is
better tolerated than erythromycin and is less toxic than clindamycin. Ampicillin
and amoxicillin seem to be as efficacious as erythromycin
in studies with random assignment of pregnant subjects.131 The failure to comply with 7 days of oral erythromycin therapy explains
a large portion of the success of ampicillin. HERPES SIMPLEX VIRUS. Herpes simplex virus poses a special risk to the fetus. In the presence
of primary disease with multiple vesicles (high inoculum), intrapartum
fetal exposure can result in neonatal infection rates of 30% to 50% with
subsequent high neonatal mortality (30% to 50%) and
severe neurologic morbidity (30% to 50%). Cesarean
section is recommended for any woman with prodromal symptoms
or lesions, regardless of primary or secondary characteristics. In the nonpregnant state, acyclovir and similar agents, famciclovir and
valacyclovir (see Table 9), are given to reduce symptoms of primary disease
and are given long-term to reduce the risk of recurrent episodes. No
dramatic increase in fetal abnormalities has been noted in women
who were exposed inadvertently to acyclovir in the first trimester.28 No risks have been associated with third-trimester exposure.132 There seems to be no justification for termination of the pregnancy based
on acyclovir exposure alone. After further study, acyclovir may play
a role in reducing viral shedding and symptoms and allowing more vaginal
deliveries.132 HIV INFECTION.
HIV infection is one of the most important health problems confronting
women of reproductive age. Women are acquiring the infection at a faster
rate than men, although they represent a smaller proportion of the total
number of infected persons (30%).133
Among infected women, minorities are affected disproportionately: 62%
African-Americans, 18% Latinas, and 18% whites. An increasing
proportion (38%) of the infection in women is transmitted heterosexually.134
The HIV seropositivity rate among pregnant women in the United States
is about 1.8 per 1000 births. The overall AIDS rate, the more advanced
disease with CD4 counts less than 200 cells/μL, is 9.3 per 100,000
women compared with 32.4 per 100,000 men. Because women tend to acquire
HIV during their reproductive years, HIV in pregnancy is common. The population
at highest risk for HIV during pregnancy includes women who receive little
or no prenatal care (OR, 4.1; 95% CI, 1.9 to 8.4), adolescents
(4.7 in 1000 births), crack cocaine users (OR, 2.3; 95% CI, 4.3
to 4.63), and women with a history of STD (OR, 2.6; 95% CI, 1.5
to 4.5).134,135,136
When the seroconversion date is known, the adjusted relative risk of progression
from HIV infection to AIDS is 0.7 (95% CI, 0.4 to 1.2).137
Maternal-infant transmission is the primary means by which young children
become infected with HIV-1. In 1% to 30% of neonates, vertical
transmission occurs through infection in utero, the labor and
delivery process, or breastfeeding (Table 19). The risk of transmission seems to be higher when the mother is viremic (early
or advanced disease [AIDS]) or when there is prolonged rupture
of membranes with histologic or clinical chorioamnionitis. Children
who are infected have a fulminant course; most die within 3 to 4 years. TABLE 19. Risk of Neonatal HIV Infection
Population | Risk of Neonatal HIV Infection (%) |
No antiretroviral therapy and vaginal delivery | 25–30 |
Breastfeeding | 15–20 |
Zidovudine monotherapy | 8–9 |
Cesarean section (intact membranes, no labor, zidovudine monotherapy) | 1–2 |
Highly active antiretroviral therapy | 1–2 |
HIV RNA level < 1000 copies per mL no therapy | 9.8 |
HIV RNA levels < 1000 copies per mL plus zidovudine monotherapy | 1 | Until November 1994, little could be done to limit the vertical transmission
of HIV. At that time, results of the Pediatric AIDS Clinical Trials
Group Protocol 076 study were reported.138 In this study, 477 HIV-positive pregnant women with CD4 counts equal to
or greater than 200 cells/μL were randomized to receive no antiretroviral
therapy or antepartum zidovudine (100 mg five times daily), intrapartum
zidovudine (2 mg/kg intravenously, then 1 mg/kg/hr intravenously), and
zidovudine for the newborn (2 mg/kg by mouth every 6 hours
for 6 weeks). The risk of perinatal transmission was reduced dramatically
by 68%: from 26% in the control group to 8.3% in
the treated group (p < .0001). Few short-term toxic effects were seen in the mother
or infant. The neonatal hemoglobin in the zidovudine group was lower than
the control neonates (p < .01). The anemia in the zidovudine-exposed neonates returned
to normal by 12 weeks. The National Institutes of Health stopped the study
after interim analysis showed significant benefit, and the protocol
has become the standard of care. Unless there is documented evidence
of zidovudine toxicity or viral resistance, zidovudine should be included
in the treatment of pregnant women and their newborns.
Since 1994, several changes in management have evolved. Highly active
antiretroviral drug therapy (HAART), cesarean section, and the recommendation
not to breastfeed (in industrialized countries) have reduced the rate
of perinatal transmission to 1% to 2% (see Table
19). Although antiretroviral therapy is initiated in nonpregnant women
based on CD4 counts (<350 cells/μL) or viral load (>50,000
copies/mL), all pregnant women should receive antiretroviral therapy.137,139 The goal of antibiotic therapy in HIV is
to reduce the HIV RNA to below the minimum detectable by ultrasensitive
antigen testing and to maintain an absolute CD4+ cell
count greater than 500 cells/cm3. Secondary goals are to reduce
the development of HIV resistance to antiretroviral antibodies and to
reduce adverse drug effects.
Combinations of three classes of antibodies (see Table 12) are used in
highly active antiretroviral therapy: nucleoside reverse-transcriptase
inhibitors, non-nucleoside reverse-transcriptase inhibitors, and protease
inhibitors. In general, combination therapy includes at least two
reverse-transcriptase inhibitor agents, including zidovudine, and a protease
inhibitor. These agents are combined during pregnancy except when
there is viral resistance or toxic effects to zidovudine.139 There is good evidence that triple-agent therapy is more effective than
monotherapy or dual-agent therapy.140 Hydroxyurea sometimes is used in nonpregnant women but has been associated
with multiple fetal abnormalities in several animal models including
primates. Hydroxyurea is contraindicated in pregnancy. The appropriate
combination of reverse-transcriptase inhibitors or protease inhibitors
is determined by the patient’s level of disease, prior exposure
to antiretroviral drugs, and previous drug reactions. A collaborative
relationship with an infectious disease expert helps decide the
best combination of antiretroviral antibiotics to use. Antiretroviral antibiotics have many side effects and may have complicated
dosing regimens. Hyperglycemia (protease inhibitors), anemia (all
agents), rash (non-nucleoside reverse transcriptase inhibitors [17%]) hepatic
failure (all agents [1.3/1000 person-years]), lactic acidosis (all
agents [8/1000 person-years]), and lipodystrophy (all agents) are
the most common serious side effects. The incidence of life-threatening
adverse reactions is 0.5% to 1%. Mild nausea, malaise, diarrhea, and
perioral tingling and numbness occur in one in
three women with HAART. As a result of side effects, more than 10% of
pregnant women are noncompliant with medications. Case management
of pregnant women with HIV infection reduces noncompliance to a minimum
and reduces high-risk sexual behavior. Baseline laboratory examinations
include CD4 count, viral load, complete blood count, chemistry
panel, 1-hour glucose challenge test (protease inhibitors), lipid panel, hepatitis
panel, Venereal Disease Research Laboratory, purified protein
derivative, and antibody testing for toxoplasmosis and cytomegalovirus. Complete
blood count, liver function tests, CD4 count, and viral
load are obtained monthly during pregnancy. Vaccination for hepatitis
B, influenza, and pneumococcus are recommended regardless of pregnancy
status if the mother’s CD4 count is greater than 200 cells/μL. If
CD4 count is less than 200 cells/μL, trimethoprim-sulfamethoxazole (1 Bactrim DS orally twice a day, three times a week) is
started for Pneumocystis carinii prophylaxis. Inhaled pentamidine, 300 mg once a month, is an alternative
agent. When the CD4 count decreases to less than 100 cells/μL
other prophylactic therapies are essential and are recommended by the
infectious disease specialist. The recommendation to use HAART in all pregnant women raises the issue
of viral resistance. Previous exposure to monotherapy using zidovudine
induces viral resistance to 15% to 25% of isolates.137 The high activity of HAART may reduce the incidence of resistance, however, by
killing most of the virus. The real issue related to resistance
is patient compliance rather than HAART itself. The fetal effects of antiretroviral therapy are a major concern of the
mother and a cause for noncompliance. Except for zidovudine monotherapy, the
number of children exposed to other agents and combinations in
the first trimester is relatively small. Zidovudine does not seem to be
associated with an increase in birth defects. The other nucleoside reverse-transcriptase
inhibitors and protease inhibitors seem to be safe
based on animal studies. Animal studies have suggested a risk of birth
defects when there is exposure to high doses of delavirdine or efavirenz, non-nucleoside
reverse-transcriptase inhibitors. In general and
because a lack of human experience, it is prudent to initiate antiretroviral
therapy after the first trimester. If the patient is on an effective
HAART regimen when pregnancy is diagnosed, the regimen should not
be changed. Previous studies of pregnant women receiving HAART suggested an increase
in the risk of fetal growth restriction and preterm delivery. An analysis
of several large U.S. trials does not support these obsevations.141 After control for CD4 count and exposure to alcohol, tobacco, and illicit
drugs, the risks were as follows: preterm birth, 15% to 17%; LBW, 13% to 16%; low 5-minute Apgar score, les
than 1%; and fetal death, less than 1%. These risks were
regardless whether the women were on no therapy, monotherapy, or HAART. There
were 3265 patients (2123 received antiretroviral therapies) included
in the analysis.141 Vaginitis Vaginitis is a common problem during pregnancy (see Table 1). Candida, trichomonas, and bacterial vaginosis account for 80%; other
STDs, such as gonococcal or chlamydial cervicitis, account for
the rest. CANDIDA INFECTIONS. The high estrogen state of pregnancy encourages the growth of yeast species. Many
species become symptomatic spontaneously or after antibiotic
therapy; yeast infections occur in 10% to 20% of pregnancies. The
treatment for pregnant women is similar to that for nonpregnant
women (Table 20); the only caution is clarity of diagnosis. STDs may have similar symptoms
with more serious consequences. Over-the-counter symptomatic treatment
by patients without diagnosis should be discouraged. TABLE 20. Specific Regimen for Treatment of Vulvovaginitis in Pregnancy
Candidiasis Miconazole, terconazole
Bacterial vaginosis Metronidazole 500 mg PO tid × 7 days
Metronidazole gel 0.75% (5 g) bid × 5 days
Clindamycin cream 2% (5 g) every night × 7 days
Trichomoniasis First trimester: metronidazole gel 0.75% (5 g) bid × 5 days
After first trimester: metronidazole 2 g PO × 1 dose
TRICHOMONIASIS.
Trichomoniasis is a common STD during pregnancy and is associated with
premature rupture of membranes and preterm delivery. Symptomatic and asymptomatic
trichomoniasis should be treated during pregnancy, and the treatment of
choice is metronidazole (see Table 20). In the 1970s,
there was concern regarding chromosomal changes found in cell culture,
and subsequently use of metronidazole in pregnancy was discouraged. On
further review of animal experiments and human experience, however, metronidazole
now is designated with a category B fetal risk classification (see Tables
7 and 8). The use of metronidazole in the second
and third trimesters seems to be safe and may prevent preterm birth. In
the first trimester, it is prudent to delay systemic metronidazole treatment
until after 13 weeks. Although not curative, metronidazole gel can provide
significant symptomatic relief until curative systemic therapy can be
administered.
BACTERIAL VAGINOSIS. Bacterial vaginosis occurs in 15% to 30% of pregnant women. It
is characterized by abnormal vaginal microflora with high concentrations
of anaerobes. To diagnose bacterial vaginosis, three or four
of the following must be present on clinical examination: clue cells on
wet smear, vaginal pH greater than 4.5, amine odor on application of 10% potassium
hydroxide to vaginal secretions, and a homogeneous
gray discharge. On Gram stain, the absence of lactobacilli, together
with large numbers of G. vaginalis and Mobiluncus, is associated with a bacterial vaginosis score of 7 to 10 and is diagnostic
of bacterial vaginosis. Symptomatic bacterial vaginosis occurs in 5% to 10% of pregnant
women and is characterized by copious gray, watery homogeneous
discharge with a fishy odor, especially after coitus. Vulvar and vaginal
irritative symptoms are present, but are generally milder than in other
forms of vaginitis. Gram stain of vaginal secretions of asymptomatic
pregnant women shows an additional 10% to 20% to have
bacterial vaginosis.
Bacterial vaginosis is associated with preterm birth, preterm labor,
premature rupture of membranes, intra-amniotic infection, endometritis,
and postsurgical infection. In general, the degree of risk for preterm
birth is 1.5-fold to 2-fold; intra-amniotic infection, 2-fold to 3-fold;
endometritis, 3-fold to 6-fold; and postsurgical infection, 3-fold to
4-fold.11,13,55,92,142
Given the frequency of bacterial vaginosis in pregnancy (20% to 25%), should
all pregnant patients with bacterial vaginosis be
treated? In 1995, McGregor and colleagues115 reported the results of a prospective controlled trial of antepartum diagnosis
and treatment of cervicovaginal pathogens, including bacterial
vaginosis. Management during two sequential time periods was evaluated. The
outcome variable was incidence of preterm birth. In the first
time period (control phase), 559 women were included, with sampling at
first prenatal visit, between 22 and 29 weeks, and after 32 weeks. Treatment
was provided for N. gonorrhoeae, C. trachomatis, and symptomatic trichomoniasis or bacterial vaginosis. In the test period (n = 579), every patient with the latter conditions was treated
and had test of cure. Bacterial vaginosis (OR, 1.6; 95% CI, 1.1 to 2.4) was
associated with preterm birth. The test group was associated
with a reduction in preterm birth (OR, 0.66; 95% CI, 0.4 to 1.0). A
large study with random assignment to metronidazole treatment
or placebo failed to show a benefit.116 Symptomatic patients, asymptomatic patients at high risk for preterm birth, or
patients undergoing surgical procedures through the vagina (e.g., cerclage, chorionic
villus sampling, abortion) should be treated. The
low-risk patient with asymptomatic bacterial vaginosis remains a
clinical question. The goal of treatment is to reduce the number and concentration of anaerobes
in the lower genital tract and to preserve the Lactobacillus, which provides competitive inhibition of pathogens (see Table 20). Metronidazole (250 mg four times daily for 5 to 7 days) cures 80% to 90% of
cases and is superior to ampicillin or triple-sulfa
vaginal cream (60% to 70% cure).142 Oral clindamycin (300 mg twice a day for 7 days) is also effective in
nonpregnant patients, and it seems to be safe in pregnancy. Vaginal topical
medications containing metronidazole or clindamycin are equally
effective (80% to 90%) but are associated with approximately
a 10% occurrence of symptomatic candidal infection as a consequence
of treatment. The maternal serum levels of vaginally administered
clindamycin or metronidazole are considerably less than when the
antibiotics are given orally; the fetal risks are expected to be minimal. Clindamycin
may have the disadvantage of reducing levels of Lactobacillus, but the clinical significance of this is not known. |